Radium: Difference between revisions
wertyuiytfdhguio[ugchfxcgjiop]i Tags: Blanking blanking |
ClueBot NG (talk | contribs) m Reverting possible vandalism by 50.206.18.72 to version by OofyProsser. Report False Positive? Thanks, ClueBot NG. (3366268) (Bot) |
||
| Line 1: | Line 1: | ||
{{about|the chemical element}} |
|||
{{Use dmy dates|date=April 2014}} |
|||
{{infobox radium}} |
|||
'''Radium''' is a [[chemical element]] with symbol '''Ra''' and [[atomic number]] 88. It is the sixth element in [[alkaline earth metal|group 2]] of the [[periodic table]], also known as the [[alkaline earth metal]]s. Pure radium is silvery-white, but it readily reacts with nitrogen (rather than oxygen) on exposure to air, forming a black surface layer of radium nitride (Ra<sub>3</sub>N<sub>2</sub>). All isotopes of radium are highly [[radioactive]], with the most stable [[isotope]] being [[radium-226]], which has a [[half-life]] of 1600 years and [[Radioactive decay|decays]] into [[radon]] gas (specifically the isotope [[radon-222]]). When radium decays, [[ionizing radiation]] is a product, which can excite [[fluorescent]] chemicals and cause [[radioluminescence]]. |
|||
Radium, in the form of [[radium chloride]], was [[discovery of the chemical elements|discovered]] by [[Marie Curie|Marie]] and [[Pierre Curie]] in 1898. They extracted the radium compound from [[uraninite]] and published the discovery at the [[French Academy of Sciences]] five days later. Radium was isolated in its [[metal]]lic state by Marie Curie and [[André-Louis Debierne]] through the [[electrolysis]] of radium chloride in 1911.<ref>{{Cite web|url=http://www.rsc.org/periodic-table/element/88/radium|title=Radium|publisher=Royal Society of Chemistry }}</ref> |
|||
In nature, radium is found in [[uranium]] and (to a lesser extent) [[thorium]] ores in trace amounts as small as a seventh of a gram per ton of uraninite. Radium is not necessary for living organisms, and adverse health effects are likely when it is incorporated into biochemical processes because of its radioactivity and chemical reactivity. Currently, other than its use in [[nuclear medicine]], radium has no commercial applications; formerly, it was used as a radioactive source for [[radioluminescent]] devices and also in [[radioactive quackery]] for its supposed curative powers. Today, these former applications are no longer in vogue because radium's toxicity has since become known, and less dangerous isotopes are used instead in radioluminescent devices. |
|||
==Bulk properties== |
|||
Radium is the heaviest known [[alkaline earth metal]] and is the only [[radioactive]] member of its group. Its physical and chemical properties most closely resemble its lighter [[congener (chemistry)|congener]] [[barium]].<ref name=Greenwood112/> |
|||
Pure radium is a [[volatility (chemistry)|volatile]] silvery-white metal, although its lighter congeners [[calcium]], [[strontium]], and [[barium]] have a slight yellow tint.<ref name=Greenwood112>Greenwood and Earnshaw, p. 112</ref> Its color rapidly vanishes in air, yielding a black layer of [[radium nitride]] (Ra<sub>3</sub>N<sub>2</sub>).<ref name=k4>Kirby et al., p. 4</ref> Its [[melting point]] is either {{convert|700|°C}} or {{convert|960|°C}}{{efn|Both values are encountered in sources and there is no agreement among scientists as to the true value of the melting point of radium.}} and its [[boiling point]] is {{convert|1737|°C}}. Both of these values are slightly lower than those of barium, confirming [[periodic trend]]s down the group 2 elements.<ref name="Lide2004">{{cite book|last = Lide|first=D. R. |title = CRC Handbook of Chemistry and Physics |edition = 84th |location = Boca Raton (FL) |publisher = CRC Press |date = 2004 |isbn = 978-0-8493-0484-2}}</ref> Like barium and the [[alkali metal]]s, radium crystallizes in the [[body-centered cubic]] structure at [[standard temperature and pressure]]: the radium–radium bond distance is 514.8 [[picometer]]s.<ref>{{cite journal | last1 = Weigel | first1 = F. | last2 = Trinkl | first2 = A. | year = 1968 | title = Zur Kristallchemie des Radiums| doi = 10.1524/ract.1968.10.12.78 | journal = Radiochim. Acta | volume = 10 | issue = | page = 78}}</ref> Radium has a density of 5.5 g/cm<sup>3</sup>, higher than that of barium, again confirming periodic trends; the radium-barium density ratio is comparable to the radium-barium atomic mass ratio,<ref name=Young>{{cite book | url = https://books.google.com/books?id=F2HVYh6wLBcC&pg=PA85 | page = 85 | chapter =Radium|author=Young, David A. |title=Phase Diagrams of the Elements|publisher=University of California Press|date=1991|isbn=0-520-91148-2}}</ref> due to the two elements' similar crystal structures.<ref name=Young/><ref>[http://wwwhomes.uni-bielefeld.de/achim/ele_structures.html "Crystal Structures of the Chemical Elements at 1 bar"]. uni-bielefeld.de.</ref><!--books.google.com/books?id=QsgmAAAAMAAJ&q="melting+point+of+radium"&dq="melting+point+of+radium"&hl=de&sa=X&ei=8j_iT72ZAYfOsgb-r91v&ved=0CD4Q6AEwAg books.google.com/books?id=1hNSAAAAMAAJ&q="melting+point+of+radium"&dq="melting+point+of+radium"&hl=de&sa=X&ei=8j_iT72ZAYfOsgb-r91v&ved=0CEgQ6AEwBA--> |
|||
==Isotopes== |
|||
{{main article|Isotopes of radium}} |
|||
[[File:Decay chain(4n+2, Uranium series).svg|thumb|upright=1.25|left|Decay chain of <sup>238</sup>U, the primordial progenitor of <sup>226</sup>Ra]] |
|||
Radium has 33 known isotopes, with [[mass number]]s from 202 to 234: all of them are [[radioactive]].<ref name=NUBASE>{{cite journal |author=G. Audi |author2=A. H. Wapstra |author3=C. Thibault|author4=J. Blachot |author5=O. Bersillon |last-author-amp=yes |year=2003 |title=The NUBASE evaluation of nuclear and decay properties |url=http://amdc.in2p3.fr/nubase/Nubase2003.pdf |journal=[[Nuclear Physics A]] |volume=729 |pages=3–128 |doi=10.1016/j.nuclphysa.2003.11.001 |bibcode=2003NuPhA.729....3A}}</ref> Four of these – <sup>223</sup>Ra ([[half-life]] 11.4 days), <sup>224</sup>Ra (3.64 days), <sup>226</sup>Ra (1600 years), and <sup>228</sup>Ra (5.75 years) – occur naturally in the [[decay chain]]s of primordial [[thorium]]-232, [[uranium-235]], and [[uranium-238]] (<sup>223</sup>Ra from uranium-235, <sup>226</sup>Ra from uranium-238, and the other two from thorium-232). These isotopes nevertheless still have half-lives too short to be [[primordial nuclide|primordial radionuclides]] and only exist in nature from these decay chains.<ref name=k3>Kirby et al., p. 3</ref> Together with the [[synthetic radioisotope|artificial]] <sup>225</sup>Ra (15 d), these are the five most stable isotopes of radium.<ref name=k3/> All other known radium isotopes have half-lives under two hours, and the majority have half-lives under a minute.<ref name=NUBASE/> At least 12 [[nuclear isomer]]s have been reported; the most stable of them is radium-205m, with a half-life of between 130 and 230 milliseconds, which is still shorter than twenty-four [[ground state|ground-state]] radium isotopes.<ref name=NUBASE/> |
|||
In the early history of the study of radioactivity, the different natural isotopes of radium were given different names. In this scheme, <sup>223</sup>Ra was named actinium X (AcX), <sup>224</sup>Ra thorium X (ThX), <sup>226</sup>Ra radium (Ra), and <sup>228</sup>Ra mesothorium 1 (MsTh<sub>1</sub>).<ref name=k3/> When it was realized that all of these are isotopes of the same element, many of these names fell out of use, and "radium" came to refer to all isotopes, not just <sup>226</sup>Ra.<ref name=k3/> Some of radium-226's decay products received historical names including "radium", ranging from radium A to radium G, with the letter indicating approximately how far they were down the chain from their parent <sup>226</sup>Ra.<ref name=k3/> |
|||
<sup>226</sup>Ra is the most stable isotope of radium and is the last isotope in the (4''n'' + 2) decay chain of uranium-238 with a half-life of over a millennium: it makes up almost all of natural radium. Its immediate decay product is the dense radioactive [[noble gas]] [[radon]], which is responsible for much of the danger of environmental radium.<ref>[http://www.epa.gov/radiation/radionuclides/radium.html Radium – Radiation Protection]. [[United States Environmental Protection Agency]].</ref> It is 2.7 million times more radioactive than the same [[amount of substance|molar amount]] of natural [[uranium]] (mostly uranium-238), due to its proportionally shorter half-life.<ref>{{cite book | url = https://books.google.com/books?id=ojaelt2o7AQC&pg=PA139 | pages = 139– | title = The Interpretation of Radium | isbn = 978-0-486-43877-1 | author1 = Soddy | first1 = Frederick | date = 25 August 2004}}</ref><ref>{{cite book | url = https://books.google.com/books?id=t-fpKQ54f44C&pg=PT115| pages = 115– | title = Radioactivity | isbn = 978-0-19-983178-4 |publisher=Oxford University Press| author1 = Malley | first1 = Marjorie C. | date = 2011}}</ref> |
|||
A sample of radium metal maintains itself at a higher [[temperature]] than its surroundings because of the radiation it emits – [[alpha particles]], [[beta particles]], and [[gamma rays]]. More specifically, natural radium (which is mostly <sup>226</sup>Ra) emits mostly alpha particles, but other steps in its decay chain (the [[Decay chain#Uranium series|uranium or radium series]]) emit alpha or beta particles, and almost all particle emissions are accompanied by gamma rays.<ref>{{cite book | url = https://books.google.com/books?id=alC0vvE-ZUwC&pg=PA133| pages = 133– | title = The Becquerel Rays and the Properties of Radium | isbn = 978-0-486-43875-7 | author1 = Strutt | first1 = R. J. | date = 7 September 2004}}</ref> |
|||
==Chemistry== |
|||
Radium, like barium, is a highly [[reactivity (chemistry)|reactive]] metal and always exhibits its group oxidation state of +2.<ref name=k4/> It forms the colorless Ra<sup>2+</sup> [[cation]] in [[aqueous solution]], which is highly [[base (chemistry)|basic]] and does not form [[coordination complex|complexes]] readily.<ref name=k4/> Most radium compounds are therefore simple [[ionic bond|ionic]] compounds,<ref name=k4/> though participation from the 6s and 6p electrons (in addition to the valence 7s electrons) is expected due to [[relativistic quantum chemistry|relativistic effects]] and would enhance the [[covalent bond|covalent]] character of radium compounds such as Ra[[fluorine|F]]<sub>2</sub> and Ra[[astatine|At]]<sub>2</sub>.<ref name=Thayer>{{cite journal |last1=Thayer |first1=John S. |title=Relativistic Effects and the Chemistry of the Heavier Main Group Elements |year=2010 |page=81 |doi=10.1007/978-1-4020-9975-5_2}}</ref> For this reason, the [[standard electrode potential]] for the half-reaction Ra<sup>2+</sup> (aq) + 2e<sup>−</sup> → Ra (s) is −2.916 [[volt|V]], even slightly lower than the value −2.92 V for barium, whereas the values had previously smoothly increased down the group (Ca: −2.84 V; Sr: −2.89 V; Ba: −2.92 V).<ref name=Greenwood111>Greenwood and Earnshaw, p. 111</ref> The values for barium and radium are almost exactly the same as those of the heavier alkali metals [[potassium]], [[rubidium]], and [[caesium]].<ref name=Greenwood111/> |
