Lead(II) oxide: Difference between revisions
Rescuing 1 sources and tagging 0 as dead.) #IABot (v2.0 |
→cite book, chem, co2, h2o, tweak cites, |
||
| Line 43: | Line 43: | ||
==Preparation== |
==Preparation== |
||
PbO may be prepared by heating lead metal in air at approximately {{convert|600|C|F|-2}}. At this temperature it is also the end product of oxidation of other [[lead oxide (disambiguation)|oxides of lead]] in air:<ref>{{Greenwood&Earnshaw2nd}}{{page needed|date=June 2017}}</ref> |
PbO may be prepared by heating lead metal in air at approximately {{convert|600|C|F|-2}}. At this temperature it is also the end product of oxidation of other [[lead oxide (disambiguation)|oxides of lead]] in air:<ref>{{Greenwood&Earnshaw2nd}}{{page needed|date=June 2017}}</ref> |
||
:PbO |
:{{chem|PbO|2}} {{overset|293 °C|→}} {{chem|Pb|12|O|19}} {{overset|351 °C|→}} {{chem|Pb|12|O|17}} {{overset|375 °C|→}} {{chem|Pb|3|O|4}} {{overset|605 °C|→}} PbO |
||
Thermal decomposition of [[lead(II) nitrate]] or [[lead carbonate|lead(II) carbonate]] also results in the formation of PbO: |
Thermal decomposition of [[lead(II) nitrate]] or [[lead carbonate|lead(II) carbonate]] also results in the formation of PbO: |
||
:2 Pb(NO |
:2 {{chem|Pb|(NO|3|)|2}} → 2 PbO + 4 {{chem|link=nitrogen dioxide|NO|2}} + {{chem|O|2}} |
||
:PbCO |
:{{chem|PbCO|3}} → PbO + {{CO2|link=yes}} |
||
PbO is produced on a large scale as an intermediate product in refining raw lead ores into metallic lead. The usual lead ore is [[galena]] ([[lead(II) sulfide]]). At a temperature of around {{convert|1000|C|F|-2}} the sulfide is converted to the oxide:<ref>{{cite journal|title=Thermal and XRD analysis of Egyptian galena|journal=Journal of Thermal Analysis and Calorimetry|year=2006|volume=86|issue=2|pp=393–401|last1=Abdel-Rehim|first1=A. M.}}</ref> |
PbO is produced on a large scale as an intermediate product in refining raw lead ores into metallic lead. The usual lead ore is [[galena]] ([[lead(II) sulfide]]). At a temperature of around {{convert|1000|C|F|-2}} the sulfide is converted to the oxide:<ref>{{cite journal|title=Thermal and XRD analysis of Egyptian galena|journal=Journal of Thermal Analysis and Calorimetry|year=2006|volume=86|issue=2|pp=393–401|last1=Abdel-Rehim|first1=A. M. |doi=10.1007/s10973-005-6785-6}}</ref> |
||
:2 PbS + 3 O |
:2 PbS + 3 {{chem|O|2}} → 2 PbO + 2 {{SO2|link=yes}} |
||
Metallic lead is obtained by reducing PbO with [[carbon monoxide]] at around {{convert|1200|C|F|-2}}:<ref>[http://universalium.academic.ru/276093/lead_processing Lead Processing @ Universalium.academic.ru]. Alt address: [http://www.enwiki.net/wiki/eb/2366/ Lead processing @ Enwiki.net].</ref> |
Metallic lead is obtained by reducing PbO with [[carbon monoxide]] at around {{convert|1200|C|F|-2}}:<ref>[http://universalium.academic.ru/276093/lead_processing Lead Processing @ Universalium.academic.ru]. Alt address: [http://www.enwiki.net/wiki/eb/2366/ Lead processing @ Enwiki.net].</ref> |
||
:PbO + CO → Pb + |
:PbO + CO → Pb + {{CO2}} |
||
==Structure== |
==Structure== |
||
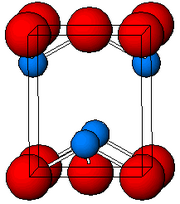
