Periodontal disease
| Periodontal disease | |
|---|---|
| Other names | Gum disease, pyorrhea, periodontitis |
 | |
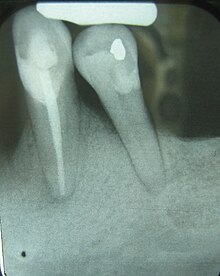
| Radiograph showing bone loss between the two roots of a tooth (black region). The spongy bone has receded due to infection under tooth, reducing the bony support for the tooth. | |
| Pronunciation |
|
| Specialty | Dentistry |
| Symptoms | Red, swollen, painful, bleeding gums, loose teeth, bad breath[1] |
| Complications | Tooth loss, gum abscess[1][2] |
| Causes | Bacteria related plaque build up[1] |
| Risk factors | Smoking, diabetes, HIV/AIDS, certain medications[1] |
| Diagnostic method | Dental examination, X-rays[1] |
| Treatment | Good oral hygiene, regular professional cleaning[3] |
| Frequency | 538 million (2015)[4] |
Periodontal disease, also known as gum disease, is a set of inflammatory conditions affecting the tissues surrounding the teeth.[3] In its early stage, called gingivitis, the gums become swollen, red, and may bleed.[3] In its more serious form, called periodontitis, the gums can pull away from the tooth, bone can be lost, and the teeth may loosen or fall out.[3] Bad breath may also occur.[1]
Periodontal disease is generally due to bacteria in the mouth infecting the tissue around the teeth.[3] Risk factors include smoking, diabetes, HIV/AIDS, family history, and certain medications.[1] Diagnosis is by inspecting the gum tissue around the teeth both visually and with a probe and X-rays looking for bone loss around the teeth.[5][1]
Treatment involves good oral hygiene and regular professional teeth cleaning.[3] Recommended oral hygiene include daily brushing and flossing.[3] In certain cases antibiotics or dental surgery may be recommended.[6] Globally 538 million people were estimated to be affected in 2015.[4] In the United States nearly half of those over the age of 30 are affected to some degree, while about 70% of those over the age of 65 have the condition.[3] Males are affected more often than females.[3]
Signs and symptoms

In the early stages, periodontitis has very few symptoms, and in many individuals the disease has progressed significantly before they seek treatment.
Symptoms may include:
- Redness or bleeding of gums while brushing teeth, using dental floss or biting into hard food (e.g., apples) (though this may occur even in gingivitis, where there is no attachment loss)
- Gum swelling that recurs
- Spitting out blood after brushing teeth
- Halitosis, or bad breath, and a persistent metallic taste in the mouth
- Gingival recession, resulting in apparent lengthening of teeth. (This may also be caused by heavy-handed brushing or with a stiff toothbrush.)
- Deep pockets between the teeth and the gums (pockets are sites where the attachment has been gradually destroyed by collagen-destroying enzymes, known as collagenases)
- Loose teeth, in the later stages (though this may occur for other reasons, as well)
Patients should realize gingival inflammation and bone destruction are largely painless. Hence, people may wrongly assume painless bleeding after teeth cleaning is insignificant, although this may be a symptom of progressing periodontitis in that patient.
Associated conditions
Periodontitis has been linked to increased inflammation in the body, such as indicated by raised levels of C-reactive protein and interleukin-6.[7][8][9][10] It is linked through this to increased risk of stroke,[11][12] myocardial infarction,[13] and atherosclerosis.[14][15][16][17][18][19][20] It also linked in those over 60 years of age to impairments in delayed memory and calculation abilities.[21][22] Individuals with impaired fasting glucose and diabetes mellitus have higher degrees of periodontal inflammation, and often have difficulties with balancing their blood glucose level owing to the constant systemic inflammatory state, caused by the periodontal inflammation.[23][24] Although no causal association was proven, a recent study showed correlation between chronic periodontitis and erectile dysfunction.[25]
Causes
Periodontitis is an inflammation of the periodontium, i.e., the tissues that support the teeth. The periodontium consists of four tissues:
- gingiva, or gum tissue,
- cementum, or outer layer of the roots of teeth,
- alveolar bone, or the bony sockets into which the teeth are anchored, and
- periodontal ligaments (PDLs), which are the connective tissue fibers that run between the cementum and the alveolar bone.
The primary cause of gingivitis is poor or ineffective oral hygiene[26], which leads to the accumulation of a mycotic[27][28][29][30] and bacterial matrix at the gum line, called dental plaque. Other contributors are poor nutrition and underlying medical issues such as diabetes.[31] Diabetics must be meticulous with their homecare to control periodontal disease.[32] New finger prick tests have been approved by the Food and Drug Administration in the US, and are being used in dental offices to identify and screen patients for possible contributory causes of gum disease, such as diabetes.
In some people, gingivitis progresses to periodontitis – with the destruction of the gingival fibers, the gum tissues separate from the tooth and deepened sulcus, called a periodontal pocket. Subgingival microorganisms (those that exist under the gum line) colonize the periodontal pockets and cause further inflammation in the gum tissues and progressive bone loss. Examples of secondary causes are those things that, by definition, cause microbic plaque accumulation, such as restoration overhangs and root proximity.

