Vaccine: Difference between revisions
Reverted 1 edit by 121.246.241.16. (TW) |
|||
| Line 174: | Line 174: | ||
=======================({{No More Links}})=============================--> |
=======================({{No More Links}})=============================--> |
||
* {{dmoz|Health/Pharmacy/Drugs_and_Medications/Vaccines_and_Antisera|Vaccines and Antisera}} |
* {{dmoz|Health/Pharmacy/Drugs_and_Medications/Vaccines_and_Antisera|Vaccines and Antisera}} |
||
* [http://healthmad.com/children/side-effects-of-vaccines-in-kids Side Effects of Vaccines in Kids] |
|||
<!--Use two spaces after External links section, so navboxes don't bunch up. --> |
<!--Use two spaces after External links section, so navboxes don't bunch up. --> |
||
Revision as of 08:58, 26 November 2009
A vaccine is a biological preparation that improves immunity to a particular disease. A vaccine typically contains a small amount of an agent that resembles a microorganism. The agent stimulates the body's immune system to recognize the agent as foreign, destroy it, and "remember" it, so that the immune system can more easily recognize and destroy any of these microorganisms that it later encounters.
Vaccines can be prophylactic (e.g. to prevent or ameliorate the effects of a future infection by any natural or "wild" pathogen), or therapeutic (e.g. vaccines against cancer are also being investigated; see cancer vaccine).
The term vaccine derives from Edward Jenner's 1796 use of the term cow pox (Latin variolæ vaccinæ, adapted from the Latin vaccīn-us, from vacca cow), which, when administered to humans, provided them protection against smallpox.
History
Sometime during the 1770s Edward Jenner heard a milkmaid boast that she would never have the often-fatal or disfiguring disease smallpox, because she had already had cowpox, which has a very mild effect in humans. In 1796 Jenner took pus from the hand of a milkmaid with cowpox, inoculated an 8-year-old boy with it, and six weeks later variolated the boy's arm with smallpox, afterwards observing that the boy did not catch smallpox.[1] Since vaccination with cowpox was much safer than smallpox inoculation,[2] the latter, though still widely practiced in England, was banned in 1840.[3] Louis Pasteur generalized Jenner's idea by developing what he called a rabies vaccine (now termed an antitoxin), and in the 19th century vaccines were considered a matter of national prestige, and compulsory vaccination laws were passed.[1]
The 20th century saw the introduction of several successful vaccines, including those against diphtheria, measles, mumps, and rubella. Major achievements included the development of the polio vaccine in the 1950s and the eradication of smallpox during the 1960s and 1970s. As vaccines became more common, many people began taking them for granted. However, vaccines remained elusive for many important diseases, including malaria and HIV.[1]
Types
Vaccines are dead or inactivated organisms or purified products derived from them.
There are several types of vaccines currently in use.[4] These represent different strategies used to try to reduce risk of illness, while retaining the ability to induce a beneficial immune response.
Killed
Vaccines containing killed microorganisms - these are previously virulent micro-organisms which have been killed with chemicals or heat. Examples are vaccines against flu, cholera, bubonic plague, polio and hepatitis A.
Attenuated
Some vaccines contain live, attenuated virus microorganisms. These are live micro-organisms that have been cultivated under conditions that disable their virulent properties, or which use closely-related but less dangerous organisms to produce a broad immune response. They typically provoke more durable immunological responses and are the preferred type for healthy adults. Examples include yellow fever, measles, rubella, and mumps. The live Mycobacterium tuberculosis vaccine developed by Calmette and Guérin is not made of a contagious strain, but contains a virulently modified strain called "BCG" used to elicit immunogenicity to the vaccine.
Toxoid
Toxoids - these are inactivated toxic compounds in cases where these (rather than the micro-organism itself) cause illness. Examples of toxoid-based vaccines include tetanus and diphtheria. Not all toxoids are for micro-organisms; for example, Crotalus atrox toxoid is used to vaccinate dogs against rattlesnake bites.