|||
===Compounds=== |
|||
Solid radium compounds are white as radium ions provide no specific coloring, but they gradually turn yellow and then dark over time due to self-[[radiolysis]] from radium's [[alpha decay]].<ref name=k4/> Insoluble radium compounds [[Coprecipitation|coprecipitate]] with all barium, most [[strontium]], and most [[lead]] compounds.<ref name=k8>Kirby et al., p. 8</ref> |
|||
[[Radium oxide]] (RaO) has not been characterized well past its existence, despite oxides being common compounds for the other alkaline earth metals. [[Radium hydroxide]] (Ra(OH)<sub>2</sub>) is the most readily soluble among the alkaline earth hydroxides and is a stronger base than its barium congener, [[barium hydroxide]].<ref name=k4to8>Kirby et al., pp. 4–8</ref> It is also more soluble than [[actinium hydroxide]] and [[thorium hydroxide]]: these three adjacent hydroxides may be separated by precipitating them with [[ammonia]].<ref name=k4to8/> |
|||
[[Radium chloride]] (RaCl<sub>2</sub>) is a colorless, luminous compound. It becomes yellow after some time due to self-damage by the [[alpha radiation]] given off by radium when it decays. Small amounts of barium impurities give the compound a rose color.<ref name=k4to8/> It is soluble in water, though less so than [[barium chloride]], and its solubility decreases with increasing concentration of [[hydrochloric acid]]. Crystallization from aqueous solution gives the dihydrate RaCl<sub>2</sub>·2H<sub>2</sub>O, isomorphous with its barium analog.<ref name=k4to8/> |
|||
[[Radium bromide]] (RaBr<sub>2</sub>) is also a colorless, luminous compound.<ref name=k4to8/> In water, it is more soluble than radium chloride. Like radium chloride, crystallization from aqueous solution gives the dihydrate RaBr<sub>2</sub>·2H<sub>2</sub>O, isomorphous with its barium analog. The ionizing radiation emitted by radium bromide excites [[nitrogen]] molecules in the air, making it glow. The [[alpha particle]]s emitted by radium quickly gain two electrons to become neutral [[helium]], with builds up inside and weakens radium bromide crystals. This effect sometimes causes the crystals to break or even explode.<ref name=k4to8/> |
|||
[[Radium nitrate]] (Ra(NO<sub>3</sub>)<sub>2</sub>) is a white compound that can be made by dissolving [[radium carbonate]] in [[nitric acid]]. As the concentration of nitric acid increases, the solubility of radium nitrate decreases, an important property for the chemical purification of radium.<ref name=k4to8/> |
|||
Radium forms much the same insoluble salts as its lighter congener barium: it forms the insoluble [[radium sulfate|sulfate]] (RaSO<sub>4</sub>, the most insoluble known sulfate), [[radium chromate|chromate]] (RaCrO<sub>4</sub>), [[radium carbonate|carbonate]] (RaCO<sub>3</sub>), [[radium iodate|iodate]] (Ra(IO<sub>3</sub>)<sub>2</sub>), [[radium tetrafluoroberyllate|tetrafluoroberyllate]] (RaBeF<sub>4</sub>), and nitrate (Ra(NO<sub>3</sub>)<sub>2</sub>). With the exception of the carbonate, all of these are less soluble in water than the corresponding barium salts, but they are all isostructural to their barium counterparts. Additionally, [[radium phosphate]], [[radium oxalate|oxalate]], and [[radium sulfite|sulfite]] are probably also insoluble, as they [[coprecipitation|coprecipitate]] with the corresponding insoluble barium salts.<ref name=k8to9>Kirby et al., pp. 8–9</ref> The great insolubility of radium sulfate (at 20 °C, only 2.1 [[milligram|mg]] will dissolve in 1 [[kilogram|kg]] of water) means that it is one of the less biologically dangerous radium compounds.<ref name=k12>Kirby et al., p. 12</ref> The large ionic radius of Ra<sup>2+</sup> (148 pm) results in weak complexation and poor extraction of radium from aqueous solutions when not at high pH.<ref name=Ullmann97/> |
|||
==Occurrence== |
|||
All isotopes of radium have half-lives much shorter than the [[age of the Earth]], so that any primordial radium would have decayed long ago. Radium nevertheless still occurs [[Radium and radon in the environment|in the environment]], as the isotopes <sup>223</sup>Ra, <sup>224</sup>Ra, <sup>226</sup>Ra, and <sup>228</sup>Ra are part of the decay chains of natural thorium and uranium isotopes; since thorium and uranium have very long half-lives, these daughters are continually being regenerated by their decay.<ref name=k3/> Of these four isotopes, the longest-lived is <sup>226</sup>Ra (half-life 1600 years), a decay product of natural uranium. Because of its relative longevity, <sup>226</sup>Ra is the most common isotope of the element, making up about one [[parts per trillion|part per trillion]] of the Earth's crust; essentially all natural radium is <sup>226</sup>Ra.<ref name=Greenwood109/> Thus, radium is found in tiny quantities in the uranium ore [[uraninite]] and various other uranium [[minerals]], and in even tinier quantities in thorium minerals. One [[ton]] of [[uraninite|pitchblende]] typically yields about one seventh of a [[gram]] of radium.<ref>[http://periodic.lanl.gov/88.shtml "Radium"], Los Alamos National Laboratory. Retrieved 5 August 2009.</ref> One kilogram of the [[Earth's crust]] contains about 900 [[picogram]]s of radium, and one [[liter]] of [[sea water]] contains about 89 [[femtogram]]s of radium.<ref name=Raabundance>Section 14, Geophysics, Astronomy, and Acoustics; Abundance of Elements in the Earth's Crust and in the Sea, in Lide, David R. (ed.), ''[[CRC Handbook of Chemistry and Physics]], 85th Edition''. CRC Press. Boca Raton, Florida (2005).</ref> |
|||
==History== |
|||
[[File:Curie and radium by Castaigne.jpg|thumb|Marie and Pierre Curie experimenting with radium, a drawing by [[André Castaigne]]]] |
|||
[[File:US radium standard 1927.jpg|thumb|Glass tube of radium chloride kept by the US Bureau of Standards that served as the primary standard of radioactivity for the United States in 1927.]] |
|||
{{Details|Marie Curie#New elements}} |
|||
Radium was [[discovery of the chemical elements|discovered]] by [[Marie Curie|Marie Sklodowska-Curie]] and her husband [[Pierre Curie]] on 21 December 1898, in a [[uraninite]] (pitchblende) sample.<ref name=crc>Hammond, C. R. "Radium" in {{RubberBible92nd}}</ref> While studying the mineral earlier, the Curies removed uranium from it and found that the remaining material was still radioactive. They separated out an element similar to [[bismuth]] from pitchblende in July 1898, that turned out to be [[polonium]]. They then separated out a radioactive mixture consisting mostly of two components: compounds of [[barium]], which gave a brilliant green flame color, and unknown radioactive compounds which gave [[carmine]] [[spectral line]]s that had never been documented before. The Curies found the radioactive compounds to be very similar to the barium compounds, except that they were more insoluble. This made it possible for the Curies to separate out the radioactive compounds and discover a new element in them. The Curies announced their discovery to the [[French Academy of Sciences]] on 26 December 1898.<ref>{{cite journal |year=1898|title=Sur une nouvelle substance fortement radio-active, contenue dans la pechblende (On a new, strongly radioactive substance contained in pitchblende)|journal=Comptes Rendus|volume= 127|pages= 1215–1217|url=http://www.aip.org/history/curie/discover.htm |accessdate=1 August 2009 |author=Curie, Pierre |author2=Curie, Marie |author3=Bémont, Gustave |last-author-amp=yes }}</ref><ref>{{cite journal | doi = 10.1021/ed010p79 | title = The discovery of the elements. XIX. The radioactive elements |year = 1933 | last1 = Weeks | first1 = Mary Elvira |authorlink1=Mary Elvira Weeks| journal = Journal of Chemical Education | volume = 10 | issue = 2 | pages = 79|bibcode = 1933JChEd..10...79W }}</ref> The naming of radium dates to about 1899, from the French word ''radium'', formed in Modern Latin from ''radius'' (''ray''): this was in recognition of radium's power of emitting energy in the form of rays.<ref>{{cite journal|author=Ball, David W. |url=http://superieur.deboeck.com/resource/extra/9782804171278/mcquarrie_interA.pdf|journal=Journal of Chemical Education| volume =62 |year=1985|pages =787–788|title=Elemental etymology: What's in a name?|doi=10.1021/ed062p787|bibcode=1985JChEd..62..787B}}</ref><ref name="Carvalho2011">{{cite journal|last1=Carvalho|first1=Fernando P.|title=Marie Curie and the Discovery of Radium|year=2011|pages=3–13|doi=10.1007/978-3-642-22122-4_1}}</ref><ref name="Weeks1933">{{cite journal|last1=Weeks|first1=Mary Elvira|title=The discovery of the elements. XIX. The radioactive elements|journal=Journal of Chemical Education|volume=10|issue=2|year=1933|pages=79|doi=10.1021/ed010p79|bibcode=1933JChEd..10...79W}}</ref> |
|||
In 1910, radium was isolated as a pure [[metal]] by Marie Curie and [[André-Louis Debierne]] through the [[electrolysis]] of a pure radium [[chloride]] (RaCl<sub>2</sub>) solution using a [[mercury (element)|mercury]] [[cathode]], producing a radium–mercury [[amalgam (chemistry)|amalgam]]. This amalgam was then heated in an atmosphere of [[hydrogen]] gas to remove the mercury, leaving pure radium metal.<ref>{{cite journal|author=Curie, Marie|author2=Debierne, André|last-author-amp=yes |year=1910|title=Sur le radium métallique" (On metallic radium)|journal=Comptes Rendus|volume=151 |pages=523–525 |url=http://visualiseur.bnf.fr/CadresFenetre?O=NUMM-3104&I=523&M=tdm |language=French|accessdate=1 August 2009}}</ref> The same year, E. Eoler isolated radium by [[thermal decomposition]] of its [[azide]], Ra(N<sub>3</sub>)<sub>2</sub>.<ref name=k3/> Radium metal was first industrially produced in the beginning of the 20th century by [[Biraco]], a subsidiary company of [[Union Minière du Haut Katanga]] (UMHK) in its [[Olen, Belgium|Olen]] plant in Belgium.<ref>{{cite book | page = 206 | url = https://books.google.com/books?id=yCkJgKwyAVoC&pg=PA206 | title = Biotechnology for waste management and site restoration: Technological, educational, business, political aspects | isbn = 978-0-7923-4769-9 | author1 = Ronneau, C. | author2 = Bitchaeva, O. | publisher = Scientific Affairs Division, North Atlantic Treaty Organization | date = 1997}}</ref> |
