SKF-83,959: Difference between revisions
Content deleted Content added
No edit summary |
rewritten |
||
| Line 38: | Line 38: | ||
}} |
}} |
||
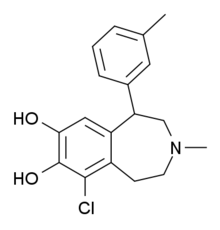
'''SKF-83,959''' is a [[chemical synthesis|synthetic]] [[benzazepine]] [[chemical derivative|derivative]] used in [[scientific research]] which acts as |
'''SKF-83,959''' is a [[chemical synthesis|synthetic]] [[benzazepine]] [[chemical derivative|derivative]] used in [[scientific research]] which acts as an agonist at the [[D1-D2 Dopamine receptor heteromer|D<sub>1</sub>-D<sub>2</sub> dopamine receptor]].<ref>{{cite journal |author=Rashid AJ, So CH, Kong MM, ''et al.'' |title=D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=104 |issue=2 |pages=654–9 |year=2007 |pmid=17194762 |pmc=1766439 |doi=10.1073/pnas.0604049104 |url=}}</ref> It behaves as a full agonist at the D<sub>1</sub> [[protomer]] and a high-affinity partial agonist at the D<sub>2</sub> protomer. SKF-83,959 is a racemate that consists of the ''R''-(+)- and ''S''-(-)-enantiomers MCL 202 and MCL 201, respectively. SKF-83,959 inhibits [[sodium channel]]s<ref>{{cite journal |author=Chu HY, Wu Q, Zhou S, ''et al.'' |title=SKF83959 suppresses excitatory synaptic transmission in rat hippocampus via a dopamine receptor-independent mechanism |journal=J. Neurosci. Res. |volume=89 |issue=8 |pages=1259–66 |year=2011 |pmid=21538463 |doi=10.1002/jnr.22653 |url=}}</ref> as well as delayed rectifier [[potassium channel]]s<ref>{{cite journal |author=Chen XQ, Zhang J, Neumeyer JL, ''et al.'' |title=Arylbenzazepines are potent modulators for the delayed rectifier K+ channel: a potential mechanism for their neuroprotective effects |journal=PLoS ONE |volume=4 |issue=6 |pages=e5811 |year=2009 |pmid=19503734 |pmc=2690691 |doi=10.1371/journal.pone.0005811 |url=}}</ref>. |
||
== References == |
== References == |
||
Revision as of 12:26, 28 June 2012
 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H20ClNO2 |
| Molar mass | 317.810 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
SKF-83,959 is a synthetic benzazepine derivative used in scientific research which acts as an agonist at the D1-D2 dopamine receptor.[1] It behaves as a full agonist at the D1 protomer and a high-affinity partial agonist at the D2 protomer. SKF-83,959 is a racemate that consists of the R-(+)- and S-(-)-enantiomers MCL 202 and MCL 201, respectively. SKF-83,959 inhibits sodium channels[2] as well as delayed rectifier potassium channels[3].
References
- ^ Rashid AJ, So CH, Kong MM; et al. (2007). "D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum". Proc. Natl. Acad. Sci. U.S.A. 104 (2): 654–9. doi:10.1073/pnas.0604049104. PMC 1766439. PMID 17194762.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Chu HY, Wu Q, Zhou S; et al. (2011). "SKF83959 suppresses excitatory synaptic transmission in rat hippocampus via a dopamine receptor-independent mechanism". J. Neurosci. Res. 89 (8): 1259–66. doi:10.1002/jnr.22653. PMID 21538463.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Chen XQ, Zhang J, Neumeyer JL; et al. (2009). "Arylbenzazepines are potent modulators for the delayed rectifier K+ channel: a potential mechanism for their neuroprotective effects". PLoS ONE. 4 (6): e5811. doi:10.1371/journal.pone.0005811. PMC 2690691. PMID 19503734.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link)