Subunit
Protein subunit - rather than introducing an inactivated or attenuated micro-organism to an immune system (which would constitute a "whole-agent" vaccine), a fragment of it can create an immune response. Characteristic examples include the subunit vaccine against Hepatitis B virus that is composed of only the surface proteins of the virus (produced in yeast) and the virus-like particle (VLP) vaccine against human papillomavirus (HPV) that is composed of the viral major capsid protein.
Conjugate
Conjugate - certain bacteria have polysaccharide outer coats that are poorly immunogenic. By linking these outer coats to proteins (e.g. toxins), the immune system can be led to recognize the polysaccharide as if it were a protein antigen. This approach is used in the Haemophilus influenzae type B vaccine.
Experimental
A number of innovative vaccines are also in development and in use:
- Recombinant Vector - by combining the physiology of one micro-organism and the DNA of the other, immunity can be created against diseases that have complex infection processes
- DNA vaccination - in recent years a new type of vaccine called DNA vaccination, created from an infectious agent's DNA, has been developed. It works by insertion (and expression, triggering immune system recognition) of viral or bacterial DNA into human or animal cells. Some cells of the immune system that recognize the proteins expressed will mount an attack against these proteins and cells expressing them. Because these cells live for a very long time, if the pathogen that normally expresses these proteins is encountered at a later time, they will be attacked instantly by the immune system. One advantage of DNA vaccines is that they are very easy to produce and store. As of 2006, DNA vaccination is still experimental.
- T-cell receptor peptide vaccines are under development for several diseases using models of Valley Fever, stomatitis, and atopic dermatitis. These peptides have been shown to modulate cytokine production and improve cell mediated immunity.
- Targeting of identified bacterial proteins that are involved in complement inhibition would neutralize the key bacterial virulence mechanism[5].
While most vaccines are created using inactivated or attenuated compounds from micro-organisms, synthetic vaccines are composed mainly or wholly of synthetic peptides, carbohydrates or antigens.
Valence
Vaccines may be monovalent (also called univalent) or multivalent (also called polyvalent). A monovalent vaccine is designed to immunize against a single antigen or single microorganism.[6] A multivalent or polyvalent vaccine is designed to immunize against two or more strains of the same microorganism, or against two or more microorganisms.[7] In certain cases a monovalent vaccine may be preferable for rapidly developing a strong immune response.[8]
Developing immunity
The immune system recognizes vaccine agents as foreign, destroys them, and 'remembers' them. When the virulent version of an agent comes along the body recognizes the protein coat on the virus, and thus is prepared to respond, by (1) neutralizing the target agent before it can enter cells, and (2) by recognizing and destroying infected cells before that agent can multiply to vast numbers.
When two or more vaccines are mixed together in the same formulation, the two vaccines can interfere. This most frequently occurs with live attenuated vaccines, where one of the vaccine components is more robust than the others and suppresses the growth and immune response to the other components. This phenomenon was first noted in the trivalent Sabin polio vaccine, where the amount of serotype 2 virus in the vaccine had to be reduced to stop it from interfering with the "take" of the serotype 1 and 2 viruses in the vaccine.[9] This phenomenon has also been found to be a problem with the dengue vaccines currently being researched, where the DEN-3 serotype was found to predominate and suppress the response to DEN-1, -2 and -4 serotypes.[10]
Vaccines have contributed to the eradication of smallpox, one of the most contagious and deadly diseases known to man. Other diseases such as rubella, polio, measles, mumps, chickenpox, and typhoid are nowhere near as common as they were a hundred years ago. As long as the vast majority of people are vaccinated, it is much more difficult for an outbreak of disease to occur, let alone spread. This effect is called herd immunity. Polio, which is transmitted only between humans, is targeted by an extensive eradication campaign that has seen endemic polio restricted to only parts of four countries (Afghanistan, India, Nigeria and Pakistan).[1] The difficulty of reaching all children as well as cultural misunderstandings, however, have caused the anticipated eradication date to be missed several times.
