Glycolysis: Difference between revisions
m - ' l' |
m →External links: added an external link to a detailed Glycolysis animation |
||
| Line 614: | Line 614: | ||
== External links == |
== External links == |
||
* [http://www.1lec.com/Biochemistry/How%20Glycolysis%20Work/index.html Glycolysis Animation] (Flash Required) |
* [http://www.1lec.com/Biochemistry/How%20Glycolysis%20Work/index.html A Simplified Glycolysis Animation] ([http://get.adobe.com/flashplayer/ Flash] Required) |
||
*[http://www.iubmb-nicholson.org/swf/glycolysis.swf A Detailed Glycolysis Animation provided by [[IUBMB]]] ([http://get.adobe.com/shockwave/ Shockwave] Required) |
|||
* [http://nist.rcsb.org/pdb/molecules/pdb50_1.html The Glycolytic enzymes in Glycolysis] at [[Protein Data Bank]] |
* [http://nist.rcsb.org/pdb/molecules/pdb50_1.html The Glycolytic enzymes in Glycolysis] at [[Protein Data Bank]] |
||
* [http://www.wdv.com/CellWorld/Biochemistry/Glycolytic Glycolytic cycle with animations] at wdv.com |
* [http://www.wdv.com/CellWorld/Biochemistry/Glycolytic Glycolytic cycle with animations] at wdv.com |
||
Revision as of 01:57, 26 April 2009
Glycolysis (from glycose, an older term[1] for glucose + -lysis degradation) is the metabolic pathway that converts glucose, C6H12O6, into pyruvate, C3H3O3-. The free energy released in this process is used to form the high energy compounds, ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).
Glycolysis is a sequence of ten reactions involving ten intermediate compounds (one of the steps involves two intermediates). The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose, glucose, and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate is a source of the glycerol that combines with fatty acids to form fat.
Glycolysis is thought to be the archetype of a universal metabolic pathway. It occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient known metabolic pathways.[2]
The most common type of glycolysis is the Embden-Meyerhof pathway, which was first discovered by Gustav Embden and Otto Meyerhof. Glycolysis also refers to other pathways, such as the Entner-Doudoroff Pathway. However, the discussion here will be limited to the Embden-Meyerhof pathway.
Overview
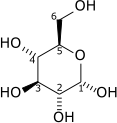
The overall reaction of glycolysis is:
| D-Glucose | Pyruvate | ||||
|
+ 2 NAD+ + 2 ADP + 2 Pi | File:Biochem reaction arrow foward NNNN horiz med.png | 2 | 
|
+ 2 NADH + 2 H+ + 2 ATP + 2 H2O |
The mnemonic "Goodness Gracious, Father Franklin Did Go By Picking Pumpkins (to) Prepare Pies" can be used to remember the intermediates in the metabolic pathway.[3]
For simple anaerobic fermentations, the metabolism of one molecule of glucose to two molecules of pyruvate has a net yield of two molecules of ATP. Most cells will then carry out further reactions to 'repay' the used NAD+ and produce a final product of ethanol or lactic acid. Many bacteria use inorganic compounds as hydrogen acceptors to regenerate the NAD+.
Cells performing aerobic respiration synthesize much more ATP, but not as part of glycolysis. These further aerobic reactions use pyruvate and NADH + H+ from glycolysis. Eukaryotic aerobic respiration produces approximately 34 additional molecules of ATP for each glucose molecule, however most of these are produced by a vastly different mechanism to the substrate-level phosphorylation in glycolysis.
The lower energy production, per glucose, of anaerobic respiration relative to aerobic respiration, results in greater flux through the pathway under hypoxic (low-oxygen) conditions, unless alternative sources of anaerobically-oxidizable substrates, such as fatty acids, are found.
Elucidation of the pathway
In 1860 Louis Pasteur discovered that microorganisms are responsible for fermentation. In 1897 Eduard Buchner found that extracts of certain cells can cause fermentation. In 1905 Arthur Harden and William Young determined that a heat-sensitive high-molecular-weight subcellular fraction (the enzymes) and a heat-insensitive low-molecular-weight cytoplasm fraction (ADP, ATP and NAD+ and other cofactors) are required together for fermentation to proceed. The details of the pathway were eventually determined by 1940, with a major input from Otto Meyerhof and some years later by Luis Leloir. The biggest difficulties in determining the intricacies of the pathway were due to the very short lifetime and low steady-state concentrations of the intermediates of the fast glycolytic reactions.
