Valproate semisodium
 | |
| Clinical data | |
|---|---|
| Trade names | Depakote |
| AHFS/Drugs.com | Multum Consumer Information |
| MedlinePlus | a682412 |
| Pregnancy category |
|
| Routes of administration | Oral |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 10-18.5% (dose-dependent) |
| Metabolism | Hepatic (30-50% via glucuronidation; 40% via β-oxidation & 15-20% via other oxidative pathways) |
| Elimination half-life | 9-16 hours (dose-dependent) |
| Excretion | Renal (<3% unchanged) |
| Identifiers | |
| |
| PubChem CID | |
| Chemical and physical data | |
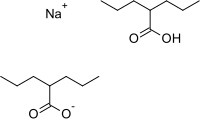
| Formula | C16H31NaO4 |
| Molar mass | 310.410 g·mol−1 |
Valproate semisodium (INN, BAN) or divalproex sodium (USAN) consists of a compound of sodium valproate and valproic acid in a 1:1 molar relationship in an enteric coated form.[1] Its chief use in medicine is as a treatment for bipolar disorder, epilepsy and in the prevention of migraines.[2]
Medical uses
It is used in the treatment of migraines, bipolar disorder and epilepsy.[2][3][4]
Adverse effects
Adverse effects by frequency:[2][3][4]
Very common (>10% frequency):
- Nausea
- Tremor
Common (1-10% frequency):
- Liver injury
- Gastralgia
- Diarrhoea
- Extrapyramidal (movement) disorder
- Stupor†
- Somnolence
- Convulsion†
- Memory impairment
- Headache
- Nystagmus
- Confusional state
- Aggression‡
- Agitation‡
- Impaired attention‡
- Hyponatraemia
- Anaemia
- Thrombocytopaenia
- Hypersensitivity
- Transient and/or dose-related hair loss
- Dysmenorrhea (missing one's periods)
- Haemorrhage (bleeding)
- Weight gain¶
Uncommon (0.01-0.1% frequency):
- Pancreatitis (sometimes lethal)
- Coma†
- Lethargy†
- SIADH
- Pancytopenia
- Leucopenia
- Rash
- Angioedema
- Amenorrhoea (absence of menstrual cycle)
- Vasculitis
- Peripheral oedema
- Reduced bone mineral density
- Osteopaenia
- Osteoporosis
- Pleural effusions
- Gingival enlargement
Rare (<0.01% frequency):
- Reversible dementia
- Reversible cerebral atrophy
- Abnormal behaviour‡
- Psychomotor hyperactivity‡
- Learning disorder‡
- Hyperammonaemia
- Hypothyroidism
- Bone marrow failure
- Male infertility
- Toxic epidermal necrolysis
- Stevens-Johnson syndrome
- Erythema multiforme
- DRESS syndrome
- Male infertility
- Polycystic ovaries
- Enuresis
- Reversible Fanconi syndrome
- Coagulation abnormalities
- Systemic lupus erythematosus
- Gynaecomastia
† Rare cases of lethargy occasionally progressing to stupor, sometimes with associated hallucinations or convulsions have been reported. Encephalopathy and coma have very rarely been observed. These cases are most often seen in association with other factors such as rapid dose escalations or withdrawal from other medications. They have usually been reversible on withdrawal of treatment or reduction of dosage. ‡ These cases most commonly occur in the paediatric population. ¶ Weight gain should be monitored closely as there is a potential link between weight gain and polycystic ovary syndrome.
Contraindications
Contraindications:[2]
- Active liver disease
- Personal or family history of drug-related liver dysfunction
- Hypersensitivity to valproate semisodium,valproate,Valproic acid any of its excipients
- Porphyria
Interactions
Drug interactions include:[2][3][4]
- MAO inhibitors, antidepressants, benzodiazepines and antipsychotics — may potentiate its effects, including its side effects.
- Phenobarbital — plasma concentrations of phenobarbital is increased. Decreases plasma concentrations of valproate.
- Phenytoin — plasma concentrations of phenytoin are reduced. Decreases plasma concentrations of valproate.
- Carbamazepine — toxic effects of carbamazepine are potentiated by this combination. Decreases plasma concentrations of valproate.
- Lamotrigine — reduces lamotrigine's half-life by half.
- Felbamate — may reduce felbamate's clearance by up to 16%. Felbamate may also reduce valproate plasma concentrations by 22-50%.
- Zidovudine — increases plasma concentrations, potentially leading to zidovudine toxicity.
- Temozolomide — slight, yet seemingly insignificant increase in temozolomide plasma concentrations.
- Vitamin K-dependent anticoagulants (e.g. warfarin) — valproate may displace these drugs from the plasma proteins hence potentiating their effects.
- Mefloquine and chloroquine induce valproate's metabolism, hence potentially reducing plasma concentrations of the drug.
- Highly protein-bound agents (e.g. aspirin) may displace valproate from plasma proteins leading, potentially, to potential valproate toxicity.
- Cimetidine and erythromycin may increase valproate plasma concentrations.
- Carbapenem antibiotics (e.g. imipenem) may reduce plasma levels of valproate by 60-100%.
- Colestyramine may reduce valproate absorption from the small intestine
- Rifampicin may decrease plasma concentrations of valproate.
- Concomitant topiramate may induce encephalopathy in patients on valproate.
Branded formulations
- Brazil – Depakote by Abbott Laboratories
- Canada – Epival by Abbott Laboratories
- Mexico – Epival and Epival ER (extended release) by Abbott Laboratories
- United Kingdom – Depakote (for psychiatric conditions) and Epilim (for epilepsy) by Sanofi-Aventis and generics
- United States – Depakote and Depakote ER (extended release) by Abbott Laboratories and generics
- India – Valance and Valance OD by Abbott Healthcare Pvt Ltd,Divalid ER by Linux laboratories Pvt Ltd,Valex ER by Sigmund Promedica, Dicorate by Sun Pharma
- Germany – Ergenyl Chrono by Sanofi-Aventis and generics
- Chile – Valcote and Valcote ER by Abbott Laboratories
- France and other European countries — Depakote
- Peru – Divalprax by AC Farma Laboratories
- China – Diprate OD
In the United States, generic versions of valproate semisodium became available on July 29, 2008.[5]
References
- ^ Valproate. Pharmaceutical Press. 17 October 2013. Retrieved 18 January 2014.
{{cite book}}:|work=ignored (help) - ^ a b c d e "Depakote 250mg Tablets - Summary of Product Characteristics". electronic Medicines Compendium. Sanofi. 28 November 2013. Retrieved 18 January 2014.
- ^ a b c "DEPAKOTE (divalproex sodium) tablet, delayed release [AbbVie Inc.]". DailyMed. AbbVie Inc. September 2013. Retrieved 18 January 2014.
- ^ a b c "Depakote (divalproex sodium) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 18 January 2014.
- ^ http://fdanews.com/newsletter/article?issueId=11836&articleId=109236
External links
- RXList.com: Depakote (Divalproex sodium)
- British National Formulary Edition 50
- Drugs.com Advanced Consumer Info: Valproic Acid
- Depakote (Official) - Abbott Laboratories
- Drug Information Online
- Mayo Clinic drug information
