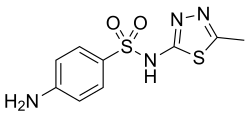
Sulfamethizole
Appearance
This article needs attention from an expert in Pharmacology. Please add a reason or a talk parameter to this template to explain the issue with the article. (August 2014) |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a682231 |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 98–99% |
| Elimination half-life | 3–8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.129 |
| Chemical and physical data | |
| Formula | C9H10N4O2S2 |
| Molar mass | 270.333 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Sulfamethizole is a sulfonamide antibiotic.
References
- Ratanajamit C, Skriver M, Nørgaard M, Jepsen P, Schønheyder H, Sørensen H (2003). "Adverse pregnancy outcome in users of sulfamethizole during pregnancy: a population-based observational study". J Antimicrob Chemother. 52 (5): 837–41. doi:10.1093/jac/dkg438. PMID 14519675.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kerrn M, Frimodt-Møller N, Espersen F (2003). "Effects of Sulfamethizole and Amdinocillin against Escherichia coli Strains (with Various Susceptibilities) in an Ascending Urinary Tract Infection Mouse Model". Antimicrob Agents Chemother. 47 (3): 1002–9. doi:10.1128/AAC.47.3.1002-1009.2003. PMC 149286. PMID 12604534.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Watanabe H, Hastings J (1990). "Inhibition of bioluminescence in Photobacterium phosphoreum by sulfamethizole and its stimulation by thymine". Biochim Biophys Acta. 1017 (3): 229–34. doi:10.1016/0005-2728(90)90189-B. PMID 2372557.