This section needs expansion. You can help by adding to it. (June 2008) |
Sequence of reactions
Preparatory phase
The first five steps are regarded as the preparatory (or investment) phase since they consume energy to convert the glucose into two three-carbon sugar phosphates (G3P).
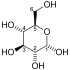
| The first step in glycolysis is phosphorylation of glucose by a family of enzymes called hexokinases to form glucose 6-phosphate (G6P). This reaction consumes ATP, but it acts to keep the glucose concentration low, promoting continuous transport of glucose into the cell through the plasma membrane transporters. In addition, it blocks the glucose from leaking out - the cell lacks transporters for G6P. Glucose may alternatively be from the phosphorolysis or hydrolysis of intracellular starch or glycogen.
In animals, an isozyme of hexokinase called glucokinase is also used in the liver, which has a much lower affinity for glucose (Km in the vicinity of normal glycemia), and differs in regulatory properties. The different substrate affinity and alternate regulation of this enzyme are a reflection of the role of the liver in maintaining blood sugar levels. Cofactors: Mg2+ |
| ||||||||||||||||||||
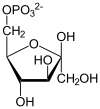
| G6P is then rearranged into fructose 6-phosphate (F6P) by glucose phosphate isomerase. Fructose can also enter the glycolytic pathway by phosphorylation at this point.
The change in structure is an isomerization, in which the G6P has been converted to F6P. The reaction requires an enzyme, phosphohexose isomerase, to proceed. This reaction is freely reversible under normal cell conditions. However, it is often driven forward because of a low concentration of F6P, which is constantly consumed during the next step of glycolysis. Under conditions of high F6P concentration this reaction readily runs in reverse. This phenomenon can be explained through Le Chatelier's Principle. |
| ||||||||||||||||||||
| The energy expenditure of another ATP in this step is justified in 2 ways: The glycolytic process (up to this step) is now irreversible, and the energy supplied destabilizes the molecule. Because the reaction catalyzed by Phosphofructokinase 1 (PFK-1) is energetically very favorable, it is essentially irreversible, and a different pathway must be used to do the reverse conversion during gluconeogenesis. This makes the reaction a key regulatory point (see below).
The same reaction can also be catalysed by pyrophosphate dependent phosphofructokinase (PFP or PPi-PFK), which is found in most plants, some bacteria, archea and protists but not in animals. This enzyme uses pyrophosphate (PPi) as a phosphate donor instead of ATP. It is a reversible reaction, increasing the flexibility of glycolytic metabolism.[4] A rarer ADP-dependent PFK enzyme variant has been identified in archaean species.[5] Cofactors: Mg2+ |
| ||||||||||||||||||||
| Destabilizing the molecule in the previous reaction allows the hexose ring to be split by aldolase into two triose sugars, dihydroxyacetone phosphate, a ketone, and glyceraldehyde 3-phosphate, an aldehyde. There are two classes of aldolases: class I aldolases, present in animals and plants, and class II aldolases which present in fungi and bacteria; the two classes use different mechanisms in cleaving the hexose ring. |
| ||||||||||||||||||||||||||
| Triosephosphate isomerase rapidly interconverts dihydroxyacetone phosphate with glyceraldehyde 3-phosphate (GADP) that proceeds further into glycolysis. This is advantageous, as it directs dihydroxyacetone phosphate down the same pathway as glyceraldehyde 3-phosphate, simplifying regulation. |
| ||||||||||||||||||||
Pay-off phase
The second half of glycolysis is known as the pay-off phase, characterised by a net gain of the energy-rich molecules ATP and NADH. Since glucose leads to two triose sugars in the preparatory phase, each reaction in the pay-off phase occurs twice per glucose molecule. This yields 2 NADH molecules and 4 ATP molecules, leading to a net gain of 2 NADH molecules and 2 ATP molecules from the glycolytic pathway per glucose.
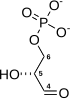
| The triose sugars are dehydrogenated and inorganic phosphate is added to them, forming 1,3-bisphosphoglycerate.