|||
The common historical unit for radioactivity, the [[curie]], is based on the radioactivity of <sup>226</sup>Ra.<ref>{{cite web | author = Frame, Paul W. | title = How the Curie Came to Be | url = http://www.orau.org/ptp/articlesstories/thecurie.htm | accessdate = 30 April 2008}}</ref> |
|||
===Historical applications=== |
|||
====Luminescent paint==== |
|||
[[File:Radium-paint.jpg|left|thumb|upright|Self-luminous white paint which contains radium on the face and hand of an old clock.]] |
|||
[[File:Radium 2.jpg|thumb|Radium watch hands under ultraviolet light]] |
|||
Radium was formerly used in [[luminescence|self-luminous]] paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials. A typical self-luminous watch that uses radium paint contains around 1 microgram of radium.<ref name="PMC2024184" /> In the mid-1920s, a lawsuit was filed against the [[United States Radium Corporation]] by five dying "[[Radium Girls]]" dial painters who had painted radium-based [[luminous paint]] on the dials of watches and clocks. The dial painters routinely licked their brushes to give them a fine point, thereby ingesting radium.<ref name=OakRidge>Frame, Paul. [http://www.orau.org/ptp/collection/radioluminescent/radioluminescentinfo.htm Radioluminescent Paint], [[Oak Ridge Associated Universities]]. Retrieved September 17, 2007.</ref> Their exposure to radium caused serious health effects which included sores, [[anemia]], and [[bone cancer]]. This is because radium is treated as [[calcium]] by the body, and [[bone seeker|deposited in the bones]], where radioactivity degrades [[bone marrow|marrow]] and can mutate [[bone cells]].<ref name=epa/> |
|||
During the litigation, it was determined that the company's scientists and management had taken considerable precautions to protect themselves from the effects of radiation, yet had not seen fit to protect their employees. Additionally, for several years the companies had attempted to cover up the effects and avoid liability by insisting that the Radium Girls were instead suffering from [[syphilis]]. This complete disregard for employee welfare had a significant impact on the formulation of [[occupational disease]] [[labor law]].<ref>{{cite web|url=http://66.147.244.135/~enviror4/people/radiumgirls/ |title=Environmental history timeline – Radium Girls|accessdate=29 December 2014}}</ref> |
|||
As a result of the lawsuit, the adverse effects of radioactivity became widely known, and radium-dial painters were instructed in proper safety precautions and provided with protective gear. In particular, dial painters no longer licked paint brushes to shape them (which caused some ingestion of radium salts). Radium was still used in dials as late as the 1960s, but there were no further injuries to dial painters. This highlighted that the harm to the Radium Girls could easily have been avoided.<ref>Rowland, R. E. (1995) [http://www.osti.gov/accomplishments/documents/fullText/ACC0029.pdf Radium in humans: a review of U.S. studies]. Argonne National Laboratory. p. 22</ref> |
|||
From the 1960s the use of radium paint was discontinued. In many cases luminous dials were implemented with non-radioactive fluorescent materials excited by light; such devices glow in the dark after exposure to light, but the glow fades.<ref name=epa/> Where long-lasting self-luminosity in darkness was required, safer radioactive [[promethium]]-147 (half-life 2.6 years) or [[tritium]] (half-life 12 years) paint was used; both continue to be used today.<ref>{{Cite book|title = Man-made and natural radioactivity in environmental pollution and radiochronology|date = 2004|page = 78|isbn = 1-4020-1860-6|last1 = Tykva|first1 = Richard|last2 = Berg|first2 = Dieter|publisher=Springer}}</ref> These had the added advantage of not degrading the phosphor over time, unlike radium.<ref>{{cite book |script-title=ru:Аналитическая химия технеция, прометия, астатина и франция |trans-title=Analytical Chemistry of Technetium, Promethium, Astatine, and Francium |language=Russian |first1=A. K. |last1=Lavrukhina |first2=A. A. |last2=Pozdnyakov |date=1966 |publisher=[[Nauka (publisher)|Nauka]] |page=118}}</ref> Tritium emits very low-energy [[beta radiation]] (even lower-energy than the beta radiation emitted by promethium)<ref name=NUBASE/> which cannot penetrate the skin,<ref>[http://www.ehso.emory.edu/content-forms/3anuclidedatasafetysheets.pdf Nuclide safety data sheet: Hydrogen-3]. ehso.emory.edu</ref> rather than the penetrating gamma radiation of radium and is regarded as safer.<ref name=ieer>{{cite web|author=Zerriffi, Hisham |date=January 1996|title=Tritium: The environmental, health, budgetary, and strategic effects of the Department of Energy's decision to produce tritium|url=http://www.ieer.org/reports/tritium.html#(11)|publisher=[[Institute for Energy and Environmental Research]]|accessdate=15 September 2010}}</ref> |
|||
Clocks, watches, and instruments dating from the first half of the 20th century, often in military applications, may have been painted with radioactive luminous paint. They are usually no longer luminous; however, this is not due to radioactive decay of the radium (which has a half-life of 1600 years) but to the fluorescence of the zinc sulfide fluorescent medium being worn out by the radiation from the radium.<ref name=emsley>{{cite book|author=Emsley, John |title=Nature's building blocks: an A-Z guide to the elements|url=https://books.google.com/books?id=j-Xu07p3cKwC&pg=PA351|date=2003|publisher=Oxford University Press|isbn=978-0-19-850340-8|pages=351–}}</ref> The appearance of an often thick layer of green or yellowish brown paint in devices from this period suggests a radioactive hazard. The radiation dose from an intact device is relatively low and usually not an acute risk; but the paint is dangerous if released and inhaled or ingested.<ref name=brit>[http://global.britannica.com/EBchecked/topic/489270/radium-Ra radium]. ''Encyclopædia Britannica''</ref><ref>[http://www.vintagewatchstraps.com/luminous.htm Luminous Radium Paint]. vintagewatchstraps.com</ref> |
|||
====Commercial use==== |
|||
[[File:Radium Water Bath Department, top floor, Hotel Will Rogers, Claremore, Okla., U.S.A (63053).jpg|thumb|Hotel postcard advertising radium baths, c.1940s]] |
|||
{{Main article|Radioactive quackery }} |
|||
Radium was once an additive in products such as toothpaste, hair creams, and even food items due to its supposed curative powers.<ref>{{cite web|accessdate=1 August 2009|title=French Web site featuring products (medicines, mineral water, even underwear) containing radium|url=http://www.dissident-media.org/infonucleaire/radieux.html}}</ref> Such products soon fell out of vogue and were prohibited by authorities in many countries after it was discovered they could have serious adverse health effects. (See, for instance, ''[[Radithor]]'' or ''[[Revigator]]'' types of "Radium water" or "Standard Radium Solution for Drinking".)<ref name=emsley/> [[Destination spa|Spas]] featuring radium-rich water are still occasionally touted as beneficial, such as those in [[Misasa, Tottori]], Japan. In the U.S., nasal radium irradiation was also administered to children to prevent middle-ear problems or enlarged tonsils from the late 1940s through the early 1970s.<ref name="Baltimore">{{cite news|url=http://baltimorechronicle.com/rupnose.html|title=Nasal Radium Irradiation of Children Has Health Fallout|last=Cherbonnier|first=Alice|date=1 October 1997|work=Baltimore Chronicle|accessdate=1 August 2009}}</ref> |
|||
====Medical use==== |
|||
Radium (usually in the form of [[radium chloride]] or [[radium bromide]]) was used in [[medicine]] to produce radon gas which in turn was used as a [[cancer]] treatment; for example, several of these radon sources were used in Canada in the 1920s and 1930s.<ref name=brit/><ref>{{cite book|url = https://books.google.com/?id=NtKUdnjaCxMC&pg=PA135| title = An Element of Hope: Radium and the Response to Cancer in Canada, 1900–1940|first = Charles|last = Hayter|publisher = McGill-Queen's Press|date = 2005|isbn = 978-0-7735-2869-7|chapter = The Politics of Radon Therapy in the 1930s}}</ref> However, many treatments that were used in the early 1900s are not used anymore because of the harmful effects radium bromide exposure caused. Some examples of these effects are [[anaemia]], cancer, and [[mutation|genetic mutations]].<ref name="Harvie">{{cite journal | doi = 10.1016/S0160-9327(99)01201-6| pmid = 10589294| title = The radium century| journal = Endeavour| volume = 23| issue = 3| pages = 100–5|year = 1999| last1 = Harvie| first1 = David I.}}</ref> Safer gamma emitters such as [[cobalt-60|<sup>60</sup>Co]], which is less costly and available in larger quantities, are usually used today to replace the historical use of radium in this application.<ref name=Ullmann97/> |
|||
Early in the 1900s, biologists used radium to induce mutations and study [[genetics]]. As early as 1904, Daniel MacDougal used radium in an attempt to determine whether it could provoke sudden large mutations and cause major evolutionary shifts. [[Thomas Hunt Morgan]] used radium to induce changes resulting in white-eyed fruit flies. |
|||
Nobel-winning biologist Hermann Muller briefly studied the effects of radium on fruit fly mutations before turning to more affordable x-ray experiments.<ref name="Hamilton">{{cite journal|last1=Hamilton|first1=Vivien|title=The Secrets of Life: Historian Luis Campos resurrects radium's role in early genetics research|journal=Distillations|date=2016|volume=2|issue=2 |pages=44–45 |url=https://www.sciencehistory.org/distillations/magazine/the-secrets-of-life|accessdate=22 March 2018}}</ref> |
|||
[[Howard Atwood Kelly]], one of the founding physicians of [[Johns Hopkins Hospital]], was a major pioneer in the medical use of radium to treat cancer.<ref>{{cite web |
|||
| url = http://www.hopkinsmedicine.org/about/history/history5.html |
|||
| title=The Four Founding Physicians |
|||
| accessdate = 10 April 2013 |
|||
