User talk:Digbymom
Hi Advanced Cell Phys students at EC,
Please do the following:
- Create an account for yourselves by clicking here and then going to the registration tab.
- Play in the sandbox for a few minutes to practice editing.
- Check out the page you've selected to edit.
- Go to this location for editing help.
- Leave me a note here. :-) Do this by going to the top and selecting the tab "edit this page."
See you on Monday. Amy
References:
School and University Projects page.
Instructions for Students.
Instructions for Faculty.
Copyrights.
Five Pillars.
Editing Policy.
Editing Cheat Sheet.
Tutorial.
I am working on the following:
| Wee1 | |||||||
|---|---|---|---|---|---|---|---|
 Crystal structure of human Wee1 | |||||||
| Identifiers | |||||||
| Symbol | Mitosis inhibitor protein kinase Wee1 | ||||||
| Alt. symbols | wee1 dual specificity protein kinase Wee1 | ||||||
| NCBI gene | 2539123 | ||||||
| UniProt | P07527 | ||||||
| Other data | |||||||
| EC number | 2.7.11.1 | ||||||
| |||||||
Wee1 is a nuclear kinase belonging to the Ser/Thr family of protein kinases in the fission yeast Schizosaccharomyces pombe (S. pombe). It has a molecular mass of 96 kDa and it is a key regulator of cell cycle progression. It influences cell size by inhibiting the entry into mitosis, through inhibiting Cdc2. It has homologues in many other organisms, including mammals.
Introduction
[edit]The regulation of cell size is critical to ensure functionality of a cell. Besides environmental factors such as nutrients, growth factors and functional load, cell size is also controlled by a cellular cell size checkpoint.
Wee1 is a component of this checkpoint. It is a kinase determining the timepoint of entry into mitosis, thus influencing the size of the daughter cells. Loss of Wee1 function will produce smaller than normal daughter cell, because cell division occurs prematurely.
Its name is derived from the Scottish slang word wee, meaning small - its discoverer Paul Nurse was working at the University of Edinburgh in Scotland at the time of discovery.[1][2]
Function
[edit]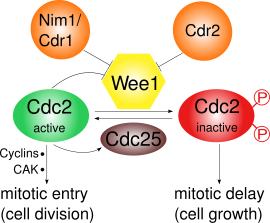
Wee1 inhibits Cdc2 by phosphorylating it on two different sites, Tyr15 and Thr14[3]. Cdc2 is crucial for the cyclin-dependent passage of the various cell cycle checkpoints. At least three checkpoints exist for which the inhibition of Cdc2 by Wee1 is important:
- G2/M checkpoint: Wee1 phosphorylates the amino acids Tyr15 and Thr14 of Cdc2, which keeps the kinase activity of Cdc2 low and prevents entry into mitosis; in S. pombe further cell growth can occur. During mitotic entry the activity of Wee1 is decreased by several regulators and thus Cdc2 activity is increased. Cdc2 itself negatively regulates Wee1 by phosphorylation, which leads to a positive feedback loop. The decreased Wee1 activity alone is not sufficient for mitotic entry: Synthesis of cyclins and an activating phosphorylation by a cyclin activating kinase (CAK) are also required.[4]
- Cell size checkpoint: There is evidence for the existence of a cell size checkpoint, which prevents small cells from entering mitois. Wee1 plays a role in this checkpoint by coordinating cell size and cell cycle progression.[5]
- DNA damage checkpoint: This checkpoint also controls the G2/M transition. In S. pombe this checkpoint delays the mitosis entry of cells with DNA damage (for example induced by gamma radiation). The lengthening of the G2 phase depends on Wee1; wee1 mutants have no prolonged G2 phase after gamma irradiation. [6]
Homologues
[edit]| human WEE1 homolog (S. pombe) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | WEE1 | ||||||
| NCBI gene | 7465 | ||||||
| HGNC | 12761 | ||||||
| OMIM | 193525 | ||||||
| RefSeq | NM_003390 | ||||||
| UniProt | P30291 | ||||||
| Other data | |||||||
| Locus | Chr. 11 p15.3-15.1 | ||||||
| |||||||
| human WEE1 homolog 2 (S. pombe) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | WEE2 | ||||||
| NCBI gene | 494551 | ||||||
| HGNC | 19684 | ||||||
| RefSeq | NM_001105558 | ||||||
| UniProt | P0C1S8 | ||||||
| Other data | |||||||
| Locus | Chr. 7 q32-q32 | ||||||
| |||||||
The WEE1 gene has two known homologues in humans, WEE1 (also known as WEE1A) and WEE2 (WEE1B). The corresponding proteins are Wee1-like protein kinase and Wee1-like protein kinase 2 which act on the human Cdc2 homologue Cdk1.
The homologue to Wee1 in budding yeast Saccharomyces cerevisiae is called Swe1.