The hydrogen is used to reduce two molecules of NAD+, a hydrogen carrier, to give NADH + H+. |
| ||||||||||||||||||||
| This step is the enzymatic transfer of a phosphate group from 1,3-bisphosphoglycerate to ADP by phosphoglycerate kinase, forming ATP and 3-phosphoglycerate. At this step, glycolysis has reached the break-even point: 2 molecules of ATP were consumed, and 2 new molecules have now been synthesized. This step, one of the two substrate-level phosphorylation steps, requires ADP; thus, when the cell has plenty of ATP (and little ADP), this reaction does not occur. Because ATP decays relatively quickly when it is not metabolized, this is an important regulatory point in the glycolytic pathway.
Cofactors: Mg2+ |
| ||||||||||||||||||||
| Phosphoglycerate mutase now forms 2-phosphoglycerate. Notice that this enzyme is a mutase and not an isomerase. Whereas an isomerase changes the oxidation state of the carbons of the compound, a mutase does not. |
| ||||||||||||||||||||
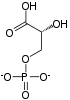
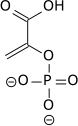
| Enolase next forms phosphoenolpyruvate from 2-phosphoglycerate.
Cofactors: 2 Mg2+: one "conformational" ion to coordinate with the carboxylate group of the substrate, and one "catalytic" ion which participates in the dehydration. |
| ||||||||||||||||||||
| A final substrate-level phosphorylation now forms a molecule of pyruvate and a molecule of ATP by means of the enzyme pyruvate kinase. This serves as an additional regulatory step, similar to the phosphoglycerate kinase step.
Cofactors: Mg2+ |
| ||||||||||||||||||||
Regulation
Glycolysis is regulated by slowing down or speeding up certain steps in the glycolysis pathway. This is accomplished by inhibiting or activating the enzymes that are involved. The steps that are regulated may be determined by calculating the change in free energy, ΔG, for each step. If a step's products and reactants are in equilibrium, then the step is assumed to not be regulated. Since the change in free energy is zero for a system at equilibrium, any step with a free energy change near zero is not being regulated. If a step is being regulated, then that step's enzyme is not converting reactants into products as fast as it could, resulting in a build-up of reactants, which would be converted to products if the enzyme were operating faster. Since the reaction is thermodynamically favorable, the change in free energy for the step will be negative. A step with a large negative change in free energy is assumed to be regulated.
Free energy changes
| Compound | Concentration / mM |
|---|---|
| glucose | 5.0 |
| glucose-6-phosphate | 0.083 |
| fructose-6-phosphate | 0.014 |
| fructose-1,6-bisphosphate | 0.031 |
| dihydroxyacetone phosphate | 0.14 |
| glyceraldehyde-3-phosphate | 0.019 |
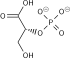
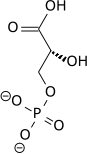
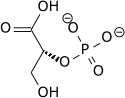
| 1,3-bisphosphoglycerate | 0.001 |
| 2,3-bisphosphoglycerate | 4.0 |
| 3-phosphoglycerate | 0.12 |
| 2-phosphoglycerate | 0.03 |
| phosphoenolpyruvate | 0.023 |
| pyruvate | 0.051 |
| ATP | 1.85 |
| ADP | 0.14 |
| Pi | 1.0 |
The change in free energy, ΔG, for each step in the glycolysis pathway can be calculated using ΔG = ΔG°' + RTln Q, where Q is the reaction quotient. This requires knowing the concentrations of the metabolites. All of these values are available for erythrocytes, with the exception of the concentrations of NAD+ and NADH. The ratio of NAD+ to NADH is approximately 1, which results in these concentrations canceling out in the reaction quotient. (Since NAD+ and NADH occur on opposite sides of the reactions, one will be in the numerator and the other in the denominator.)