}}</ref> His first patient was his own aunt in 1904, who died shortly after surgery.<ref name="DasturTank2011">{{cite journal|last1=Dastur|first1=Adi E.|last2=Tank|first2=P. D.|title=Howard Atwood Kelly: much beyond the stitch|journal=The Journal of Obstetrics and Gynecology of India|volume=60|issue=5|year=2011|pages=392–394|doi=10.1007/s13224-010-0064-6|pmc=3394615}}</ref> Kelly was known to use excessive amounts of radium to treat various cancers and tumors. As a result, some of his patients died from radium exposure.<ref name="AronowitzRobison2010">{{cite journal|last1=Aronowitz|first1=Jesse N.|last2=Robison|first2=Roger F.|title=Howard Kelly establishes gynecologic brachytherapy in the United States|journal=Brachytherapy|volume=9|issue=2|year=2010|pages=178–184|doi=10.1016/j.brachy.2009.10.001|pmid=20022564}}</ref> His method of radium application was inserting a radium capsule near the affected area, then sewing the radium "points" directly to the [[tumor]].<ref name="AronowitzRobison2010" /> This was the same method used to treat [[Henrietta Lacks]], the host of the original [[HeLa cells]], for [[cervical cancer]].<ref name="Skloot2010">{{cite book|author=Rebecca Skloot|title=The Immortal Life of Henrietta Lacks|url=https://books.google.com/books?id=LBBhikJpLjwC|accessdate=8 April 2013|date=2 February 2010|publisher=Random House Digital, Inc.|isbn=978-0-307-58938-5}}</ref> Currently, safer and more available radioisotopes are used instead.<ref name=epa/> |
|||
==Production== |
|||
Uranium had no large scale application in the late 19th century and therefore no large uranium mines existed. In the beginning the only large source for uranium ore was the [[silver]] mines in Joachimsthal, [[Austria-Hungary]] (now [[Jáchymov]], Czech Republic).<ref name=crc/> The uranium ore was only a [[byproduct]] of the mining activities.<ref name=Ceranski>{{cite journal | doi = 10.1007/s00048-008-0308-z | title = Tauschwirtschaft, Reputationsökonomie, Bürokratie |year = 2008 | last1 = Ceranski | first1 = Beate | journal = NTM Zeitschrift für Geschichte der Wissenschaften, Technik und Medizin | volume = 16 | issue = 4 | pages = 413–443}}</ref> |
|||
In the first extraction of radium Curie used the residues after extraction of uranium from pitchblende. The uranium had been extracted by dissolution in sulfuric acid leaving radium sulfate, which is similar to barium sulfate but even less soluble in the residues. The residues also contained rather substantial amounts of barium sulfate which thus acted as a carrier for the radium sulfate. The first steps of the radium extraction process involved boiling with sodium hydroxide followed by hydrochloric acid treatment to remove as much as possible of other compounds. The remaining residue was then treated with sodium carbonate to convert the barium sulfate into barium carbonate carrying the radium, thus making it soluble in hydrochloric acid. After dissolution the barium and radium are reprecipitated as sulfates and this was repeated one or few times, for further purification of the mixed sulfate. Some impurities, that form insoluble sulfides, were removed by treating the chloride solution with hydrogen sulfide followed by filtering. When the mixed sulfate were pure enough they were once more converted to mixed chloride and barium and radium were separated by [[fractional crystallization (chemistry)|fractional crystallisation]] while monitoring the progress using a [[spectroscope]] (radium gives characteristic red lines in contrast to the green barium lines), and the [[electroscope]].<ref>[http://lateralscience.blogspot.se/2012/11/marie-curie-method-of-extraction-of.html "Lateral Science"]. ''lateralscience.blogspot.se''. November 2012</ref> |
|||
After the isolation of radium by Marie and Pierre Curie from uranium ore from Joachimsthal several scientists started to isolate radium in small quantities. Later small companies purchased mine tailings from Joachimsthal mines and started isolating radium. In 1904 the Austrian government [[nationalization|nationalised]] the mines and stopped exporting raw ore. For some time the radium availability was low.<ref name="Ceranski"/> |
|||
The formation of an Austrian monopoly and the strong urge of other countries to have access to radium led to a worldwide search for uranium ores. The United States took over as leading producer in the early 1910s. The [[Carnotite]] sands in [[Colorado]] provide some of the element, but richer ores are found in the [[Democratic Republic of the Congo|Congo]] and the area of the [[Great Bear Lake]] and the [[Great Slave Lake]] of northwestern Canada.<ref name=crc/><ref>{{cite journal | jstor = 40796935|author=Just, Evan|author2=Swain, Philip W.|author3=Kerr, William A.|last-author-amp=yes |journal=Financial Analysts Journal|volume=8|issue=1|year=1952 |pages=85–93|title=Peacetíme Impact of Atomíc Energy | doi = 10.2469/faj.v8.n1.85}}</ref> Neither of the deposits is mined for radium but the uranium content makes mining profitable. |
|||
The Curies' process was still used for industrial radium extraction in 1940, but mixed bromides were then used for the fractionation.<ref>{{Cite journal | doi = 10.1021/ed017p417| title = Extraction of radium from Canadian pitchblende| journal = Journal of Chemical Education| volume = 17| issue = 9| pages = 417| year = 1940| last1 = Kuebel | first1 = A. | bibcode = 1940JChEd..17..417K}}</ref> If the barium content of the uranium ore is not high enough it is easy to add some to carry the radium. These processes were applied to high grade uranium ores but may not work well with low grade ores. |
|||
Small amounts of radium were still extracted from uranium ore by this method of mixed precipitation and ion exchange as late as the 1990s,<ref name=Greenwood109>Greenwood and Earnshaw, pp. 109–110</ref> but today they are extracted only from spent nuclear fuel.<ref name=nbb>{{cite book| page=437| title =Nature's building blocks: an A-Z guide to the elements|first =John|last=Emsley| publisher=Oxford University Press| isbn = 9780199605637| date=2011}}</ref> In 1954, the total worldwide supply of purified radium amounted to about {{convert|5|lb|kg}}<ref name="PMC2024184">{{cite journal| title=Radium in the healing arts and in industry: Radiation exposure in the United States|pmc=2024184| year=1954| volume=69| issue=3| pmid=13134440| last1=Terrill Jr| first1=J. G.| last2=Ingraham Sc| first2=2nd|last3=Moeller|first3=D. W.| pages=255–62| journal=Public Health Reports|doi=10.2307/4588736}}</ref> and it is still in this range today, while the annual production of pure radium compounds is only about 100 g in total today.<ref name=Greenwood109/> The chief radium-producing countries are Belgium, Canada, the Czech Republic, [[Slovakia]], the United Kingdom, and [[Russia]].<ref name=Greenwood109/> The amounts of radium produced were and are always relatively small; for example, in 1918, 13.6 g of radium were produced in the United States. <ref>{{cite journal | doi = 10.1126/science.49.1262.227 | title = Radium Production |year = 1919 | last1 = Viol | first1 = C. H. | journal = Science | volume = 49 | issue = 1262 | pages = 227–8 | pmid = 17809659|bibcode = 1919Sci....49..227V }}</ref> The metal is isolated by reducing radium oxide with aluminium metal in a vacuum at 1200 °C.<ref name=Ullmann97/> |
|||
==Modern applications== |
|||
Some of the few practical uses of radium are derived from its radioactive properties. More recently discovered [[radioisotope]]s, such as [[cobalt-60]] and [[caesium-137]], are replacing radium in even these limited uses because several of these isotopes are more powerful emitters, safer to handle, and available in more concentrated form.<ref>{{cite book | url = https://books.google.com/books?id=3cT2REdXJ98C&pg=PA24| page =24 | title = Radiation source use and replacement: Abbreviated version | isbn = 978-0-309-11014-3 | author1 = Committee On Radiation Source Use And Replacement | first1 = National Research Council (U.S.) | last2 = Nuclear And Radiation Studies Board | first2 = National Research Council (U.S.) | date = January 2008}}</ref><ref>{{cite book | url = https://books.google.com/books?id=bk0go_-FO5QC&pg=PA8| page =8 | title = Radiation therapy planning | isbn = 978-0-07-005115-7 | author1 = Bentel | first1 = Gunilla Carleson | date = 1996}}</ref> |
|||
The isotope <sup>223</sup>Ra (under the trade name [[Xofigo]]) was approved by the United States [[Food and Drug Administration]] in 2013 for use in [[medicine]] as a [[cancer]] treatment of bone [[metastasis]].<ref name=FBT-FDA2013>{{Cite web|title=FDA OKs pinpoint prostate cancer radiation drug Xofigo from Bayer, Algeta |url=http://www.fiercebiotech.com/story/breaking-fda-oks-pinpoint-prostate-cancer-radiation-drug-xofigo-bayer-alget/2013-05-15 |archiveurl=https://www.webcitation.org/6Gdfdbr1u |archivedate=2013-05-15 |deadurl=no }}</ref><ref>[http://www.cancer.org/cancer/news/news/fda-approves-xofigo-for-advanced-prostate-cancer "FDA Approves Xofigo for Advanced Prostate Cancer"]. cancer.org. (2013-05-15)</ref> The main indication of treatment with [[Xofigo]] is the therapy of bony metastases from castration-resistant prostate cancer due to the favourable characteristics of this alpha-emitter radiopharmaceutical.<ref>{{cite journal |pmid=26222274 | volume=59 | title=New radiopharmaceutical agents for the treatment of castration-resistant prostate cancer | year=2015 | journal=Q J Nucl Med Mol Imaging | pages=420–38 | last1 = Maffioli | first1 = L. | last2 = Florimonte | first2 = L. | last3 = Costa | first3 = D. C. | last4 = Correia Castanheira | first4 = J. | last5 = Grana | first5 = C. | last6 = Luster | first6 = M. | last7 = Bodei | first7 = L. | last8 = Chinol | first8 = M.}}</ref> <sup>225</sup>Ra has also been used in experiments concerning therapeutic irradiation, as it is the only reasonably long-lived radium isotope which does not have radon as one of its daughters.<ref>{{cite book |first=Wolfgang |last=Stoll |chapter=Thorium and Thorium Compounds |doi=10.1002/14356007.a27_001 |title=Ullmann's Encyclopedia of Industrial Chemistry |publisher=[[Wiley-VCH]] |year=2005 |isbn=978-3-527-31097-5 |page=717}}</ref> |
|||