Schedule
- See also: Vaccination policy
In order to provide best protection, children are recommended to receive vaccinations as soon as their immune systems are sufficiently developed to respond to particular vaccines, with additional 'booster' shots often required to achieve 'full immunity'. This has led to the development of complex vaccination schedules. In the United States, the Advisory Committee on Immunization Practices, which recommends schedule additions for the Centers for Disease Control and Prevention, recommends routine vaccination of children against: hepatitis A, hepatitis B, polio, mumps, measles, rubella, diphtheria, pertussis, tetanus, HiB, chickenpox, rotavirus, influenza, meningococcal disease and pneumonia. The large number of vaccines and boosters recommended (up to 24 injections by age two) has led to problems with achieving full compliance. In order to combat declining compliance rates, various notification systems have been instituted and a number of combination injections are now marketed (e.g., Pneumococcal conjugate vaccine and MMRV vaccine), which provide protection against multiple diseases.
Besides recommendations for infant vaccinations and boosters, many specific vaccines are recommended at other ages or for repeated injections throughout life—most commonly for measles, tetanus, influenza, and pneumonia. Pregnant women are often screened for continued resistance to rubella. The human papillomavirus vaccine is currently recommended in the U.S. and UK for ages 11–25. Vaccine recommendations for the elderly concentrate on pneumonia and influenza, which are more deadly to that group. In 2006, a vaccine was introduced against shingles, a disease caused by the chickenpox virus, which usually affects the elderly.
In Australia, a massive increase in vaccination rates was observed when the federal government made certain benefits (such as the universal 'Family Allowance' welfare payments for parents of children) dependent upon vaccination compliance. As well, children were not allowed into school unless they were either vaccinated or their parents completed a statutory declaration refusing to immunize them, after discussion with a doctor, and other bureaucracy. (Similar school-entry vaccination regulations have been in place in some parts of Canada for several years.) It became easier and cheaper to vaccinate one's children than not to. When faced with the annoyance, many more casual objectors simply gave in.[citation needed]
Effectiveness
Vaccines do not guarantee complete protection from a disease.[11] Sometimes this is because the host's immune system simply doesn't respond adequately or at all. This may be due to a lowered immunity in general (diabetes, steroid use, HIV infection) or because the host's immune system does not have a B cell capable of generating antibodies to that antigen.
Even if the host develops antibodies, the human immune system is not perfect and in any case the immune system might still not be able to defeat the infection.
Adjuvants are typically used to boost immune response. Adjuvants are sometimes called the dirty little secret of vaccines [2] in the scientific community, as not much is known about how adjuvants work. Most often aluminium adjuvants are used, but adjuvants like squalene are also used in some vaccines and more vaccines with squalene and phosphate adjuvants are being tested. The efficacy or performance of the vaccine is dependent on a number of factors:
- the disease itself (for some diseases vaccination performs better than for other diseases)
- the strain of vaccine (some vaccinations are for different strains of the disease) [3]
- whether one kept to the timetable for the vaccinations (see Vaccination schedule)
- some individuals are 'non-responders' to certain vaccines, meaning that they do not generate antibodies even after being vaccinated correctly
- other factors such as ethnicity or genetic predisposition
When a vaccinated individual does develop the disease vaccinated against, the disease is likely to be milder than without vaccination.[citation needed]
The following are important considerations in the effectiveness of a vaccination program:[citation needed]
- careful modelling to anticipate the impact that an immunization campaign will have on the epidemiology of the disease in the medium to long term
- ongoing surveillance for the relevant disease following introduction of a new vaccine and
- maintaining high immunization rates, even when a disease has become rare.