Smoking is another factor that increases the occurrence of periodontitis, directly or indirectly,[33][34][35] and may interfere with or adversely affect its treatment.[36][37][38] It is arguably the most important environmental risk factor for periodontitis. Research has shown that smokers have more bone loss, attachment loss and tooth loss compared to non-smokers.[39] The reason for this is that smoking has several effects on the immune response including:
- Decreased wound healing
- Suppresses antibody production
- It reduces neutrophilic phagocytosis [39]
Ehlers–Danlos syndrome is a periodontitis risk factor and so is the Papillon–Lefèvre syndrome also known as palmoplantar keratoderma.
If left undisturbed, microbial plaque calcifies to form calculus, which is commonly called tartar. Calculus above and below the gum line must be removed completely by the dental hygienist or dentist to treat gingivitis and periodontitis. Although the primary cause of both gingivitis and periodontitis is the microbial plaque that adheres to the tooth surfaces, there are many other modifying factors. A very strong risk factor is one's genetic susceptibility. Several conditions and diseases, including Down syndrome, diabetes, and other diseases that affect one's resistance to infection, also increase susceptibility to periodontitis.
Another factor that makes periodontitis a difficult disease to study is that human host response can also affect the alveolar bone resorption. Host response to the bacterial-mycotic insult is mainly determined by genetics; however, immune development may play some role in susceptibility.
According to some researchers periodontitis may be associated with higher stress.[40] Periodontitis occurs more often in people from the lower end of the socioeconomic scale than people from the upper end of the socioeconomic scale.[41]
Genetics appear to play a role in determining the risk for periodontitis. It is believed genetics could explain why some patients with good plaque control have advanced periodontitis, whilst some others with poor oral hygiene are free from the disease. Genetic factors which could modify the risk of a patient developing periodontitis include:
- Defects of Phagocytosis: patient may have a hypo-responsive phagocytes.
- Hyper-production of interleukins, prostaglandins and cytokines. This results in a massively exaggerated immune response.
- Interleukin 1 (IL-1) gene polymorphism: Patients with this polymorphism produce more IL-1, and subsequently are more at risk of developing chronic periodontitis.[39]
Diabetes appears to exacerbates the onset, progression, and severity of periodontitis.[42] Although the majority of research has focused on type 2 diabetes, type 1 diabetes appears to have an identical effect on the risk for periodontitis.[43] The extent of the increased risk of periodontitis is dependent on the level of glycaemic control. Therefore, in well managed diabetes there seems to be a small effect of diabetes on the risk for periodontitis. However, the risk increases exponentially as glycaemic control worsens.[43] Overall, the increased risk of periodontitis in diabetics is estimated to be between 2-3 times higher.[42] So far, the mechanisms underlying the link are not fully understood, but it’s known to involve aspects of inflammation, immune functioning, neutrophil activity, and cytokine biology.[43][44]
Mechanism
As dental plaque or biofilm accumulates on the teeth near and below the gums that is some dysbiosis of the normal oral microbiome.[45] As of 2017 it was not certain what species are most responsible for causing harm but gram-negative anaerobic bacteria, spirochetes, and viruses have been suggested; in individual people it is sometimes clear that one or more species is driving disease.[45] Current research has indicated 3 species gram negative anerobic species: Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Bacteroides forsythus and Eikenella corrodens.[39]
Plaque may be soft and uncalcified, hard and calcified, or both; for plaques that are on teeth the calcium comes from saliva; for plaques below the gumline, it comes from blood via oozing of inflamed gums.[45]
The damage to teeth and gums comes from the immune system as it attempts to destroy the microbes that are disrupting the normal symbiosis between the oral tissues and the oral microbe community. As in other tissues, Langerhans cells in the epithelium take up antigens from the microbes, and present them to the immune system, leading to movement of white blood cells into the affected tissues. This process in turn activates osteoclasts which begin to destroy bone, and it activates matrix metalloproteinases that destroy ligaments.[45] So, in summary, it is bacteria which initiates the disease, but key destructive events are brought about by the exaggerated response from the host's immune system.[39]
Diagnosis
Classification
The 1999 classification system for periodontal diseases and conditions listed seven major categories of periodontal diseases,[46] of which 2–6 are termed destructive periodontal disease, because the damage is essentially irreversible. The seven categories are as follows:
- Gingivitis
- Chronic periodontitis
- Aggressive periodontitis
- Periodontitis as a manifestation of systemic disease
- Necrotizing ulcerative gingivitis/periodontitis
- Abscesses of the periodontium
- Combined periodontic-endodontic lesions
Moreover, terminology expressing both the extent and severity of periodontal diseases are appended to the terms above to denote the specific diagnosis of a particular patient or group of patients.
Extent
The "extent" of disease refers to the proportion of the dentition affected by the disease in terms of percentage of sites. Sites are defined as the positions at which probing measurements are taken around each tooth and, generally, six probing sites around each tooth are recorded, as follows:
- mesiobuccal
- mid-buccal
- distobuccal
- mesiolingual
- mid-lingual
- distolingual
If up to 30% of sites in the mouth are affected, the manifestation is classified as "localized"; for more than 30%, the term "generalized" is used.