Radium is still used today as a radiation source in some [[industrial radiography]] devices to check for flawed metallic parts, similarly to [[X-ray imaging]].<ref name=epa/> When mixed with [[beryllium]], radium acts as a [[neutron source]].<ref name=emsley/><ref>{{cite book | url = https://books.google.com/books?id=YpEiPPFlNAAC&pg=PA261 | pages = 260–261 | chapter = Alpha particle induced nuclear reactions | title = Radioactivity: Introduction and history | isbn = 978-0-444-52715-8 | author1 = l'Annunziata | first1 = Michael F. | date = 2007|publisher=Elsevier}}</ref> Radium-beryllium neutron sources are still sometimes used even today,<ref name=epa>[http://www.epa.gov/radiation/radionuclides/radium.html Radium: Radiation Protection] – US EPA</ref><ref>{{Cite journal |
|||
| pmid = 15069300 |
|||
| year = 2004 |
|||
| author1 = Holden |
|||
| first1 = N. E. |
|||
| title = Radiation dosimetry of a graphite moderated radium-beryllium source |
|||
| journal = Health physics |
|||
| volume = 86 |
|||
| issue = 5 Suppl |
|||
| pages = S110–2 |
|||
| last2 = Reciniello |
|||
| first2 = R. N. |
|||
| last3 = Hu |
|||
| first3 = J. P. |
|||
| last4 = Rorer |
|||
| first4 = David C. |
|||
| bibcode = 2003rdtc.conf..484H |
|||
| doi = 10.1142/9789812705563_0060 |
|||
| url = http://www.bnl.gov/isd/documents/24293.pdf |
|||
}}</ref> but other materials such as [[polonium]] are now more common: about 1500 polonium-beryllium neutron sources, with an individual activity of {{convert|1850|Ci|TBq|abbr=on}}, have been used annually in [[Russia]].<ref>[http://www.stringer.ru/publication.mhtml?Part=50&PubID=6767 Красивая версия "самоубийства" Литвиненко вследствие криворукости] (in Russian). stringer.ru (2006-11-26).</ref> These RaBeF<sub>4</sub>-based (α, n) neutron sources have been deprecated despite the high number of neutrons they emit (1.84×10<sup>6</sup> neutrons per second) in favour of <sup>241</sup>[[americium|Am]]–Be sources.<ref name=Ullmann97/> Today, the isotope <sup>226</sup>Ra is mainly used to form <sup>227</sup>[[actinium|Ac]] by [[neutron irradiation]] in a nuclear reactor.<ref name=Ullmann97/> |
|||
==Hazards== |
|||
Radium is highly radioactive and its immediate daughter, [[radon]] gas, is also radioactive. When ingested, 80% of the ingested radium leaves the body through the [[feces]], while the other 20% goes into the [[bloodstream]], mostly accumulating in the bones.<ref name=epa/> Exposure to radium, internal or external, can cause cancer and other disorders, because radium and radon emit alpha and [[gamma ray]]s upon their decay, which kill and mutate cells.<ref name=epa/> At the time of the [[Manhattan Project]] in 1944, the "tolerance dose" for workers was set at 0.1 micrograms of ingested radium.<ref>{{cite book|author=Weisgall, Jonathan M.|title=Operation crossroads: the atomic tests at Bikini Atoll|url=https://books.google.com/books?id=K63bAAAAMAAJ|accessdate=20 August 2011|date=1994|publisher=Naval Institute Press|isbn=978-1-55750-919-2|page=238}}</ref><ref>{{cite journal | doi = 10.2307/3579805| first =Shirley A. | last =Fry| title = Supplement: Madame Curie's Discovery of Radium (1898): A Commemoration by Women in Radiation Sciences | journal =Radiation Research | volume= 150 | issue = 5 |year = 1998 | pages = S21–S29 | pmid = 9806606}}</ref><!-- http://www.osti.gov/accomplishments/documents/fullText/ACC0029.pdf--> |
|||
Some of the biological effects of radium were apparent from the start. The first case of so-called "radium-dermatitis" was reported in 1900, only 2 years after the element's discovery. The French physicist [[Antoine Becquerel]] carried a small ampoule of radium in his waistcoat pocket for 6 hours and reported that his skin became [[Ulcer (dermatology)|ulcerated]]. Pierre and Marie Curie were so intrigued by radiation that they sacrificed their own health to learn more about it. Pierre Curie attached a tube filled with radium to his arm for ten hours, which resulted in the appearance of a skin lesion, suggesting the use of radium to attack cancerous tissue as it had attacked healthy tissue.<ref>{{cite book|last1=Redniss|first1=Lauren|title=Radioactive: Marie & Pierre Curie: A Tale Of Love And Fallout|date=2011|publisher=HarperCollins|location=New York, NY|isbn=978-0-06-135132-7|page=70}}</ref> Handling of radium has been blamed for Marie Curie's death due to [[aplastic anemia]]. A significant amount of radium's danger comes from its daughter radon: being a gas, it can enter the body far more readily than can its parent radium.<ref name=epa/> |
|||
Today, <sup>226</sup>Ra is considered to be the most toxic of the quantity radioelements, and it must be handled in tight glove boxes with significant airstream circulation that is then treated to avoid escape of its daughter <sup>222</sup>Rn to the environment. Old ampoules containing radium solutions must be opened with care because radiolytic decomposition of water can produce an overpressure of hydrogen and oxygen gas.<ref name=Ullmann97>Ullmann, pp. 97–98</ref> |
|||
==See also== |
|||
{{Subject bar |
|||
|portal1=Chemistry |
|||
|portal2=Medicine |
|||
|book1=Radium |
|||
|book2=Period 7 elements |
|||
|book3=Alkaline earth metals |
|||
|book4=Chemical elements (sorted alphabetically) |
|||
|book5=Chemical elements (sorted by number) |
|||
}} |
|||
==Notes== |
|||
{{notelist}} |
|||
==References== |
|||
{{Reflist|35em}} |
|||
==Bibliography== |
|||
* {{cite book | url = http://library.lanl.gov/cgi-bin/getfile?rc000041.pdf| title = The Radiochemistry of Radium|ref=Kirby | last1 = Kirby | first1 = H. W. | last2 = Salutsky | first2 = Murrell L. | date = 1964 | publisher=National Academies Press}} |
|||
* {{Greenwood&Earnshaw2nd}} |
|||
* {{Ullmann | first1=Cornelius |last1=Keller |first2=Walter |last2=Wolf |first3=Jashovam |last3=Shani | title = Radionuclides, 2. Radioactive Elements and Artificial Radionuclides | doi = 10.1002/14356007.o22_o15}} |
|||
==Further reading== |
|||
* {{cite book|title=Guide to the Elements – Revised Edition|author=Albert Stwertka|publisher=Oxford University Press|date=1998|isbn=0-19-508083-1}} |
|||
* {{cite news|url=https://www.nytimes.com/library/national/science/100698sci-radium.html|title=A Glow in the Dark, and a Lesson in Scientific Peril|date=6 October 1998|author=Denise Grady|accessdate=25 December 2007|work=The New York Times}} |
|||
* {{cite web|url=http://nobelprize.org/nobel_prizes/physics/articles/curie/index.html|title=Marie and Pierre Curie and the Discovery of Polonium and Radium|publisher=Nobel Foundation|author=Nanny Fröman|date=1 December 1996|accessdate=25 December 2007}} |
|||
* {{cite journal|title = The great radium scandal|author = Macklis, R. M.|journal = Scientific American|year = 1993|volume = 269|issue = 2|pages = 94–99|pmid = 8351514|doi = 10.1038/scientificamerican0893-94|bibcode = 1993SciAm.269b..94M}} |
|||
* {{cite book|title = Radium Girls: Women and Industrial Health Reform, 1910–1935|author = Clark, Claudia|date = 1987|publisher = University of North Carolina Press|isbn = 0-8078-4640-6}} |
|||
* {{cite book|last=Curie |first=Marie |authorlink=Marie Curie|title=[[s:The Discovery of Radium|The Discovery of Radium]]|year=1921|publisher=Vassar College|location=Poughkeepsie}} |
|||
==External links== |
|||
{{Sister project links |wikt=radium |commons=radium |commonscat=yes |n=no |q=no |s=no |b=no |v=Radium atom}} |
|||
* {{cite web|title=Lateral Science: The Discovery of Radium|url=http://www.lateralscience.co.uk/radium/RaDisc.html|accessdate=13 May 2017|archiveurl=https://web.archive.org/web/20160309040715/http://lateralscience.blogspot.co.uk/2012/07/the-discovery-of-radium-by-marie-curie.html|archivedate=March 9, 2016|date=July 8, 2012}} |
|||
* [http://www.markwshead.com/stuffHappens/radium.html Photos of Radium Water Bath in Oklahoma] |
|||
* [http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@na+@rel+radium,+radioactive NLM Hazardous Substances Databank – Radium, Radioactive] |
|||
* [http://alsos.wlu.edu/qsearch.aspx?browse=science/Radium Annotated bibliography for radium from the Alsos Digital Library for Nuclear Issues] |
|||
* [http://www.japantoday.com/category/jt-experts/view/the-poisoner-next-door The Poisoner Next Door – Japan Today, 10/20/2001] |
|||
* [http://www.periodicvideos.com/videos/088.htm Radium] at ''[[The Periodic Table of Videos]]'' (University of Nottingham) |
|||
* [http://www.radioactivity.eu.com/en/site/pages/Radium.htm Radioactivity.eu.com] (Created and maintained by physicists) |
|||
{{Compact periodic table}} |
|||
{{Authority control}} |
|||
[[Category:Radium| ]] |
|||
[[Category:Alkaline earth metals]] |
|||
[[Category:Chemical elements]] |
|||
Revision as of 20:10, 1 May 2018
 Radium electroplated on a very small sample of copper foil and covered with polyurethane to prevent reaction with the air | ||||||||||||||||||||||||||||||||||||
| Radium | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈreɪdiəm/ | |||||||||||||||||||||||||||||||||||
| Appearance | silvery white metallic | |||||||||||||||||||||||||||||||||||
| Mass number | [226] | |||||||||||||||||||||||||||||||||||
| Radium in the periodic table | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 88 | |||||||||||||||||||||||||||||||||||
| Group | group 2 (alkaline earth metals) | |||||||||||||||||||||||||||||||||||
| Period | period 7 | |||||||||||||||||||||||||||||||||||
| Block | s-block | |||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 7s2 | |||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 8, 2 | |||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||
| Melting point | 973 K (700 °C, 1292 °F) (disputed) | |||||||||||||||||||||||||||||||||||
| Boiling point | 2010 K (1737 °C, 3159 °F) | |||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 5.5 g/cm3 | |||||||||||||||||||||||||||||||||||
| Heat of fusion | 8.5 kJ/mol | |||||||||||||||||||||||||||||||||||
| Heat of vaporization | 113 kJ/mol | |||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||
| Oxidation states | common: +2, | |||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 0.9 | |||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||
| Covalent radius | 221±2 pm | |||||||||||||||||||||||||||||||||||
| Van der Waals radius | 283 pm | |||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||
| Natural occurrence | from decay | |||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc) (cF4) | |||||||||||||||||||||||||||||||||||
| Lattice constant | a = 514.8 pm (near r.t.)[1] | |||||||||||||||||||||||||||||||||||
| Thermal conductivity | 18.6 W/(m⋅K) | |||||||||||||||||||||||||||||||||||
| Electrical resistivity | 1 µΩ⋅m (at 20 °C) | |||||||||||||||||||||||||||||||||||
| Magnetic ordering | nonmagnetic | |||||||||||||||||||||||||||||||||||