In 1958 there were 763,094 cases of measles and 552 deaths in the United States.[12][13] With the help of new vaccines, the number of cases dropped to fewer than 150 per year (median of 56).[13] In early 2008, there were 64 suspected cases of measles. 54 out of 64 infections were associated with importation from another country, although only 13% were actually acquired outside of the United States; 63 of these 64 individuals either had never been vaccinated against measles, or were uncertain whether they had been vaccinated.[13]
Controversy

Opposition to vaccination, from a wide array of vaccine critics, has existed since the earliest vaccination campaigns.[14] Disputes have arisen over the morality, ethics, effectiveness, and safety of vaccination. The mainstream medical opinion is that the benefits of preventing suffering and death from serious infectious diseases greatly outweigh the risks of rare adverse effects following immunization.[15][16] Some vaccination critics say that vaccines are ineffective against disease[17] or that vaccine safety studies are inadequate.[16][17] Some religious groups do not allow vaccination,[18] and some political groups oppose mandatory vaccination on the grounds of individual liberty.[14]
Economics of development
One challenge in vaccine development is economic: many of the diseases most demanding a vaccine, including HIV, malaria and tuberculosis, exist principally in poor countries. Pharmaceutical firms and biotechnology companies have little incentive to develop vaccines for these diseases, because there is little revenue potential. Even in more affluent countries, financial returns are usually minimal and the financial and other risks are great.[19]
Most vaccine development to date has relied on 'push' funding by government, universities and non-profit organizations.[20] Many vaccines have been highly cost effective and beneficial for public health.[19] The number of vaccines actually administered has risen dramatically in recent decades. This increase, particularly in the number of different vaccines administered to children before entry into schools[21] may be due to government mandates and support, rather than economic incentive.[citation needed]
Many researchers and policymakers are calling for a different approach, using 'pull' mechanisms to motivate industry. Mechanisms such as prizes, tax credits, or advance market commitments could ensure a financial return to firms that successfully developed a HIV vaccine. If the policy were well-designed, it might also ensure people have access to a vaccine if and when it is developed.[citation needed]
Patents
The filing of patents on vaccine development processes can also be viewed as an obstacle to the development of new vaccines. Because of the weak protection offered through a patent on the final product, the protection of the innovation regarding vaccines is often made through the patent of processes used on the development of new vaccines as well as the protection of secrecy.[22]
Production
Vaccine production has several stages. First, the antigen itself is generated. Viruses are grown either on primary cells such as chicken eggs (e.g., for influenza), or on continuous cell lines such as cultured human cells (e.g., for hepatitis A). Bacteria are grown in bioreactors (e.g., Haemophilus influenzae type b). Alternatively, a recombinant protein derived from the viruses or bacteria can be generated in yeast, bacteria, or cell cultures. After the antigen is generated, it is isolated from the cells used to generate it. A virus may need to be inactivated, possibly with no further purification required. Recombinant proteins need many operations involving ultrafiltration and column chromatography. Finally, the vaccine is formulated by adding adjuvant, stabilizers, and preservatives as needed. The adjuvant enhances the immune response of the antigen, stabilizers increase the storage life, and preservatives allow the use of multidose vials.[23] Combination vaccines are harder to develop and produce, because of potential incompatibilities and interactions among the antigens and other ingredients involved.[24]
Vaccine production techniques are evolving. Cultured mammalian cells are expected to become increasingly important, compared to conventional options such as chicken eggs, due to greater productivitity and few problems with contamination. Recombination technology that produces genetically detoxified vaccine is expected to grow in popularity for the production of bacterial vaccines that use toxoids. Combination vaccines are expected to reduce the quantities of antigens they contain, and thereby decrease undesirable interactions, by using pathogen-associated molecular patterns.[24]