Using the measured concentrations of each step, and the standard free energy changes, the actual free energy change can be calculated.
| Reaction | ΔG°' / (kJ/mol) | ΔG / (kJ/mol) |
|---|---|---|
| glucose + ATP4- → glucose-6-phosphate2- + ADP3- + H+ | -16.7 | -34 |
| glucose-6-phosphate2- → fructose-6-phosphate2- | 1.67 | -2.9 |
| fructose-6-phosphate2- + ATP4- → fructose-1,6-bisphosphate4- + ADP3- + H+ | -14.2 | -19 |
| fructose-1,6-bisphosphate4- → dihydroxyacetone phosphate2- + glyceraldehyde-3-phosphate2- | 23.9 | -0.23 |
| dihydroxyacetone phosphate2- → glyceraldehyde-3-phosphate2- | 7.56 | 2.4 |
| glyceraldehyde-3-phosphate2- + Pi2- + NAD+ → 1,3-bisphosphoglycerate4- + NADH + H+ | 6.30 | -1.29 |
| 1,3-bisphosphoglycerate4- + ADP3- → 3-phosphoglycerate3- + ATP4- | -18.9 | 0.09 |
| 3-phosphoglycerate3- → 2-phosphoglycerate3- | 4.4 | 0.83 |
| 2-phosphoglycerate3- → phosphoenolpyruvate3- + H2O | 1.8 | 1.1 |
| phosphoenolpyruvate3- + ADP3- + H+ → pyruvate- + ATP4- | -31.7 | -23.0 |
The figure below shows the change in free energy for each step of glycolysis. Step 5 is shown behind the other steps, because that step is a side reaction that can decrease or increase the concentration of the intermediate, glyceraldehyde-3-phosphate. That compound is converted to dihydroxyacetone phosphate by the enzyme, triose phosphate isomerase, which is a catalytically perfect enzyme; its rate is so fast that the reaction can be assumed to be in equilibrium. The fact that ΔG is not zero indicates that the actual concentrations in the erythrocyte are not accurately known.
Given the uncertainties in the calculated ΔG values, the figure suggests that seven of the steps in glycolysis are in equilibrium and unregulated, and that three of the steps—the ones with large negative free energy changes—are regulated. The regulated steps are referred to as irreversible. (Presumably, if the enzymes controlling these steps were not regulated, these steps would be reversible, but the equilibrium would strongly favor the product's side of the reaction.)
Biochemical logic
The existence of more than one point of regulation indicates that intermediates between those points enter and leave the glycolysis pathway by other processes. For example, in the first regulated step, hexokinase converts glucose into glucose-6-phosphate. Instead of continuing through the glycolysis pathway, this intermediate can be converted into glucose storage molecules, such as glycogen or starch. The reverse reaction, breaking down, e.g., glycogen, produces mainly glucose-6-phosphate; very little free glucose is formed in the reaction. The glucose-6-phosphate so produced can enter glycolysis after the first control point.
In the second regulated step (the third step of glycolysis) phosphofructokinase converts fructose-6-phosphate into fructose-1,6-bisphosphate, which then is converted into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. The dihydroxyacetone phosphate can be removed from glycolysis by conversion into glycerol-3-phosphate, which can be used to form triglycerides.[8] Conversely, triglycerides can be broken down into fatty acids and glycerol; the latter, in turn, can be converted into dihydroxyacetone phosphate, which can enter glycolysis after the second control point.
Regulation
The three regulated enzymes are hexokinase, phosphofructokinase, and pyruvate kinase.
The flux through the glycolytic pathway is adjusted in response to conditions both inside and outside the cell. The rate in liver is regulated to meet major cellular needs: (1) the production of ATP, (2) the provision of building blocks for biosynthetic reactions, and (3) to lower blood glucose, one of the major functions of the liver. When blood sugar falls, glycolysis is halted in liver to allow the reverse process, gluconeogenesis. In glycolysis, the reactions catalyzed by hexokinase, phosphofructokinase, and pyruvate kinase are effectively irreversible in most organisms. In metabolic pathways, such enzymes are potential sites of control, and all three enzymes serve this purpose in glycolysis.
Hexokinase

In animals, regulation of blood glucose levels by the liver is a vital part of homeostasis. In liver cells, extra G6P may be converted to G1P for conversion to glycogen, or it is alternatively converted by glycolysis to acetyl-CoA and then citrate. Excess citrate is exported to the cytosol, where ATP citrate lyase will regenerate acetyl-CoA and OAA. The acetyl-CoA is then used for fatty acid and cholesterol synthesis, two important ways of utilizing excess glucose when its concentration is high in blood. Liver contains both hexokinase and glucokinase; the latter catalyses the phosphorylation of glucose to G6P and is not inhibited by G6P. Thus it allows glucose to be converted into glycogen, fatty acids, and cholesterol even when hexokinase activity is low.[9] This is important when blood glucose levels are high. During hypoglycemia, the glycogen can be converted back to G6P and then converted to glucose by a liver-specific enzyme glucose 6-phosphatase. This reverse reaction is an important role of liver cells to maintain blood sugars levels during fasting. This is critical for brain function, since the brain utilizes glucose as an energy source under most conditions.