| CAS Number | 7440-14-4 | |||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||
| Discovery | Pierre and Marie Curie (1898) | |||||||||||||||||||||||||||||||||||
| First isolation | Marie Curie (1910) | |||||||||||||||||||||||||||||||||||
| Isotopes of radium | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
Radium is a chemical element with symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather than oxygen) on exposure to air, forming a black surface layer of radium nitride (Ra3N2). All isotopes of radium are highly radioactive, with the most stable isotope being radium-226, which has a half-life of 1600 years and decays into radon gas (specifically the isotope radon-222). When radium decays, ionizing radiation is a product, which can excite fluorescent chemicals and cause radioluminescence.
Radium, in the form of radium chloride, was discovered by Marie and Pierre Curie in 1898. They extracted the radium compound from uraninite and published the discovery at the French Academy of Sciences five days later. Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne through the electrolysis of radium chloride in 1911.[3]
In nature, radium is found in uranium and (to a lesser extent) thorium ores in trace amounts as small as a seventh of a gram per ton of uraninite. Radium is not necessary for living organisms, and adverse health effects are likely when it is incorporated into biochemical processes because of its radioactivity and chemical reactivity. Currently, other than its use in nuclear medicine, radium has no commercial applications; formerly, it was used as a radioactive source for radioluminescent devices and also in radioactive quackery for its supposed curative powers. Today, these former applications are no longer in vogue because radium's toxicity has since become known, and less dangerous isotopes are used instead in radioluminescent devices.
Bulk properties
Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Its physical and chemical properties most closely resemble its lighter congener barium.[4]
Pure radium is a volatile silvery-white metal, although its lighter congeners calcium, strontium, and barium have a slight yellow tint.[4] Its color rapidly vanishes in air, yielding a black layer of radium nitride (Ra3N2).[5] Its melting point is either 700 °C (1,292 °F) or 960 °C (1,760 °F)[a] and its boiling point is 1,737 °C (3,159 °F). Both of these values are slightly lower than those of barium, confirming periodic trends down the group 2 elements.[6] Like barium and the alkali metals, radium crystallizes in the body-centered cubic structure at standard temperature and pressure: the radium–radium bond distance is 514.8 picometers.[7] Radium has a density of 5.5 g/cm3, higher than that of barium, again confirming periodic trends; the radium-barium density ratio is comparable to the radium-barium atomic mass ratio,[8] due to the two elements' similar crystal structures.[8][9]
Isotopes
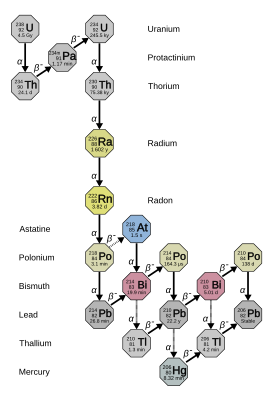
Radium has 33 known isotopes, with mass numbers from 202 to 234: all of them are radioactive.[10] Four of these – 223Ra (half-life 11.4 days), 224Ra (3.64 days), 226Ra (1600 years), and 228Ra (5.75 years) – occur naturally in the decay chains of primordial thorium-232, uranium-235, and uranium-238 (223Ra from uranium-235, 226Ra from uranium-238, and the other two from thorium-232). These isotopes nevertheless still have half-lives too short to be primordial radionuclides and only exist in nature from these decay chains.[11] Together with the artificial 225Ra (15 d), these are the five most stable isotopes of radium.[11] All other known radium isotopes have half-lives under two hours, and the majority have half-lives under a minute.[10] At least 12 nuclear isomers have been reported; the most stable of them is radium-205m, with a half-life of between 130 and 230 milliseconds, which is still shorter than twenty-four ground-state radium isotopes.[10]
In the early history of the study of radioactivity, the different natural isotopes of radium were given different names. In this scheme, 223Ra was named actinium X (AcX), 224Ra thorium X (ThX), 226Ra radium (Ra), and 228Ra mesothorium 1 (MsTh1).[11] When it was realized that all of these are isotopes of the same element, many of these names fell out of use, and "radium" came to refer to all isotopes, not just 226Ra.[11] Some of radium-226's decay products received historical names including "radium", ranging from radium A to radium G, with the letter indicating approximately how far they were down the chain from their parent 226Ra.[11]
226Ra is the most stable isotope of radium and is the last isotope in the (4n + 2) decay chain of uranium-238 with a half-life of over a millennium: it makes up almost all of natural radium. Its immediate decay product is the dense radioactive noble gas radon, which is responsible for much of the danger of environmental radium.[12] It is 2.7 million times more radioactive than the same molar amount of natural uranium (mostly uranium-238), due to its proportionally shorter half-life.[13][14]
A sample of radium metal maintains itself at a higher temperature than its surroundings because of the radiation it emits – alpha particles, beta particles, and gamma rays. More specifically, natural radium (which is mostly 226Ra) emits mostly alpha particles, but other steps in its decay chain (the uranium or radium series) emit alpha or beta particles, and almost all particle emissions are accompanied by gamma rays.[15]
Chemistry
Radium, like barium, is a highly reactive metal and always exhibits its group oxidation state of +2.[5] It forms the colorless Ra2+ cation in aqueous solution, which is highly basic and does not form complexes readily.[5] Most radium compounds are therefore simple ionic compounds,[5] though participation from the 6s and 6p electrons (in addition to the valence 7s electrons) is expected due to relativistic effects and would enhance the covalent character of radium compounds such as RaF2 and RaAt2.[16] For this reason, the standard electrode potential for the half-reaction Ra2+ (aq) + 2e− → Ra (s) is −2.916 V, even slightly lower than the value −2.92 V for barium, whereas the values had previously smoothly increased down the group (Ca: −2.84 V; Sr: −2.89 V; Ba: −2.92 V).[17] The values for barium and radium are almost exactly the same as those of the heavier alkali metals potassium, rubidium, and caesium.[17]
Compounds
Solid radium compounds are white as radium ions provide no specific coloring, but they gradually turn yellow and then dark over time due to self-radiolysis from radium's alpha decay.[5] Insoluble radium compounds coprecipitate with all barium, most strontium, and most lead compounds.[18]
Radium oxide (RaO) has not been characterized well past its existence, despite oxides being common compounds for the other alkaline earth metals. Radium hydroxide (Ra(OH)2) is the most readily soluble among the alkaline earth hydroxides and is a stronger base than its barium congener, barium hydroxide.[19] It is also more soluble than actinium hydroxide and thorium hydroxide: these three adjacent hydroxides may be separated by precipitating them with ammonia.[19]
Radium chloride (RaCl2) is a colorless, luminous compound. It becomes yellow after some time due to self-damage by the alpha radiation given off by radium when it decays. Small amounts of barium impurities give the compound a rose color.[19] It is soluble in water, though less so than barium chloride, and its solubility decreases with increasing concentration of hydrochloric acid. Crystallization from aqueous solution gives the dihydrate RaCl2·2H2O, isomorphous with its barium analog.[19]
Radium bromide (RaBr2) is also a colorless, luminous compound.[19] In water, it is more soluble than radium chloride. Like radium chloride, crystallization from aqueous solution gives the dihydrate RaBr2·2H2O, isomorphous with its barium analog. The ionizing radiation emitted by radium bromide excites nitrogen molecules in the air, making it glow. The alpha particles emitted by radium quickly gain two electrons to become neutral helium, with builds up inside and weakens radium bromide crystals. This effect sometimes causes the crystals to break or even explode.[19]
Radium nitrate (Ra(NO3)2) is a white compound that can be made by dissolving radium carbonate in nitric acid. As the concentration of nitric acid increases, the solubility of radium nitrate decreases, an important property for the chemical purification of radium.[19]
Radium forms much the same insoluble salts as its lighter congener barium: it forms the insoluble sulfate (RaSO4, the most insoluble known sulfate), chromate (RaCrO4), carbonate (RaCO3), iodate (Ra(IO3)2), tetrafluoroberyllate (RaBeF4), and nitrate (Ra(NO3)2). With the exception of the carbonate, all of these are less soluble in water than the corresponding barium salts, but they are all isostructural to their barium counterparts. Additionally, radium phosphate, oxalate, and sulfite are probably also insoluble, as they coprecipitate with the corresponding insoluble barium salts.[20] The great insolubility of radium sulfate (at 20 °C, only 2.1 mg will dissolve in 1 kg of water) means that it is one of the less biologically dangerous radium compounds.[21] The large ionic radius of Ra2+ (148 pm) results in weak complexation and poor extraction of radium from aqueous solutions when not at high pH.[22]
Occurrence
All isotopes of radium have half-lives much shorter than the age of the Earth, so that any primordial radium would have decayed long ago. Radium nevertheless still occurs in the environment, as the isotopes 223Ra, 224Ra, 226Ra, and 228Ra are part of the decay chains of natural thorium and uranium isotopes; since thorium and uranium have very long half-lives, these daughters are continually being regenerated by their decay.[11] Of these four isotopes, the longest-lived is 226Ra (half-life 1600 years), a decay product of natural uranium. Because of its relative longevity, 226Ra is the most common isotope of the element, making up about one part per trillion of the Earth's crust; essentially all natural radium is 226Ra.[23] Thus, radium is found in tiny quantities in the uranium ore uraninite and various other uranium minerals, and in even tinier quantities in thorium minerals. One ton of pitchblende typically yields about one seventh of a gram of radium.[24] One kilogram of the Earth's crust contains about 900 picograms of radium, and one liter of sea water contains about 89 femtograms of radium.[25]