As determined by [[X-ray crystallography]], both polymorphs, [[tetragonal]] and [[orthorhombic]] feature a pyramidal four-coordinate lead center. In the tetragonal form the four lead–oxygen bonds have the same length, but in the orthorhombic two are shorter and two longer. The pyramidal nature indicates the presence of a [[stereochemistry|stereochemically]] active [[lone pair]] of electrons.<ref>{{Wells5th}}{{page needed|date=June 2017}}</ref> When PbO occurs in tetragonal lattice structure it is called [[litharge]]; and when the PbO has orthorhombic lattice structure it is called [[massicot]]. The PbO can be changed from massicot to litharge or vice versa by controlled heating and cooling.<ref>A simple example is given in |
As determined by [[X-ray crystallography]], both polymorphs, [[tetragonal]] and [[orthorhombic]] feature a pyramidal four-coordinate lead center. In the tetragonal form the four lead–oxygen bonds have the same length, but in the orthorhombic two are shorter and two longer. The pyramidal nature indicates the presence of a [[stereochemistry|stereochemically]] active [[lone pair]] of electrons.<ref>{{Wells5th}}{{page needed|date=June 2017}}</ref> When PbO occurs in tetragonal lattice structure it is called [[litharge]]; and when the PbO has orthorhombic lattice structure it is called [[massicot]]. The PbO can be changed from massicot to litharge or vice versa by controlled heating and cooling.<ref>A simple example is given in {{cite book |author=Anil Kumar De |title=A Textbook Of Inorganic Chemistry |url=https://books.google.com/books?id=PpTi_JAx7PgC&pg=PA383 |year=2007 |publisher=New Age International |isbn=978-81-224-1384-7 |pages=383 |chapter=§9.2.6 Lead (Pb): Lead Monoxide PbO }} A more complex example is in {{cite book |first=N.Y. |last=Turova |title=The Chemistry of Metal Alkoxides |url=https://books.google.com/books?id=rPzaMRjK8pQC&pg=PA115 |date=2002 |publisher=Springer |isbn=978-0-7923-7521-0 |pages=115 |chapter=§9.4 Germanium, tin, lead alkoxides}}</ref> The tetragonal form is usually red or orange color, while the orthorhombic is usually yellow or orange, but the color is not a very reliable indicator of the structure.<ref>{{cite book |first=David John |last=Rowe |title=Lead Manufacturing in Britain: A History |url=https://books.google.com/books?id=ZL4OAAAAQAAJ&pg=PA16 |date=1983 |publisher=Croom Helm |isbn=978-0-7099-2250-6 |pages=16}}</ref> The tetragonal and orthorhombic [[Polymorphism (materials science)|forms]] of PbO occur naturally as rare minerals. |
||
==Reactions== |
==Reactions== |
||
| Line 62: | Line 62: | ||
:PbO<sub>(red)</sub> → PbO<sub>(yellow)</sub> {{pad|5em}} Δ''H'' = 1.6 kJ/mol |
:PbO<sub>(red)</sub> → PbO<sub>(yellow)</sub> {{pad|5em}} Δ''H'' = 1.6 kJ/mol |
||
PbO is [[amphoterism|amphoteric]], which means that it reacts with both acids and with bases. With acids, it forms salts of Pb |
PbO is [[amphoterism|amphoteric]], which means that it reacts with both acids and with bases. With acids, it forms salts of {{chem|Pb|2+}} via the intermediacy of oxo [[cluster chemistry|cluster]]s such as {{chem|[Pb|6|O(OH)|6|]|4+}}. With strong bases, PbO dissolves to form [[plumbite]] (also called plumbate(II)) salts:<ref name="Holl">{{Holleman&Wiberg}}{{page needed|date=June 2017}}</ref> |
||
:PbO + |