Preservatives
Many vaccines need preservatives to prevent serious adverse effects such as the Staphylococcus infection that, in one 1928 incident, killed 12 of 21 children inoculated with a diphtheria vaccine that lacked a preservative.[25] Several preservatives are available, including thiomersal, phenoxyethanol, and formaldehyde. Thiomersal is more effective against bacteria, has better shelf life, and improves vaccine stability, potency, and safety, but in the U.S., the European Union, and a few other affluent countries, it is no longer used as a preservative in childhood vaccines, as a precautionary measure due to its mercury content.[26] Although controversial claims have been made that thiomersal contributes to autism, no convincing scientific evidence supports these claims.[27]
Delivery systems

There are several new delivery systems in development, which will hopefully make vaccines more efficient to deliver. Possible methods include liposomes and ISCOM (immune stimulating complex).[28]
The latest developments in vaccine delivery technologies have resulted in oral vaccines. A polio vaccine was developed and tested by volunteer vaccinations with no formal training; the results were very positive in that the ease of the vaccines increased dramatically. With an oral vaccine, there is no risk of blood contamination. Oral vaccines are likely to be solid which have proven to be more stable and less likely to freeze; this stability eliminates the need for a "cold chain": the resources required to keep vaccines within a restricted temperature range from the manufacturing stage to the point of administration, which, in turn, will decrease costs of vaccines. Finally, a microneedle approach, which is still in stages of development, seems to be the vaccine of the future, the microneedle, which is "pointed projections fabricated into arrays that can create vaccine delivery pathways through the skin".[29]
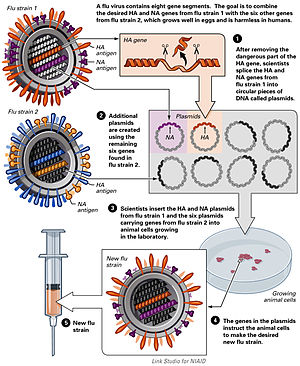
Plasmids
The use of plasmids has been validated in preclinical studies as a protective vaccine strategy for cancer and infectious diseases. However, the crossover application into human studies has been met with poor results based on the inability to provide clinically relevant benefit. The overall efficacy of plasmid DNA immunization depends on increasing the plasmid's immunogenicity while also correcting for factors involved in the specific activation of immune effector cells.[30]
Use in nonhumans
Vaccinations of animals are used both to prevent their contracting diseases and to prevent transmission of disease to humans[31]. Both animals kept as pets and animals raised as livestock are routinely vaccinated. In some instances, wild populations may be vaccinated. This is sometimes accomplished with vaccine-laced food spread in a disease-prone area and has been used to attempt to control rabies in raccoons.
Where rabies occurs, rabies vaccination of dogs may be required by law. Other canine vaccines include canine distemper, canine parvovirus, infectious canine hepatitis, adenovirus-2, leptospirosis, bordatella, canine parainfluenza virus, and Lyme disease among others.
Trends
Vaccine development has several trends:[32]
- Until now, most vaccines have been aimed at infants and children, but adolescents and adults are increasingly being targeted.
- Combinations of vaccines are becoming more common; vaccines containing five or more components are used in many parts of the world.
- New methods of administering vaccines are being developed, such as skin patches, aerosols via inhalation devices, and eating genetically engineered plants.
- Vaccines are being designed to stimulate innate immune responses, as well as adaptive.
- Attempts are being made to develop vaccines to help cure chronic infections, as opposed to preventing disease.
- Vaccines are being developed to defend against bioterrorist attacks such as anthrax, plague, and smallpox.
Principles that govern the immune response can now be used in tailor-made vaccines against many noninfectious human diseases, such as cancers and autoimmune disorders.[33] For example, the experimental vaccine CYT006-AngQb has been investigated as a possible treatment for high blood pressure.[34] Factors that have impact on the trends of vaccine development include progress in translatory medicine, demographics, regulatory science, political, cultural, and social responses.[35]
See also
- Influenza vaccine
- Immune system
- OPV AIDS hypothesis, a refuted hypothesis that the AIDS pandemic emerged from polio vaccine manufacture.