History


Radium was discovered by Marie Sklodowska-Curie and her husband Pierre Curie on 21 December 1898, in a uraninite (pitchblende) sample.[26] While studying the mineral earlier, the Curies removed uranium from it and found that the remaining material was still radioactive. They separated out an element similar to bismuth from pitchblende in July 1898, that turned out to be polonium. They then separated out a radioactive mixture consisting mostly of two components: compounds of barium, which gave a brilliant green flame color, and unknown radioactive compounds which gave carmine spectral lines that had never been documented before. The Curies found the radioactive compounds to be very similar to the barium compounds, except that they were more insoluble. This made it possible for the Curies to separate out the radioactive compounds and discover a new element in them. The Curies announced their discovery to the French Academy of Sciences on 26 December 1898.[27][28] The naming of radium dates to about 1899, from the French word radium, formed in Modern Latin from radius (ray): this was in recognition of radium's power of emitting energy in the form of rays.[29][30][31]
In 1910, radium was isolated as a pure metal by Marie Curie and André-Louis Debierne through the electrolysis of a pure radium chloride (RaCl2) solution using a mercury cathode, producing a radium–mercury amalgam. This amalgam was then heated in an atmosphere of hydrogen gas to remove the mercury, leaving pure radium metal.[32] The same year, E. Eoler isolated radium by thermal decomposition of its azide, Ra(N3)2.[11] Radium metal was first industrially produced in the beginning of the 20th century by Biraco, a subsidiary company of Union Minière du Haut Katanga (UMHK) in its Olen plant in Belgium.[33]
The common historical unit for radioactivity, the curie, is based on the radioactivity of 226Ra.[34]
Historical applications
Luminescent paint


Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials. A typical self-luminous watch that uses radium paint contains around 1 microgram of radium.[35] In the mid-1920s, a lawsuit was filed against the United States Radium Corporation by five dying "Radium Girls" dial painters who had painted radium-based luminous paint on the dials of watches and clocks. The dial painters routinely licked their brushes to give them a fine point, thereby ingesting radium.[36] Their exposure to radium caused serious health effects which included sores, anemia, and bone cancer. This is because radium is treated as calcium by the body, and deposited in the bones, where radioactivity degrades marrow and can mutate bone cells.[37]
During the litigation, it was determined that the company's scientists and management had taken considerable precautions to protect themselves from the effects of radiation, yet had not seen fit to protect their employees. Additionally, for several years the companies had attempted to cover up the effects and avoid liability by insisting that the Radium Girls were instead suffering from syphilis. This complete disregard for employee welfare had a significant impact on the formulation of occupational disease labor law.[38]
As a result of the lawsuit, the adverse effects of radioactivity became widely known, and radium-dial painters were instructed in proper safety precautions and provided with protective gear. In particular, dial painters no longer licked paint brushes to shape them (which caused some ingestion of radium salts). Radium was still used in dials as late as the 1960s, but there were no further injuries to dial painters. This highlighted that the harm to the Radium Girls could easily have been avoided.[39]
From the 1960s the use of radium paint was discontinued. In many cases luminous dials were implemented with non-radioactive fluorescent materials excited by light; such devices glow in the dark after exposure to light, but the glow fades.[37] Where long-lasting self-luminosity in darkness was required, safer radioactive promethium-147 (half-life 2.6 years) or tritium (half-life 12 years) paint was used; both continue to be used today.[40] These had the added advantage of not degrading the phosphor over time, unlike radium.[41] Tritium emits very low-energy beta radiation (even lower-energy than the beta radiation emitted by promethium)[10] which cannot penetrate the skin,[42] rather than the penetrating gamma radiation of radium and is regarded as safer.[43]
Clocks, watches, and instruments dating from the first half of the 20th century, often in military applications, may have been painted with radioactive luminous paint. They are usually no longer luminous; however, this is not due to radioactive decay of the radium (which has a half-life of 1600 years) but to the fluorescence of the zinc sulfide fluorescent medium being worn out by the radiation from the radium.[44] The appearance of an often thick layer of green or yellowish brown paint in devices from this period suggests a radioactive hazard. The radiation dose from an intact device is relatively low and usually not an acute risk; but the paint is dangerous if released and inhaled or ingested.[45][46]
Commercial use

Radium was once an additive in products such as toothpaste, hair creams, and even food items due to its supposed curative powers.[47] Such products soon fell out of vogue and were prohibited by authorities in many countries after it was discovered they could have serious adverse health effects. (See, for instance, Radithor or Revigator types of "Radium water" or "Standard Radium Solution for Drinking".)[44] Spas featuring radium-rich water are still occasionally touted as beneficial, such as those in Misasa, Tottori, Japan. In the U.S., nasal radium irradiation was also administered to children to prevent middle-ear problems or enlarged tonsils from the late 1940s through the early 1970s.[48]
Medical use
Radium (usually in the form of radium chloride or radium bromide) was used in medicine to produce radon gas which in turn was used as a cancer treatment; for example, several of these radon sources were used in Canada in the 1920s and 1930s.[45][49] However, many treatments that were used in the early 1900s are not used anymore because of the harmful effects radium bromide exposure caused. Some examples of these effects are anaemia, cancer, and genetic mutations.[50] Safer gamma emitters such as 60Co, which is less costly and available in larger quantities, are usually used today to replace the historical use of radium in this application.[22]
Early in the 1900s, biologists used radium to induce mutations and study genetics. As early as 1904, Daniel MacDougal used radium in an attempt to determine whether it could provoke sudden large mutations and cause major evolutionary shifts. Thomas Hunt Morgan used radium to induce changes resulting in white-eyed fruit flies. Nobel-winning biologist Hermann Muller briefly studied the effects of radium on fruit fly mutations before turning to more affordable x-ray experiments.[51]
Howard Atwood Kelly, one of the founding physicians of Johns Hopkins Hospital, was a major pioneer in the medical use of radium to treat cancer.[52] His first patient was his own aunt in 1904, who died shortly after surgery.[53] Kelly was known to use excessive amounts of radium to treat various cancers and tumors. As a result, some of his patients died from radium exposure.[54] His method of radium application was inserting a radium capsule near the affected area, then sewing the radium "points" directly to the tumor.[54] This was the same method used to treat Henrietta Lacks, the host of the original HeLa cells, for cervical cancer.[55] Currently, safer and more available radioisotopes are used instead.[37]
Production
Uranium had no large scale application in the late 19th century and therefore no large uranium mines existed. In the beginning the only large source for uranium ore was the silver mines in Joachimsthal, Austria-Hungary (now Jáchymov, Czech Republic).[26] The uranium ore was only a byproduct of the mining activities.[56]
In the first extraction of radium Curie used the residues after extraction of uranium from pitchblende. The uranium had been extracted by dissolution in sulfuric acid leaving radium sulfate, which is similar to barium sulfate but even less soluble in the residues. The residues also contained rather substantial amounts of barium sulfate which thus acted as a carrier for the radium sulfate. The first steps of the radium extraction process involved boiling with sodium hydroxide followed by hydrochloric acid treatment to remove as much as possible of other compounds. The remaining residue was then treated with sodium carbonate to convert the barium sulfate into barium carbonate carrying the radium, thus making it soluble in hydrochloric acid. After dissolution the barium and radium are reprecipitated as sulfates and this was repeated one or few times, for further purification of the mixed sulfate. Some impurities, that form insoluble sulfides, were removed by treating the chloride solution with hydrogen sulfide followed by filtering. When the mixed sulfate were pure enough they were once more converted to mixed chloride and barium and radium were separated by fractional crystallisation while monitoring the progress using a spectroscope (radium gives characteristic red lines in contrast to the green barium lines), and the electroscope.[57]
After the isolation of radium by Marie and Pierre Curie from uranium ore from Joachimsthal several scientists started to isolate radium in small quantities. Later small companies purchased mine tailings from Joachimsthal mines and started isolating radium. In 1904 the Austrian government nationalised the mines and stopped exporting raw ore. For some time the radium availability was low.[56]
The formation of an Austrian monopoly and the strong urge of other countries to have access to radium led to a worldwide search for uranium ores. The United States took over as leading producer in the early 1910s. The Carnotite sands in Colorado provide some of the element, but richer ores are found in the Congo and the area of the Great Bear Lake and the Great Slave Lake of northwestern Canada.[26][58] Neither of the deposits is mined for radium but the uranium content makes mining profitable.