:PbO + {{H2O}} + {{chem|OH|−}} → {{chem|[Pb(OH)|3|]|−}} |
||
==Applications== |
==Applications== |
||
Revision as of 00:13, 2 April 2020

| |
| |
| Names | |
|---|---|
| IUPAC name
Lead(II) oxide
| |
| Other names | |
| Identifiers | |
| ChemSpider | |
| ECHA InfoCard | 100.013.880 |
PubChem CID
|
|
| RTECS number |
|
| UN number | 3288 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| PbO | |
| Molar mass | 223.20 g/mol |
| Appearance | red or yellow powder |
| Density | 9.53 g/cm3 |
| Melting point | 888 °C (1,630 °F; 1,161 K) |
| Boiling point | 1,477 °C (2,691 °F; 1,750 K) |
| 0.017 g/L[1] | |
| Solubility | insoluble in dilute alkalis, alcohol soluble in concentrated alkalis soluble in HCl, ammonium chloride |
| 4.20×10−5 cm3/mol | |
| Structure | |
| Tetragonal, tP4 | |
| P4/nmm, No. 129 | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LDLo (lowest published)
|
1400 mg/kg (dog, oral)[2] |
| Safety data sheet (SDS) | ICSC 0288 |
| Related compounds | |
Other anions
|
Lead sulfide Lead selenide Lead telluride |
Other cations
|
Carbon monoxide Silicon monoxide Tin(II) oxide |
| Lead(II,II,IV) oxide Lead dioxide | |
Related compounds
|
Thallium(III) oxide Bismuth(III) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lead(II) oxide, also called lead monoxide, is the inorganic compound with the molecular formula PbO. PbO occurs in two polymorphs: litharge having a tetragonal crystal structure, and massicot having an orthorhombic crystal structure. Modern applications for PbO are mostly in lead-based industrial glass and industrial ceramics, including computer components. It is an amphoteric oxide.[3]
Preparation
PbO may be prepared by heating lead metal in air at approximately 600 °C (1,100 °F). At this temperature it is also the end product of oxidation of other oxides of lead in air:[4]
- PbO
2 Pb
12O
19 Pb
12O
17 Pb
3O
4 PbO
Thermal decomposition of lead(II) nitrate or lead(II) carbonate also results in the formation of PbO:
PbO is produced on a large scale as an intermediate product in refining raw lead ores into metallic lead. The usual lead ore is galena (lead(II) sulfide). At a temperature of around 1,000 °C (1,800 °F) the sulfide is converted to the oxide:[5]
- 2 PbS + 3 O
2 → 2 PbO + 2 SO2
Metallic lead is obtained by reducing PbO with carbon monoxide at around 1,200 °C (2,200 °F):[6]
- PbO + CO → Pb + CO2
Structure
As determined by X-ray crystallography, both polymorphs, tetragonal and orthorhombic feature a pyramidal four-coordinate lead center. In the tetragonal form the four lead–oxygen bonds have the same length, but in the orthorhombic two are shorter and two longer. The pyramidal nature indicates the presence of a stereochemically active lone pair of electrons.[7] When PbO occurs in tetragonal lattice structure it is called litharge; and when the PbO has orthorhombic lattice structure it is called massicot. The PbO can be changed from massicot to litharge or vice versa by controlled heating and cooling.[8] The tetragonal form is usually red or orange color, while the orthorhombic is usually yellow or orange, but the color is not a very reliable indicator of the structure.[9] The tetragonal and orthorhombic forms of PbO occur naturally as rare minerals.