- Immunology
- Immunization
- Inoculation
- Bacterin
- TA-CD, a vaccine which negates the effects of cocaine
- The Horse Named Jim
- Virosome
- Vaccination
- List of vaccine topics
- Reverse vaccinology
- Flying syringe
- Pandemic vaccine (incl. Pre-pandemic vaccine)
References
- ^ a b c Stern AM, Markel H (2005). "The history of vaccines and immunization: familiar patterns, new challenges". Health Aff. 24 (3): 611–21. doi:10.1377/hlthaff.24.3.611. PMID 15886151.
- ^ Van Sant JE (2008). "The Vaccinators: Smallpox, Medical Knowledge, and the 'Opening' of Japan". J Hist Med Allied Sci. 63 (2): 276–9. doi:10.1093/jhmas/jrn014.
- ^ Dudgeon JA (1963). "Development of smallpox vaccine in England in the eighteenth and nineteenth centuries". BMJ (5342): 1367–72. doi:10.1136/bmj.1.5342.1367.
- ^ The Main Types of Vaccines
- ^ Meri S, Jördens M, Jarva H. Microbial complement inhibitors as vaccines. Vaccine. 2008 Dec 30;26 Suppl 8:I113-7. Review.PMID: 19388175
- ^ "Monovalent" at Dorland's Medical Dictionary
- ^ Polyvalent vaccine at Dorlands Medical Dictionary
- ^ "Questions and answers on monovalent oral polio vaccine type 1 (mOPV1) "Issued jointly by WHO and UNICEF"".
- ^ Sutter RW, Cochi SL, Melnick JL (1999). "Live attenuated polio vaccines". In Plotkin SA, Orenstein WA (eds.) (ed.). Vaccines. Philadelphia: W. B. Saunders. pp. 364–408.
{{cite book}}:|editor=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Kanesa-thasan N, Sun W, Kim-Ahn G; et al. (2001). "Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers". Vaccine. 19 (23–24): 3179–3188. doi:10.1016/S0264-410X(01)00020-2. PMID 11312014.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Grammatikos AP, Mantadakis E, Falagas ME. Meta-analyses on pediatric infections and vaccines. Infect Dis Clin North Am. 2009; 23(2):431-57.PMID 19393917
- ^ Orenstein WA, Papania MJ, Wharton ME (2004). "Measles elimination in the United States". J Infect Dis. 189 (Suppl 1): S1–3. doi:10.1086/377693. PMID 15106120.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c "Measles--United States, January 1-April 25, 2008". MMWR Morb. Mortal. Wkly. Rep. 57 (18): 494–8. 2008. PMID 18463608.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Wolfe R, Sharp L (2002). "Anti-vaccinationists past and present". BMJ. 325 (7361): 430–2. doi:10.1136/bmj.325.7361.430. PMID 12193361.
- ^ Bonhoeffer J, Heininger U (2007). "Adverse events following immunization: perception and evidence". Curr Opin Infect Dis. 20 (3): 237–46. doi:10.1097/QCO.0b013e32811ebfb0. PMID 17471032.
- ^ a b Demicheli V, Jefferson T, Rivetti A, Price D (2005). "Vaccines for measles, mumps and rubella in children". Cochrane Database Syst Rev. 19 (4). doi:10.1002/14651858.CD004407.pub2. PMID 16235361.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Halvorsen R (2007). The Truth about Vaccines. Gibson Square. ISBN 9781903933923.
- ^ Sinal SH, Cabinum-Foeller E, Socolar R (2008). "Religion and medical neglect". South Med J. 101 (7): 703–6. doi:10.1097/SMJ.0b013e31817997c9. PMID 18580731.