Severity
The "severity" of disease refers to the amount of periodontal ligament fibers that have been lost, termed "clinical attachment loss". According to the American Academy of Periodontology, the classification of severity is as follows:[47]
- Mild: 1–2 mm (0.039–0.079 in) of attachment loss
- Moderate: 3–4 mm (0.12–0.16 in) of attachment loss
- Severe: ≥ 5 mm (0.20 in) of attachment loss
Prevention
Daily oral hygiene measures to prevent periodontal disease include:
- Brushing properly on a regular basis (at least twice daily), with the patient attempting to direct the toothbrush bristles underneath the gumline, helps disrupt the bacterial-mycotic growth and formation of subgingival plaque.[citation needed]
- Flossing daily and using interdental brushes (if the space between teeth is large enough), as well as cleaning behind the last tooth, the third molar, in each quarter[citation needed]
- Using an antiseptic mouthwash: Chlorhexidine gluconate-based mouthwash in combination with careful oral hygiene may cure gingivitis, although they cannot reverse any attachment loss due to periodontitis.[citation needed]
- Using periodontal trays to maintain dentist-prescribed medications at the source of the disease: The use of trays allows the medication to stay in place long enough to penetrate the biofilms where the microorganism are found.
- Regular dental check-ups and professional teeth cleaning as required: Dental check-ups serve to monitor the person's oral hygiene methods and levels of attachment around teeth, identify any early signs of periodontitis, and monitor response to treatment.
- Microscopic evaluation of biofilm may serve as a guide to regaining commensal health flora.[48]
Typically, dental hygienists (or dentists) use special instruments to clean (debride) teeth below the gumline and disrupt any plaque growing below the gumline. This is a standard treatment to prevent any further progress of established periodontitis. Studies show that after such a professional cleaning (periodontal debridement), microbial plaque tends to grow back to precleaning levels after about three to four months. Nonetheless, the continued stabilization of a patient's periodontal state depends largely, if not primarily, on the patient's oral hygiene at home, as well as on the go. Without daily oral hygiene, periodontal disease will not be overcome, especially if the patient has a history of extensive periodontal disease.[citation needed]
Management

The cornerstone of successful periodontal treatment starts with establishing excellent oral hygiene. This includes twice-daily brushing with daily flossing. Also, the use of an interdental brush is helpful if space between the teeth allows. For smaller spaces, products such as narrow picks with soft rubber bristles provide excellent manual cleaning. Persons with dexterity problems, such as arthritis, may find oral hygiene to be difficult and may require more frequent professional care and/or the use of a powered toothbrush. Persons with periodontitis must realize it is a chronic inflammatory disease and a lifelong regimen of excellent hygiene and professional maintenance care with a dentist/hygienist or periodontist is required to maintain affected teeth.
Initial therapy
Removal of microbial plaque and calculus is necessary to establish periodontal health. The first step in the treatment of periodontitis involves nonsurgical cleaning below the gumline with a procedure called Root Surface Instrumentation or RSI, this causes a mechanical disturbance to the bacterial biofilm below the gumline.[39] This procedure involves the use of specialized curettes to mechanically remove plaque and calculus from below the gumline, and may require multiple visits and local anesthesia to adequately complete. In addition to initial Root Surface Instrumentation, it may also be necessary to adjust the occlusion (bite) to prevent excessive force on teeth that have reduced bone support. Also, it may be necessary to complete any other dental needs, such as replacement of rough, plaque-retentive restorations, closure of open contacts between teeth, and any other requirements diagnosed at the initial evaluation. It is important to note that RSI is different to Scaling and Root planing: RSI only removes the calculus, whilst scaling and root planing removes the calculus as well as underlying softened dentine, which leaves behind a smooth and glassy surface, which is not a requiste for periodontal healing. Therefore RSI is now advocated over root planing.[39]
Reevaluation
Nonsurgical scaling and root planing are usually successful if the periodontal pockets are shallower than 4–5 mm (0.16–0.20 in).[49][50][51] The dentist or hygienist must perform a re-evaluation four to six weeks after the initial scaling and root planing, to determine if the patient's oral hygiene has improved and inflammation has regressed. Probing should be avoided then, and an analysis by gingival index should determine the presence or absence of inflammation. The monthly reevaluation of periodontal therapy should involve periodontal charting as a better indication of the success of treatment, and to see if other courses of treatment can be identified. Pocket depths of greater than 5–6 mm (0.20–0.24 in) which remain after initial therapy, with bleeding upon probing, indicate continued active disease and will very likely lead to further bone loss over time. This is especially true in molar tooth sites where furcations (areas between the roots) have been exposed.