Phosphofructokinase
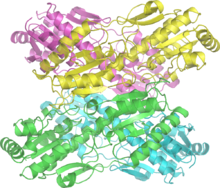
Phosphofructokinase is an important control point in the glycolytic pathway, since it is one of the irreversible steps and has key allosteric effectors, AMP and fructose 1,6-bisphosphate (F1,6BP).
Fructose 2,6-bisphosphate (F2,6BP) is a very potent activator of phosphofructokinase (PFK-1) that is synthesised when F6P is phosphorylated by a second phosphofructokinase (PFK2). In liver, when blood sugar is low and glucagon elevates cAMP, PFK2 is phosphorylated by protein kinase A. The phosphorylation inactivates PFK2, and another domain on this protein becomes active as fructose 2,6-bisphosphatase, which converts F2,6BP back to F6P. Both glucagon and epinephrine cause high levels of cAMP in the liver. The result of lower levels of liver fructose-2,6-bisphosphate is a decrease in activity of phosphofructokinase and an increase in activity of fructose 1,6-bisphosphatase, so that gluconeogenesis (essentially "glycolysis in reverse") is favored. This is consistent with the role of the liver in such situations, since the response of the liver to these hormones is to release glucose to the blood.
ATP competes with AMP for the allosteric effector site on the PFK enzyme. ATP concentrations in cells are much higher than AMP, typically 100-fold higher,[10] but the concentration of ATP does not change more than about 10% under physiological conditions, whereas a 10% drop in ATP results in a 6-fold increase in AMP.[11] Thus, the relevance of ATP as an allosteric effector is questionable. An increase in AMP is a consequence of a decrease in energy charge in the cell.
Citrate inhibits phosphofructokinase when tested in vitro by enhancing the inhibitory effect of ATP. However, it is doubtful that this is a meaningful effect in vivo, because citrate in the cytosol is mainly utilized for conversion to acetyl-CoA for fatty acid and cholesterol synthesis.
Pyruvate kinase

This enzyme catalyzes the last step of glycolysis, in which pyruvate and ATP are formed. Regulation of this enzyme is discussed in the main topic, pyruvate kinase.
Post-glycolysis processes
The overall process of glycolysis is:
- glucose + 2 NAD+ + 2 ADP + 2 Pi → 2 pyruvate + 2 NADH + 2 H+ + 2 ATP + 2 H2O
If glycolysis were to continue indefinitely, all of the NAD+ would be used up, and glycolysis would stop. To allow glycolysis to continue, organisms must be able to oxidize NADH back to NAD+.
One method of doing this is to simply have the pyruvate do the oxidation; in this process the pyruvate is converted to lactate (the conjugate base of lactic acid) in a process called lactic acid fermentation:
- pyruvate + NADH + H+ → lactate + NAD+
This process occurs in the bacteria involved in making yogurt (the lactic acid causes the milk to curdle). This process also occurs in animals under hypoxic (or partially-anaerobic) conditions, found, for example, in overworked muscles that are starved of oxygen, or in infarcted heart muscle cells. In many tissues, this is a cellular last resort for energy; most animal tissue cannot maintain anaerobic respiration for an extended length of time.
Some organisms, such as yeast, convert NADH back to NAD+ in a process called ethanol fermentation. In this process the pyruvate is converted first to acetaldehyde and carbon dioxide, then to ethanol.
Lactic acid fermentation and ethanol fermentation can occur in the absence of oxygen. This anaerobic fermentation allows many single-celled organisms to use glycolysis as their only energy source.
In the above two examples of fermentation, NADH is oxidized by transferring two electrons to pyruvate. However, anaerobic bacteria use a wide variety of compounds as the terminal electron acceptors in cellular respiration: nitrogenous compounds, such as nitrates and nitrites; sulfur compounds, such as sulfates, sulfites, sulfur dioxide, and elemental sulfur; carbon dioxide; iron compounds; manganese compounds; cobalt compounds; and uranium compounds.