{{cite journal}}: Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ a b Goodman, Jesse L. (2005-05-04). "Statement of Jesse L. Goodman, M.D., M.P.H. Director, Center for Biologics, Evaluation and Research Before the Committee on Energy and Commerce United States House of Representatives". Retrieved 2008-06-15.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Olesen OF, Lonnroth A, Mulligan B (2009). "Human vaccine research in the European Union". Vaccine. 27 (5): 640–5. doi:10.1016/j.vaccine.2008.11.064. PMID 19059446.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ihara T. The strategy for prevention of measles and rubella prevalence with measles-rubella (MR) vaccine in Japan. Vaccine. 2009 Mar 5. [Epub ahead of print]PMID: 19366578
- ^ Hardman Reis T (2006). "The role of intellectual property in the global challenge for immunization". J World Intellect Prop. 9 (4): 413–25. doi:10.1111/j.1422-2213.2006.00284.x.
- ^ Muzumdar JM, Cline RR (2009). "Vaccine supply, demand, and policy: a primer". J Am Pharm Assoc. 49 (4): e87–99. doi:10.1331/JAPhA.2009.09007. PMID 19589753.
- ^ a b Bae K, Choi J, Jang Y, Ahn S, Hur B (2009). "Innovative vaccine production technologies: the evolution and value of vaccine production technologies". Arch Pharm Res. 32 (4): 465–80. doi:10.1007/s12272-009-1400-1. PMID 19407962.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Thimerosal in vaccines". Center for Biologics Evaluation and Research, U.S. Food and Drug Administration. 2007-09-06. Retrieved 2007-10-01.
- ^ Bigham M, Copes R (2005). "Thiomersal in vaccines: balancing the risk of adverse effects with the risk of vaccine-preventable disease". Drug Saf. 28 (2): 89–101. doi:10.2165/00002018-200528020-00001. PMID 15691220.
- ^ Offit PA (2007). "Thimerosal and vaccines—a cautionary tale". N Engl J Med. 357 (13): 1278–9. doi:10.1056/NEJMp078187. PMID 17898096.
- ^ Morein B, Hu KF, Abusugra I (2004). "Current status and potential application of ISCOMs in veterinary medicine". Adv Drug Deliv Rev. 56 (10): 1367–82. doi:10.1016/j.addr.2004.02.004. PMID 15191787.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Giudice EL, Campbell JD (2006). "Needle-free vaccine delivery". Adv Drug Deliv Rev. 58 (1): 68–89. doi:10.1016/j.addr.2005.12.003. PMID 16564111.
- ^ Lowe; et al. (2008). "Plasmid DNA as Prophylactic and Therapeutic vaccines for Cancer and Infectious Diseases". Plasmids: Current Research and Future Trends. Caister Academic Press. ISBN 978-1-904455-35-6.
{{cite book}}: Explicit use of et al. in:|author=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Patel JR, Heldens JG. Immunoprophylaxis against important virus disease of horses, farm animals and birds. Vaccine. 2009 Mar 13;27(12):1797-1810. Review. PMID: 19402200
- ^ Plotkin SA (2005). "Vaccines: past, present and future". Nat Med. 11 (4 Suppl): S5–11. doi:10.1038/nm1209. PMID 15812490.
- ^ Spohn G, Bachmann MF (2008). "Exploiting viral properties for the rational design of modern vaccines". Expert Rev Vaccines. 7 (1): 43–54. doi:10.1586/14760584.7.1.43. PMID 18251693.
- ^ Samuelsson O, Herlitz H (2008). "Vaccination against high blood pressure: a new strategy". Lancet. 371 (9615): 788–9. doi:10.1016/S0140-6736(08)60355-4. PMID 18328909.
- ^ Poland GA, Jacobson RM, Ovsyannikova IG. Trends affecting the future of vaccine development and delivery: The role of demographics, regulatory science, the anti-vaccine movement, and vaccinomics. Vaccine. 2009 May 26;27(25-26):3240-4. Epub 2009 Feb 5.PMID: 19200833
External links