Surgery
If nonsurgical therapy is found to have been unsuccessful in managing signs of disease activity, periodontal surgery may be needed to stop progressive bone loss and regenerate lost bone where possible. Many surgical approaches are used in the treatment of advanced periodontitis, including open flap debridement and osseous surgery, as well as guided tissue regeneration and bone grafting. The goal of periodontal surgery is access for definitive calculus removal and surgical management of bony irregularities which have resulted from the disease process to reduce pockets as much as possible. Long-term studies have shown, in moderate to advanced periodontitis, surgically treated cases often have less further breakdown over time and, when coupled with a regular post-treatment maintenance regimen, are successful in nearly halting tooth loss in nearly 85% of patients.[52][53]
Local drug delivery
Local drug deliverys in periodontology has gained acceptance and popularity compared to systemic drugs due to decreased risk in development of resistant flora and other side effects.[54] A meta analysis of local tetracycline found improvement.[55] Local application of statin may be useful.[56]
Maintenance
Once successful periodontal treatment has been completed, with or without surgery, an ongoing regimen of "periodontal maintenance" is required. This involves regular checkups and detailed cleanings every three months to prevent repopulation of periodontitis-causing microorganisms, and to closely monitor affected teeth so early treatment can be rendered if the disease recurs. Usually, periodontal disease exists due to poor plaque control, therefore if the brushing techniques are not modified, a periodontal recurrence is probable.
Other
Most alternative "at-home" gum disease treatments involve injecting antimicrobial solutions, such as hydrogen peroxide, into periodontal pockets via slender applicators or oral irrigators. This process disrupts anaerobic micro-organism colonies and is effective at reducing infections and inflammation when used daily. A number of other products, functionally equivalent to hydrogen peroxide, are commercially available, but at substantially higher cost. However, such treatments do not address calculus formations, and so are short-lived, as anaerobic microbial colonies quickly regenerate in and around calculus.
Doxycycline may be given alongside the primary therapy of scaling (see § initial therapy).[57] Doxycycline has been shown to improve indicators of disease progression (namely probing depth and attachment level).[57] Its mechanism of action involves inhibition of matrix metalloproteinases (such as collagenase), which degrade the teeth's supporting tissues (periodontium) under inflammatory conditions.[57] To avoid killing beneficial oral microbes, only small doses of doxycycline (20 mg) are used.[57]
Prognosis
Dentists and dental hygienists measure periodontal disease using a device called a periodontal probe. This thin "measuring stick" is gently placed into the space between the gums and the teeth, and slipped below the gumline. If the probe can slip more than 3 mm (0.12 in) below the gumline, the patient is said to have a gingival pocket if no migration of the epithelial attachment has occurred or a periodontal pocket if apical migration has occurred. This is somewhat of a misnomer, as any depth is, in essence, a pocket, which in turn is defined by its depth, i.e., a 2-mm pocket or a 6-mm pocket. However, pockets are generally accepted as self-cleansable (at home, by the patient, with a toothbrush) if they are 3 mm or less in depth. This is important because if a pocket is deeper than 3 mm around the tooth, at-home care will not be sufficient to cleanse the pocket, and professional care should be sought. When the pocket depths reach 6 to 7 mm (0.24 to 0.28 in) in depth, the hand instruments and cavitrons used by the dental professionals may not reach deeply enough into the pocket to clean out the microbial plaque that causes gingival inflammation. In such a situation, the bone or the gums around that tooth should be surgically altered or it will always have inflammation which will likely result in more bone loss around that tooth. An additional way to stop the inflammation would be for the patient to receive subgingival antibiotics (such as minocycline) or undergo some form of gingival surgery to access the depths of the pockets and perhaps even change the pocket depths so they become 3 mm or less in depth and can once again be properly cleaned by the patient at home with his or her toothbrush.
If patients have 7-mm or deeper pockets around their teeth, then they would likely risk eventual tooth loss over the years. If this periodontal condition is not identified and the patients remain unaware of the progressive nature of the disease, then years later, they may be surprised that some teeth will gradually become loose and may need to be extracted, sometimes due to a severe infection or even pain.
According to the Sri Lankan tea laborer study, in the absence of any oral hygiene activity, approximately 10% will suffer from severe periodontal disease with rapid loss of attachment (>2 mm/year). About 80% will suffer from moderate loss (1–2 mm/year) and the remaining 10% will not suffer any loss.[58][59]
Epidemiology

| no data <3.5 3.5–4 4–4.5 4.5–5 5–5.5 5.5–6 | 6–6.5 6.5–7 7–7.5 7.5–8 8–8.5 >8.5 |
Periodontitis is very common, and is widely regarded as the second most common dental disease worldwide, after dental decay, and in the United States has a prevalence of 30–50% of the population, but only about 10% have severe forms.