In aerobic organisms, a complex mechanism has evolved to use the oxygen in air as the final electron acceptor of respiration.
- First, pyruvate is converted to acetyl-CoA and CO2 within the mitochondria in a process called pyruvate decarboxylation.
- Second, the acetyl-CoA enters the citric acid cycle, where it is fully oxidized to carbon dioxide and water, producing yet more NADH.
- Third, the NADH is oxidized to NAD+ by the electron transport chain, using oxygen as the final electron acceptor. This process creates a "hydrogen ion gradient" across the inner membrane of the mitochondria.
- Fourth, the proton gradient is used to produce a large amount of ATP in a process called oxidative phosphorylation.
Intermediates for other pathways
This article concentrates on the catabolic role of glycolysis with regard to converting potential chemical energy to usable chemical energy during the oxidation of glucose to pyruvate. However, many of the metabolites in the glycolytic pathway are also used by anabolic pathways, and, as a consequence, flux through the pathway is critical to maintain a supply of carbon skeletons for biosynthesis.
In addition, not all carbon entering the pathway leaves as pyruvate and may be extracted at earlier stages to provide carbon compounds for other pathways.
These metabolic pathways are all strongly reliant on glycolysis as a source of metabolites:
- Gluconeogenesis
- Lipid metabolism
- Pentose phosphate pathway
- Citric acid cycle, which in turn leads to:
From an anabolic metabolism perspective, the NADH has a role to drive synthetic reactions, doing so by directly or indirectly reducing the pool of NADP+ in the cell to NADPH, which is another important reducing agent for biosynthetic pathways in a cell.
Glycolysis in disease
Genetic diseases
Glycolytic mutations are generally rare due to importance of the metabolic pathway, however some mutations are seen.
This section needs expansion. You can help by adding to it. (June 2008) |
Cancer
Malignant rapidly-growing tumor cells typically have glycolytic rates that are up to 200 times higher than those of their normal tissues of origin. There are two common explanations. The classical explanation is that there is poor blood supply to tumors causing local depletion of oxygen. There is also evidence that attributes some of these high aerobic glycolytic rates to an overexpressed form of mitochondrially-bound hexokinase[12] responsible for driving the high glycolytic activity. This phenomenon was first described in 1930 by Otto Warburg, and hence it is referred to as the Warburg effect. Warburg hypothesis claims that cancer is primarily caused by dysfunctionality in mitochondrial metabolism, rather than because of uncontrolled growth of cells. There is ongoing research to affect mitochondrial metabolism and treat cancer by starving cancerous cells in various new ways, including a ketogenic diet.
This high glycolysis rate has important medical applications, as high aerobic glycolysis by malignant tumors is utilized clinically to diagnose and monitor treatment responses of cancers by imaging uptake of 2-18F-2-deoxyglucose (FDG) (a radioactive modified hexokinase substrate) with positron emission tomography (PET).[13][14]
Alzheimer's disease
Disfunctioning glycolysis or glucose metabolism in fronto-temporo-parietal and cingulate cortices has been associated with Alzheimer's disease [15], probably due to the decreased amyloid β (1-42) (Aβ42) and increased tau, phosphorylated tau in cerebrospinal fluid (CSF) [16]
Alternative nomenclature
Some of the metabolites in glycolysis have alternative names and nomenclature. In part, this is because some of them are common to other pathways, such as the Calvin cycle.