Regulation
[edit]In S. pombe, Wee1 is phosphorylated
Cdc2 and cyclin B make up the maturation promoting factor (MPF) which promotes the entry into mitosis. It is inactivated by phosphorylation through Wee1 and activated by the phosphatase Cdc25. Cdc25 in turn is activated by Polo kinase and inactivated by Chk1.[5] Thus in S. pombe Wee1 regulation is mainly under the control of phosphorylation.[7][8][9][10]
At the G2/M transition, Cdc2 is activated by Cdc25 through dephosphorylation of Tyr15. At the same time, Wee1 is inactivated through phosphorylation at its C-terminal catalytic domain by Nim1/Cdr1.[9] Also, the active MPF will promote its own activity by activating Cdc25 and inactivating Wee1, creating a positive feedback loop, though this is not yet understood in detail.[5]
Higher eukaryotes regulate Wee1 via phosphorylation and degradation
In higher eukaryotes, Wee1 inactivation occurs both by phosphorylation and degradation.[11]
The protein complex[nb 1] SCFβ-TrCP1/2 is an E3 ubiquitin ligase that functions in Wee1A ubiquitination. The M-phase kinases Polo-like kinase (Plk1) and Cdc2 phosphorylate two serine residues in Wee1A which are recognized by SCFβ-TrCP1/2.[12]
S. cerevisiae homologue Swe1
In S. cerevisiae, cyclin-dependent kinase Cdc28 (Cdc2 homologue) is phosphorylated by Swe1 (Wee1 homologue) and dephosphorylated by Mih1 (Cdc25 homologue). Nim1/Cdr1 homologue in S. cerevisiae, Hsl1, together with its related kinases Gin4 and Kcc4 localize Swe1 to the bud-neck. Bud-neck associating kinases Cla4 and Cdc5 (polo kinase homologue) phosphorylate Swe1 at different stages of the cell cycle. Swe1 is also phosphorylated by Clb2-Cdc28 which serves as a recognition for further phosphorylation by Cdc5.
The S. cerevisiae protein Swe1 is also regulated by degradation. Swe1 is hyperphosphorylated by Clb2-Cdc28 and Cdc5 which may be a signal for ubiquitination and degradation by SCF E3 ubiquitin ligase complex as in higher eukaryotes.[13]
Role in cancer
[edit]The mitosis promoting factor MPF also regulates DNA-damage induced apoptosis. Negative regulation of MPF by WEE1 causes aberrant mitosis and thus resistance to DNA-damage induced apoptosis. Kruppel-like factor 2 (KLF2) negatively regulates human WEE1, thus increasing sensitivity to DNA-damage induced apoptosis in cancer cells.[14]
Mutant phenotype
[edit]Wee1 acts as a dosage-dependent inhibitor of mitosis.[15] Thus, the amount of Wee1 protein correlates with the size of the cells:
The fission yeast mutant wee1, also called wee1-, divides at a significantly smaller cell size than wildtype cells. Since Wee1 inhibits entry into mitosis, its absence will lead to division at a premature stage and sub-normal cell size. Conversely, when Wee1 expression is increased, mitosis is delayed and cells grow to a large size before dividing.
See also
[edit]Notes
[edit]- ^ β-transducin repeat-containing protein 1/2 (β-TrCP1/2) F-box protein-containing SKP1/Cul1/F-box protein complex
References
[edit]- ^ Nurse P (2004). "Wee beasties". Nature. 432 (7017): 557. doi:10.1038/432557a. PMID 15577889.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Nurse P, Thuriaux P (1980). "Regulatory genes controlling mitosis in the fission yeast Schizosaccharomyces pombe". Genetics. 96 (3): 627–37. PMC 1214365. PMID 7262540.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Den Haese GJ, Walworth N, Carr AM, Gould KL (1995). "The Wee1 protein kinase regulates T14 phosphorylation of fission yeast Cdc2". Mol Biol Cell. 6 (4): 371–85. doi:10.1038/356353a0. PMID 7626804.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Coleman TR, Dunphy WG (1994). "Cdc2 regulatory factors". Curr Opin Cell Biol. 6 (6): 877–82. doi:10.1016/0955-0674(94)90060-4. PMID 7880537.
- ^ a b c Kellogg DR (2003). "Wee1-dependent mechanisms required for coordination of cell growth and cell division". J Cell Sci. 116 (24): 4883–90. doi:10.1242/jcs.00908. PMID 14625382.
- ^ Rowley R, Hudson J, Young PG (1992). "The wee1 protein kinase is required for radiation-induced mitotic delay". Nature. 356 (6367): 353–5. doi:10.1038/356353a0. PMID 1549179.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Boddy MN, Furnari B, Mondesert O, Russell P (1998). "Replication checkpoint enforced by kinases Cds1 and Chk1". Science. 280 (5365): 909–12. doi:10.1126/science.280.5365.909. PMID 9572736.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wu L, Russell P (1993). "Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase". Nature. 363 (6431): 738–41. doi:10.1038/363738a0. PMID 8515818.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Coleman TR, Tang Z, Dunphy WG (1993). "Negative regulation of the wee1 protein kinase by direct action of the nim1/cdr1 mitotic inducer". Cell. 72 (6): 919–29. doi:10.1016/0092-8674(93)90580-J. PMID 7681363.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Tang Z, Coleman TR, Dunphy WG (1993). "Two distinct mechanisms for negative regulation of the Wee1 protein kinase". Embo J. 12 (9): 3427–36. PMC 413619. PMID 7504624.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Watanabe N, Broome M, Hunter T (1995). "Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle". Embo J. 14 (9): 1878–91. PMC 398287. PMID 7743995.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Watanabe N, Arai H, Nishihara Y; et al. (2004). "M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP". Proc. Natl. Acad. Sci. U.S.A. 101 (13): 4419–24. doi:10.1073/pnas.0307700101. PMC 384762. PMID 15070733.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lee KS, Asano S, Park JE, Sakchaisri K, Erikson RL (2005). "Monitoring the cell cycle by multi-kinase-dependent regulation of Swe1/Wee1 in budding yeast". Cell Cycle. 4 (10): 1346–9. PMID 16123596.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wang F, Zhu Y, Huang Y; et al. (2005). "Transcriptional repression of WEE1 by Kruppel-like factor 2 is involved in DNA damage-induced apoptosis". Oncogene. 24 (24): 3875–85. doi:10.1038/sj.onc.1208546. PMID 15735666.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Russell P, Nurse P (1987). "Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog". Cell. 49 (4): 559–67. doi:10.1016/0092-8674(87)90458-2. PMID 3032459.
{{cite journal}}: Unknown parameter|month=ignored (help)