Chronic periodontitis affects about 750 million people or about 10.8% of the population as of 2010.[61]
Like other conditions intimately related to access to hygiene and basic medical monitoring and care, periodontitis tends to be more common in economically disadvantaged populations or regions. Its occurrence decreases with a higher standard of living. In Israeli population, individuals of Yemenite, North-African, South Asian, or Mediterranean origin have higher prevalence of periodontal disease than individuals from European descent.[62] Periodontitis is frequently reported to be socially patterned, i.e. people from the lower end of the socioeconomic scale suffer more often from it than people from the upper end of the socioeconomic scale.[63]
Society and culture
Etymology
The word "periodontitis" (Greek: περιοδοντίτις) comes from the Greek peri, "around", odous (GEN odontos), "tooth", and the suffix -itis, in medical terminology "inflammation".[64] The word pyorrhea (alternative spelling: pyorrhoea) comes from the Greek pyorrhoia (πυόρροια), "discharge of matter", itself from pyon, "discharge from a sore", rhoē, "flow", and the suffix -ia.[65] In English this term can describe, as in Greek, any discharge of pus; i.e. it is not restricted to these diseases of the teeth.[66]
Economics
It is estimated that periodontitis results in worldwide productivity losses in the size of about US$54 billion yearly.[67]
Vaccine research
A therapeutic vaccine against Porphyromonas gingivalis has been tested in mice and is now planning for human trials in 2018.[68]
Other animals
Periodontal disease is the most common disease found in dogs and affects more than 80% of dogs aged three years or older. Its prevalence in dogs increases with age, but decreases with increasing body weight; i.e., toy and miniature breeds are more severely affected. Recent research undertaken at the Waltham Centre for Pet Nutrition has established that the bacteria associated with gum disease in dogs are not the same as in humans.[69] Systemic disease may develop because the gums are very vascular (have a good blood supply). The blood stream carries these anaerobic micro-organisms, and they are filtered out by the kidneys and liver, where they may colonize and create microabscesses. The microorganisms traveling through the blood may also attach to the heart valves, causing vegetative infective endocarditis (infected heart valves). Additional diseases that may result from periodontitis include chronic bronchitis and pulmonary fibrosis.[70]
Footnotes
- ^ a b c d e f g h "Gum Disease". National Institute of Dental and Craniofacial Research. February 2018. Retrieved 13 March 2018.
- ^ "Gum Disease Complications". nhs.uk. Retrieved 13 March 2018.
- ^ a b c d e f g h i "Periodontal Disease". CDC. 10 March 2015. Retrieved 13 March 2018.
- ^ a b GBD 2015 Disease and Injury Incidence and Prevalence, Collaborators. (8 October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
{{cite journal}}:|first1=has generic name (help)CS1 maint: numeric names: authors list (link) - ^ Savage, Amir; Eaton, Kenneth A.; Moles, David R.; Needleman, Ian (2009). "A systematic review of definitions of periodontitis and methods that have been used to identify this disease". Journal of Clinical Periodontology. 36 (6): 458–67. doi:10.1111/j.1600-051X.2009.01408.x. PMID 19508246.
- ^ "Gum Disease Treatment". nhs.uk. Retrieved 13 March 2018.
- ^ D'Aiuto, Francesco; Parkar, Mohammed; Andreou, Georgios; Suvan, Hannu; Brett, Peter M.; Ready, Derren; Tonetti, Maurizio S. (2004). "Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers". J Dent Res. 83 (2): 156–60. doi:10.1177/154405910408300214. PMID 14742655.
- ^ Nibali, Luigi; D'Aiuto, Francesco; Griffiths, Gareth; Patel, Kalpesh; Suvan, Jean; Tonetti, Maurizio S. (2007). "Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case-control study". Journal of Clinical Periodontology. 34 (11): 931–37. doi:10.1111/j.1600-051X.2007.01133.x. PMID 17877746.
- ^ Paraskevas, Spiros; Huizinga, John D.; Loos, Bruno G. (2008). "A systematic review and meta-analyses on C-reactive protein in relation to periodontitis". Journal of Clinical Periodontology. 35 (4): 277–90. doi:10.1111/j.1600-051X.2007.01173.x. PMID 18294231.
- ^ D'Aiuto, Francesco; Ready, Derren; Tonetti, Maurizio S. (2004). "Periodontal disease and C-reactive protein-associated cardiovascular risk". Journal of Periodontal Research. 39 (4): 236–41. doi:10.1111/j.1600-0765.2004.00731.x. PMID 15206916.
- ^ Pussinen PJ, Alfthan G, Jousilahti P, Paju S, Tuomilehto J (2007). "Systemic exposure to Porphyromonas gingivalis predicts incident stroke". Atherosclerosis. 193 (1): 222–28. doi:10.1016/j.atherosclerosis.2006.06.027. PMID 16872615.
- ^ Pussinen PJ, Alfthan G, Rissanen H, Reunanen A, Asikainen S, Knekt P (September 2004). "Antibodies to periodontal pathogens and stroke risk". Stroke. 35 (9): 2020–23. doi:10.1161/01.STR.0000136148.29490.fe. PMID 15232116.
- ^ Pussinen PJ, Alfthan G, Tuomilehto J, Asikainen S, Jousilahti P (October 2004). "High serum antibody levels to Porphyromonas gingivalis predict myocardial infarction". European Journal of Cardiovascular Prevention & Rehabilitation. 11 (5): 408–11. doi:10.1097/01.hjr.0000129745.38217.39. PMID 15616414.
- ^ Ford PJ, Gemmell E, Timms P, Chan A, Preston FM, Seymour GJ (2007). "Anti-P. gingivalis response correlates with atherosclerosis". J Dent Res. 86 (1): 35–40. doi:10.1177/154405910708600105. PMID 17189460.
- ^ Beck, James D.; Eke, Paul; Heiss, Gerardo; Madianos, Phoebus; Couper, David; Lin, Dongming; Moss, Kevin; Elter, John; Offenbacher, Steven (2005). "Periodontal Disease and Coronary Heart Disease : A Reappraisal of the Exposure". Circulation. 112 (1): 19–24. doi:10.1161/CIRCULATIONAHA.104.511998. PMID 15983248.