| This article | Alternative names | Alternative nomenclature | ||
|---|---|---|---|---|
| 1 | glucose | Glc | dextrose | |
| 3 | fructose 6-phosphate | F6P | ||
| 4 | fructose 1,6-bisphosphate | F1,6BP | fructose 1,6-diphosphate | FBP, FDP, F1,6DP |
| 5 | dihydroxyacetone phosphate | DHAP | glycerone phosphate | |
| 6 | glyceraldehyde 3-phosphate | GADP | 3-phosphoglyceraldehyde | PGAL, G3P, GALP,GAP,TP |
| 7 | 1,3-bisphosphoglycerate | 1,3BPG | glycerate 1,3-bisphosphate, glycerate 1,3-diphosphate, 1,3-diphosphoglycerate |
PGAP, BPG, DPG |
| 8 | 3-phosphoglycerate | 3PG | glycerate 3-phosphate | PGA, GP |
| 9 | 2-phosphoglycerate | 2PG | glycerate 2-phosphate | |
| 10 | phosphoenolpyruvate | PEP | ||
| 11 | pyruvate | Pyr | pyruvic acid | |
See also
- Pentose phosphate pathway
- Gluconeogenesis
- Fermentation (biochemistry)
- Pyruvate decarboxylation
- Citric acid cycle
- Triose kinase
- carbohydrate catabolism
- Cori cycle
External links
- A Simplified Glycolysis Animation (Flash Required)
- A Detailed Glycolysis Animation provided by IUBMB (Shockwave Required)
- The Glycolytic enzymes in Glycolysis at Protein Data Bank
- Glycolytic cycle with animations at wdv.com
- Metabolism, Cellular Respiration and Photosynthesis - The Virtual Library of Biochemistry and Cell Biology at biochemweb.org
- notes on glycolysis at rahulgladwin.com
- The chemical logic behind glycolysis at ufp.pt
- Expasy biochemical pathways poster at ExPASy
- MedicalMnemonics.com: 317 5468
References
- ^ Webster's New International Dictionary of the English Language, 2nd ed. (1937) Merriam Company, Springfield, Mass.
- ^ Romano AH, Conway T. (1996) Evolution of carbohydrate metabolic pathways. Res Microbiol. 147(6-7):448-55 PMID 9084754
- ^ lavin. "Glycolysis pathway". lifehugger. Retrieved 2008-09-28.
- ^ Reeves, R. E. (1974). "Pyrophosphate: D-fructose 6-phosphate 1-phosphotransferase. A new enzyme with the glycolytic function 6-phosphate 1-phosphotransferase". J Biol Chem. 249: 7737–7741. PMID 4372217.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Selig, M. (1997). "Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium Thermotoga". Arch Microbiol. 167: 217–232. PMID 9075622.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Garrett, R.; Grisham, C. M. (2005), Biochemistry (3rd ed.), Belmont, CA: Thomson Brooks/Cole, p. 584, ISBN 0-534-49011-6
{{citation}}: Check|isbn=value: checksum (help) - ^ Garrett, R.; Grisham, C. M. (2005), Biochemistry (3rd ed.), Belmont, CA: Thomson Brooks/Cole, pp. 582–583, ISBN 0-534-49011-6
{{citation}}: Check|isbn=value: checksum (help) - ^ Berg, J. M.; Tymoczko, J. L.; Stryer, L. (2007), Biochemistry (6th ed.), New York: Freeman, p. 622, ISBN 0-534-49011-6
{{citation}}: Check|isbn=value: checksum (help) - ^ Voet D., and Voet J. G. (2004). Biochemistry 3rd Edition (New York, John Wiley & Sons, Inc.)
- ^ Beis I., and Newsholme E. A. (1975). The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem J 152, 23-32.
- ^ Voet D., and Voet J. G. (2004). Biochemistry 3rd Edition (New York, John Wiley & Sons, Inc.).
- ^ "High Aerobic Glycolysis of Rat Hepatoma Cells in Culture: Role of Mitochondrial Hexokinase -- Bustamante and Pedersen 74 (9): 3735 -- Proceedings of the National Academy of Sciences". Retrieved December 5 2005.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|dateformat=ignored (help) - ^ "PET Scan: PET Scan Info Reveals ..." Retrieved December 5 2005.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|dateformat=ignored (help) - ^ "4320139 549..559" (PDF). Retrieved December 5 2005.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|dateformat=ignored (help) - ^ Hunt, A .; et al. (2007). "Reduced cerebral glucose metabolism in patients at risk for Alzheimer's disease". Psychiatry Research: Neuroimaging. 155 (2): 147–154. doi:10.1016/j.pscychresns.2006.12.003.
{{cite journal}}: Cite has empty unknown parameters:|accessyear=and|coauthors=(help); Explicit use of et al. in:|first=(help) - ^ Hunt, A .; et al. (2008). "CSF and MRI markers independently contribute to the diagnosis of Alzheimer's disease". Neurobiology of Aging. 29 (5): 669–675. doi:10.1016/j.neurobiolaging.2006.11.018.
{{cite journal}}: Cite has empty unknown parameters:|accessyear=and|coauthors=(help); Explicit use of et al. in:|first=(help)