Reactions
The red and yellow forms of this material are related by a small change in enthalpy:
- PbO(red) → PbO(yellow) ΔH = 1.6 kJ/mol
PbO is amphoteric, which means that it reacts with both acids and with bases. With acids, it forms salts of Pb2+
via the intermediacy of oxo clusters such as [Pb
6O(OH)
6]4+
. With strong bases, PbO dissolves to form plumbite (also called plumbate(II)) salts:[10]
- PbO + H2O + OH−
→ [Pb(OH)
3]−
Applications
The kind of lead in lead glass is normally PbO, and PbO is used extensively in making glass. Depending on the glass, the benefit of using PbO in glass can be one or more of increasing the refractive index of the glass, decreasing the viscosity of the glass, increasing the electrical resistivity of the glass, and increasing the ability of the glass to absorb X-rays. Adding PbO to industrial ceramics (as well as glass) makes the materials more magnetically and electrically inert (by raising their Curie temperature) and it is often used for this purpose.[11] Historically PbO was also used extensively in ceramic glazes for household ceramics, and it is still used, but not extensively any more. Other less dominant applications include the vulcanization of rubber and the production of certain pigments and paints.[3] PbO is used in cathode ray tube glass to block X-ray emission, but mainly in the neck and funnel because it can cause discoloration when used in the faceplate. Strontium oxide is preferred for the faceplate.[citation needed]
The consumption of lead, and hence the processing of PbO, correlates with the number of automobiles, because it remains the key component of automotive lead-acid batteries.[12]
Niche or declining uses
A mixture of PbO with glycerine sets to a hard, waterproof cement that has been used to join the flat glass sides and bottoms of aquariums, and was also once used to seal glass panels in window frames. It is a component of lead paints.
PbO was used to speed up the process to turn more profit for less time and artificially increase the quality of century eggs, a type of Chinese preserved egg. It was an unscrupulous practice in some small factories but it became rampant in China and forced many honest manufacturers to label their boxes "lead-free" after the scandal went mainstream in 2013.
In powdered tetragonal litharge form, it can be mixed with linseed oil and then boiled to create a weather-resistant sizing used in gilding. The litharge would give the sizing a dark red color that made the gold leaf appear warm and lustrous, while the linseed oil would impart adhesion and a flat durable binding surface.
PbO is used in certain condensation reactions in organic synthesis.[13]
PbO is the input photoconductor in a video camera tube called the Plumbicon.
Health issues

Lead oxide may be fatal if swallowed or inhaled. It causes irritation to skin, eyes, and respiratory tract. It affects gum tissue, central nervous system, kidneys, blood, and reproductive system. It can bioaccumulate in plants and in mammals.[14]
References
- ^ Blei(II)-oxid. Merck
- ^ "Lead compounds (as Pb)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Carr, Dodd S. (2005). "Lead Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_249. ISBN 978-3527306732.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.[page needed]
- ^ Abdel-Rehim, A. M. (2006). "Thermal and XRD analysis of Egyptian galena". Journal of Thermal Analysis and Calorimetry. 86 (2): 393–401. doi:10.1007/s10973-005-6785-6.
- ^ Lead Processing @ Universalium.academic.ru. Alt address: Lead processing @ Enwiki.net.
- ^ Wells, A. F. (1984), Structural Inorganic Chemistry (5th ed.), Oxford: Clarendon Press, ISBN 0-19-855370-6[page needed]
- ^ A simple example is given in Anil Kumar De (2007). "§9.2.6 Lead (Pb): Lead Monoxide PbO". A Textbook Of Inorganic Chemistry. New Age International. p. 383. ISBN 978-81-224-1384-7. A more complex example is in Turova, N.Y. (2002). "§9.4 Germanium, tin, lead alkoxides". The Chemistry of Metal Alkoxides. Springer. p. 115. ISBN 978-0-7923-7521-0.
- ^ Rowe, David John (1983). Lead Manufacturing in Britain: A History. Croom Helm. p. 16. ISBN 978-0-7099-2250-6.
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5[page needed]
- ^ Chapter 9, "Lead Compounds", in the book Ceramic and Glass Materials: Structure, Properties and Processing, published by Springer, year 2008.
- ^ Sutherland, Charles A.; Milner, Edward F.; Kerby, Robert C.; Teindl, Herbert; Melin, Albert; Bolt, Hermann M. "Lead". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_193.pub2. ISBN 978-3527306732.
- ^ Corson, B. B. (1936). "1,4-Diphenylbutadiene". Organic Syntheses. 16: 28; Collected Volumes, vol. 2, p. 229.
- ^ "Lead(II) oxide". International Occupational Safety and Health Information Centre. Archived from the original on 2011-12-15. Retrieved 2009-06-06.