- ^ Scannapieco, Frank A.; Bush, Renee B.; Paju, Susanna (2003). "Associations Between Periodontal Disease and Risk for Atherosclerosis, Cardiovascular Disease, and Stroke. A Systematic Review". Annals of Periodontology. 8 (1): 38–53. doi:10.1902/annals.2003.8.1.38. PMID 14971247.
- ^ Wu, Tiejian; Trevisan, Maurizio; Genco, Robert J.; Dorn, Joan P.; Falkner, Karen L.; Sempos, Christopher T. (2000). "Periodontal Disease and Risk of Cerebrovascular Disease: The First National Health and Nutrition Examination Survey and Its Follow-up Study". Archives of International Medicine. 160 (18): 2749–55. doi:10.1001/archinte.160.18.2749. PMID 11025784.
- ^ Beck, James D.; Elter, John R.; Heiss, Gerardo; Couper, David; Mauriello, Sally M.; Offenbacher, Steven (2001). "Relationship of Periodontal Disease to Carotid Artery Intima-Media Wall Thickness : The Atherosclerosis Risk in Communities (ARIC) Study". Arteriosclerosis, Thrombosis, and Vascular Biology. 21 (11): 1816–22. doi:10.1161/hq1101.097803.
- ^ Elter, John R.; Champagne, Catherine M.E.; Beck, James D.; Offenbacher, Steven (2004). "Relationship of Periodontal Disease and Tooth Loss to Prevalence of Coronary Heart Disease". Journal of Periodontology. 75 (6): 782–90. doi:10.1902/jop.2004.75.6.782. PMID 15295942.
- ^ Humphrey, Linda L.; Fu, Rongwei; Buckley, David I.; Freeman, Michele; Helfand, Mark (2008). "Periodontal Disease and Coronary Heart Disease Incidence: A Systematic Review and Meta-analysis". Journal of General Internal Medicine. 23 (12): 2079–86. doi:10.1007/s11606-008-0787-6. PMC 2596495. PMID 18807098.
- ^ Noble JM, Borrell LN, Papapanou PN, Elkind MS, Scarmeas N, Wright CB (2009). "Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III". J Neurol Neurosurg Psychiatry. 80 (11): 1206–11. doi:10.1136/jnnp.2009.174029. PMC 3073380. PMID 19419981.
- ^ Kaye, Elizabeth Krall; Valencia, Aileen; Baba, Nivine; Spiro III, Avron; Dietrich, Thomas; Garcia, Raul I. (2010). "Tooth Loss and Periodontal Disease Predict Poor Cognitive Function in Older Men". Journal of the American Geriatrics Society. 58 (4): 713–18. doi:10.1111/j.1532-5415.2010.02788.x. PMC 3649065. PMID 20398152.
- ^ Zadik Y, Bechor R, Galor S, Levin L (May 2010). "Periodontal disease might be associated even with impaired fasting glucose". Br Dent J. 208 (10): e20. doi:10.1038/sj.bdj.2010.291. PMID 20339371.
- ^ Soskolne WA, Klinger A (December 2001). "The relationship between periodontal diseases and diabetes: an overview". Ann Periodontol. 6 (1): 91–98. doi:10.1902/annals.2001.6.1.91. PMID 11887477.
- ^ Zadik Y, Bechor R, Galor S, Justo D, Heruti RJ (April 2009). "Erectile dysfunction might be associated with chronic periodontal disease: two ends of the cardiovascular spectrum". J Sex Med. 6 (4): 1111–16. doi:10.1111/j.1743-6109.2008.01141.x. PMID 19170861.
- ^ "Gingivitis". Mayo Clinic. Rochester, Minnesota: MFMER. 2017-08-04. Retrieved 10 May 2018.
- ^ Crich, Aubrey (1932). "Blastomycosis of the gingiva and jaw". Can Med Assoc J. 26 (6): 662–65. PMC 402380. PMID 20318753.
- ^ Urzúa B, Hermosilla G, Gamonal J, Morales-Bozo I, Canals M, Barahona S, Cóccola C, Cifuentes V (2008). "Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets". Med Mycol. 46 (8): 783–93. doi:10.1080/13693780802060899. PMID 18608938.
- ^ Matsuo T, Nakagawa H, Matsuo N (1995). "Endogenous Aspergillus endophthalmitis associated with periodontitis". Ophthalmologica. 209 (2): 109–11. doi:10.1159/000310592. PMID 7746643.
- ^ Migliari, Dante A.; Sugaya, Norberto N.; Mimura, Maria A.; Cucé, Luiz Carlos (1998). "Periodontal aspects of the juvenile form of paracoccidioidomycosis". Revista do Instituto de Medicina Tropical de São Paulo. 40 (1). doi:10.1590/S0036-46651998000100004. ISSN 0036-4665.
- ^ Lalla, Evanthia; Cheng, Bin; Lal, Shantanu; Kaplan, Selma; Softness, Barney; Greenberg, Ellen; Goland, Robin S.; Lamster, Ira B. (2007). "Diabetes mellitus promotes periodontal destruction in children". Journal of Clinical Periodontology. 34 (4): 294–98. doi:10.1111/j.1600-051X.2007.01054.x. PMID 17378885.
- ^ "Diabetes and Periodontal Disease". WebMD.
- ^ Obeid, Patrick; Bercy, P. (2000). "Effects of smoking on periodontal health: A review". Advances in Therapy. 17 (5): 230–37. doi:10.1007/BF02853162. PMID 11186143.
- ^ Tomar, Scott L.; Asma, Samira (2000). "Smoking-Attributable Periodontitis in the United States: Findings From NHANES III". Journal of Periodontology. 71 (5): 743–51. doi:10.1902/jop.2000.71.5.743. PMID 10872955.
- ^ Ryder, Mark I. (2007). "The influence of smoking on host responses in periodontal infections". Periodontology 2000. 43 (1): 267–77. doi:10.1111/j.1600-0757.2006.00163.x. PMID 17214844.
- ^ Pauletto, Nathalie C.; Liede, Kirsti; Nieminen, Anja; Larjava, Hannu; Uitto, Veli-Jukka (2000). "Effect of Cigarette Smoking on Oral Elastase Activity in Adult Periodontitis Patients". Journal of Periodontology. 71 (1): 58–62. doi:10.1902/jop.2000.71.1.58. PMID 10695939.
- ^ Persson, Lena; Bergström, Jan; Gustafsson, Anders (2003). "Effect of Tobacco Smoking on Neutrophil Activity Following Periodontal Surgery". Journal of Periodontology. 74 (10): 1475–82. doi:10.1902/jop.2003.74.10.1475. PMID 14653394.
- ^ Bergström, Jan; Boström, Lennart (2001). "Tobacco smoking and periodontal hemorrhagic responsiveness". Journal of Clinical Periodontology. 28 (7): 680–85. doi:10.1034/j.1600-051x.2001.028007680.x. PMID 11422590.
- ^ a b c d e f g "Dental Update: Archive - Article: Current Concepts in Periodontal Pathogenesis". www.dental-update.co.uk. Retrieved 2018-02-22.
- ^ Peruzzo, Daiane C.; Benatti, Bruno B.; Ambrosano, Glaucia M.B.; Nogueira-Filho, Getúlio R.; Sallum, Enilson A.; Casati, Márcio Z.; Nociti Jr., Francisco H. (2007). "A Systematic Review of Stress and Psychological Factors as Possible Risk Factors for Periodontal Disease". Journal of Periodontology. 78 (8): 1491–1504. doi:10.1902/jop.2007.060371. PMID 17668968.
- ^ Watt RG, Listl S, Peres MA, Heilmann A, editors. "Social inequalities in oral health: from evidence to action". London: International Centre for Oral Health Inequalities Research & Policy
- ^ a b Teeuw, Wijnand J.; Kosho, Madeline X. F.; Poland, Dennis C. W.; Gerdes, Victor E. A.; Loos, Bruno G. (2017-01-01). "Periodontitis as a possible early sign of diabetes mellitus". BMJ Open Diabetes Research and Care. 5 (1): e000326. doi:10.1136/bmjdrc-2016-000326. ISSN 2052-4897.
- ^ a b c Casanova, L.; Hughes, F. J.; Preshaw, P. M. (October 2014). "Diabetes and periodontal disease: a two-way relationship". British Dental Journal. 217 (8): 433–437. doi:10.1038/sj.bdj.2014.907. ISSN 1476-5373.
- ^ Taylor, John J.; Preshaw, Philip M.; Lalla, Evanthia (2013-04-01). "A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes". Journal of Clinical Periodontology. 40: S113–S134. doi:10.1111/jcpe.12059. ISSN 1600-051X.
- ^ a b c d Kinane, DF; Stathopoulou, PG; Papapanou, PN (22 June 2017). "Periodontal diseases". Nature Reviews. Disease Primers. 3: 17038. doi:10.1038/nrdp.2017.38. PMID 28805207.
- ^ Armitage, Gary C. (1999). "Development of a classification system for periodontal diseases and conditions". Annals of Periodontology. Vol. 4, no. 1. pp. 1–6. doi:10.1902/annals.1999.4.1.1.
- ^ "The Periodontal Disease Classification System of the American Academy of Periodontology – An Update". American Academy of Periodontology.
- ^ Bonner M. To Kiss or Not to Kiss. A cure for gum disease. Amyris Editions, 2013 EAN : 978-28755-2016-6
- ^ Stambaugh RV, Dragoo M, Smith DM, Carasali L (1981). "The limits of subgingival scaling". Int J Periodontics Restorative Dent. 1 (5): 30–41. PMID 7047434.
- ^ Waerhaug J (January 1978). "Healing of the dento-epithelial junction following subgingival plaque control. I. As observed in human biopsy material". J Periodontol. 49 (1): 1–8. doi:10.1902/jop.1978.49.1.1. PMID 340634.
- ^ Waerhaug J (March 1978). "Healing of the dento-epithelial junction following subgingival plaque control. II: As observed on extracted teeth". J Periodontol. 49 (3): 119–34. doi:10.1902/jop.1978.49.3.119. PMID 288899.
- ^ Kaldahl WB, Kalkwarf KL, Patil KD, Molvar MP, Dyer JK (February 1996). "Long-term evaluation of periodontal therapy: II. Incidence of sites breaking down". J. Periodontol. 67 (2): 103–8. doi:10.1902/jop.1996.67.2.103. PMID 8667129.
- ^ Hirschfeld L, Wasserman B (May 1978). "A long-term survey of tooth loss in 600 treated periodontal patients". J. Periodontol. 49 (5): 225–37. doi:10.1902/jop.1978.49.5.225. PMID 277674.
- ^ Kalsi, R (2011). "Effect of local drug delivery in chronic periodontitis patients: A meta-analysis". J Indian Soc Periodontol. 15: 304–9. doi:10.4103/0972-124X.92559. PMC 3283924. PMID 22368351.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Nadig PS, Shah MA (2016). "Tetracycline as local drug delivery in treatment of chronic periodontitis: A systematic review and meta-analysis". J Indian Soc Periodontol. 20 (6): 576–583. doi:10.4103/jisp.jisp_97_17. PMC 5713079. PMID 29238136.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Bertl, K; Parllaku, A; Pandis, N; Buhlin, K; Klinge, B; Stavropoulos, A (1 November 2017). "The effect of local and systemic statin use as an adjunct to non-surgical and surgical periodontal therapy-A systematic review and meta-analysis". Journal of dentistry. 67: 18–28. doi:10.1016/j.jdent.2017.08.011. PMID 28855141.
- ^ a b c d Caton J, Ryan ME (2011). "Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD)". Pharmacol. Res. 63 (2): 114–20. doi:10.1016/j.phrs.2010.12.003.
- ^ Preus HR, Anerud A, Boysen H, Dunford RG, Zambon JJ, Loe H (1995). "The natural history of periodontal disease. The correlation of selected microbiological parameters with disease severity in Sri Lankan tea workers". J Clin Periodontol. 22 (9): 674–78. doi:10.1111/j.1600-051X.1995.tb00825.x. PMID 7593696.
- ^ Ekanayaka, Asoka (1984). "Tooth mortality in plantation workers and residents in Sri Lanka". Community Dent Oral Epidemiol. 12 (2): 128–35. doi:10.1111/j.1600-0528.1984.tb01425.x. PMID 6584263.
- ^ "Mortality and Burden of Disease Estimates for WHO Member States in 2002" (xls). World Health Organization. 2002.
- ^ Vos, T; Flaxman, Abraham D; Naghavi, Mohsen; Lozano, Rafael; Michaud, Catherine; Ezzati, Majid; Shibuya, Kenji; Salomon, Joshua A; Abdalla, Safa; Aboyans, Victor; Abraham, Jerry; Ackerman, Ilana; Aggarwal, Rakesh; Ahn, Stephanie Y; Ali, Mohammed K; Almazroa, Mohammad A; Alvarado, Miriam; Anderson, H Ross; Anderson, Laurie M; Andrews, Kathryn G; Atkinson, Charles; Baddour, Larry M; Bahalim, Adil N; Barker-Collo, Suzanne; Barrero, Lope H; Bartels, David H; Basáñez, Maria-Gloria; Baxter, Amanda; Bell, Michelle L; et al. (Dec 15, 2012). "Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2163–96. doi:10.1016/S0140-6736(12)61729-2. PMID 23245607.
- ^ Zadik Y, Bechor R, Shochat Z, Galor S (2008). "[Ethnic origin and alveolar bone loss in Israeli adults]". Refuat Hapeh Vehashinayim (1993) (in Hebrew). 25 (2): 19–22, 72. PMID 18780541.
- ^ Watt RG, Listl S, Peres MA, Heilmann A, editors. Social inequalities in oral health: from evidence to action. London: International Centre for Oral Health Inequalities Research & Policy; www.icohirp.com
- ^ Harper, Douglas. "periodontitis". Online Etymology Dictionary. Harper, Douglas. "periodontal". Online Etymology Dictionary. ὀδούς, ὀδών. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project.
- ^ πυόρροια, πύον, ῥοή; cf. πυορροέω in Liddell and Scott. Harper, Douglas. "-ia". Online Etymology Dictionary.
- ^ "pyorrhea". Merriam-Webster Online.
- ^ Listl, S.; Galloway, J.; Mossey, P. A.; Marcenes, W. (28 August 2015). "Global Economic Impact of Dental Diseases". Journal of Dental Research. 94: 1355–61. doi:10.1177/0022034515602879.
- ^ "World-first therapeutic dental vaccine: Periodontitis therapeutic vaccine trials could start in 2018". ScienceDaily. Retrieved 30 May 2018.
- ^ Dewhirst FE, Klein EA, Thompson EC, Blanton JM, Chen T, Milella L, Buckley CM, Davis IJ, Bennett ML, Marshall-Jones ZV (2012). "The canine oral microbiome". PLoS ONE. 7 (4): e36067. doi:10.1371/journal.pone.0036067. PMC 3338629. PMID 22558330.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Muller-Esnault, Susan, DVM. "Periodontal Disease in the Dog and Cat" (2009). http://www.critterology.com/articles/periodontal-disease-dog-and-cat
External links
- Canadian Academy of Periodontology — What is periodontitis?
- Periodontal disease in dogs and cats — Blue Cross Animal Hospital's write up on periodontal disease.