The Curies' process was still used for industrial radium extraction in 1940, but mixed bromides were then used for the fractionation.[59] If the barium content of the uranium ore is not high enough it is easy to add some to carry the radium. These processes were applied to high grade uranium ores but may not work well with low grade ores.
Small amounts of radium were still extracted from uranium ore by this method of mixed precipitation and ion exchange as late as the 1990s,[23] but today they are extracted only from spent nuclear fuel.[60] In 1954, the total worldwide supply of purified radium amounted to about 5 pounds (2.3 kg)[35] and it is still in this range today, while the annual production of pure radium compounds is only about 100 g in total today.[23] The chief radium-producing countries are Belgium, Canada, the Czech Republic, Slovakia, the United Kingdom, and Russia.[23] The amounts of radium produced were and are always relatively small; for example, in 1918, 13.6 g of radium were produced in the United States. [61] The metal is isolated by reducing radium oxide with aluminium metal in a vacuum at 1200 °C.[22]
Modern applications
Some of the few practical uses of radium are derived from its radioactive properties. More recently discovered radioisotopes, such as cobalt-60 and caesium-137, are replacing radium in even these limited uses because several of these isotopes are more powerful emitters, safer to handle, and available in more concentrated form.[62][63]
The isotope 223Ra (under the trade name Xofigo) was approved by the United States Food and Drug Administration in 2013 for use in medicine as a cancer treatment of bone metastasis.[64][65] The main indication of treatment with Xofigo is the therapy of bony metastases from castration-resistant prostate cancer due to the favourable characteristics of this alpha-emitter radiopharmaceutical.[66] 225Ra has also been used in experiments concerning therapeutic irradiation, as it is the only reasonably long-lived radium isotope which does not have radon as one of its daughters.[67]
Radium is still used today as a radiation source in some industrial radiography devices to check for flawed metallic parts, similarly to X-ray imaging.[37] When mixed with beryllium, radium acts as a neutron source.[44][68] Radium-beryllium neutron sources are still sometimes used even today,[37][69] but other materials such as polonium are now more common: about 1500 polonium-beryllium neutron sources, with an individual activity of 1,850 Ci (68 TBq), have been used annually in Russia.[70] These RaBeF4-based (α, n) neutron sources have been deprecated despite the high number of neutrons they emit (1.84×106 neutrons per second) in favour of 241Am–Be sources.[22] Today, the isotope 226Ra is mainly used to form 227Ac by neutron irradiation in a nuclear reactor.[22]
Hazards
Radium is highly radioactive and its immediate daughter, radon gas, is also radioactive. When ingested, 80% of the ingested radium leaves the body through the feces, while the other 20% goes into the bloodstream, mostly accumulating in the bones.[37] Exposure to radium, internal or external, can cause cancer and other disorders, because radium and radon emit alpha and gamma rays upon their decay, which kill and mutate cells.[37] At the time of the Manhattan Project in 1944, the "tolerance dose" for workers was set at 0.1 micrograms of ingested radium.[71][72]
Some of the biological effects of radium were apparent from the start. The first case of so-called "radium-dermatitis" was reported in 1900, only 2 years after the element's discovery. The French physicist Antoine Becquerel carried a small ampoule of radium in his waistcoat pocket for 6 hours and reported that his skin became ulcerated. Pierre and Marie Curie were so intrigued by radiation that they sacrificed their own health to learn more about it. Pierre Curie attached a tube filled with radium to his arm for ten hours, which resulted in the appearance of a skin lesion, suggesting the use of radium to attack cancerous tissue as it had attacked healthy tissue.[73] Handling of radium has been blamed for Marie Curie's death due to aplastic anemia. A significant amount of radium's danger comes from its daughter radon: being a gas, it can enter the body far more readily than can its parent radium.[37]
Today, 226Ra is considered to be the most toxic of the quantity radioelements, and it must be handled in tight glove boxes with significant airstream circulation that is then treated to avoid escape of its daughter 222Rn to the environment. Old ampoules containing radium solutions must be opened with care because radiolytic decomposition of water can produce an overpressure of hydrogen and oxygen gas.[22]
See also
Notes
- ^ Both values are encountered in sources and there is no agreement among scientists as to the true value of the melting point of radium.
References
- ^ Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ "Radium". Royal Society of Chemistry.
- ^ a b Greenwood and Earnshaw, p. 112
- ^ a b c d e Kirby et al., p. 4
- ^ Lide, D. R. (2004). CRC Handbook of Chemistry and Physics (84th ed.). Boca Raton (FL): CRC Press. ISBN 978-0-8493-0484-2.
- ^ Weigel, F.; Trinkl, A. (1968). "Zur Kristallchemie des Radiums". Radiochim. Acta. 10: 78. doi:10.1524/ract.1968.10.12.78.
- ^ a b Young, David A. (1991). "Radium". Phase Diagrams of the Elements. University of California Press. p. 85. ISBN 0-520-91148-2.
- ^ "Crystal Structures of the Chemical Elements at 1 bar". uni-bielefeld.de.
- ^ a b c d G. Audi; A. H. Wapstra; C. Thibault; J. Blachot; O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729: 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ a b c d e f g Kirby et al., p. 3
- ^ Radium – Radiation Protection. United States Environmental Protection Agency.
- ^ Soddy, Frederick (25 August 2004). The Interpretation of Radium. pp. 139–. ISBN 978-0-486-43877-1.
- ^ Malley, Marjorie C. (2011). Radioactivity. Oxford University Press. pp. 115–. ISBN 978-0-19-983178-4.
- ^ Strutt, R. J. (7 September 2004). The Becquerel Rays and the Properties of Radium. pp. 133–. ISBN 978-0-486-43875-7.
- ^ Thayer, John S. (2010). "Relativistic Effects and the Chemistry of the Heavier Main Group Elements": 81. doi:10.1007/978-1-4020-9975-5_2.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b Greenwood and Earnshaw, p. 111
- ^ Kirby et al., p. 8
- ^ a b c d e f g Kirby et al., pp. 4–8
- ^ Kirby et al., pp. 8–9
- ^ Kirby et al., p. 12
- ^ a b c d e f Ullmann, pp. 97–98
- ^ a b c d Greenwood and Earnshaw, pp. 109–110
- ^ "Radium", Los Alamos National Laboratory. Retrieved 5 August 2009.
- ^ Section 14, Geophysics, Astronomy, and Acoustics; Abundance of Elements in the Earth's Crust and in the Sea, in Lide, David R. (ed.), CRC Handbook of Chemistry and Physics, 85th Edition. CRC Press. Boca Raton, Florida (2005).
- ^ a b c Hammond, C. R. "Radium" in Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1-4398-5511-0.
- ^ Curie, Pierre; Curie, Marie; Bémont, Gustave (1898). "Sur une nouvelle substance fortement radio-active, contenue dans la pechblende (On a new, strongly radioactive substance contained in pitchblende)". Comptes Rendus. 127: 1215–1217. Retrieved 1 August 2009.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ Weeks, Mary Elvira (1933). "The discovery of the elements. XIX. The radioactive elements". Journal of Chemical Education. 10 (2): 79. Bibcode:1933JChEd..10...79W. doi:10.1021/ed010p79.
- ^ Ball, David W. (1985). "Elemental etymology: What's in a name?" (PDF). Journal of Chemical Education. 62: 787–788. Bibcode:1985JChEd..62..787B. doi:10.1021/ed062p787.
- ^ Carvalho, Fernando P. (2011). "Marie Curie and the Discovery of Radium": 3–13. doi:10.1007/978-3-642-22122-4_1.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Weeks, Mary Elvira (1933). "The discovery of the elements. XIX. The radioactive elements". Journal of Chemical Education. 10 (2): 79. Bibcode:1933JChEd..10...79W. doi:10.1021/ed010p79.
- ^ Curie, Marie; Debierne, André (1910). "Sur le radium métallique" (On metallic radium)". Comptes Rendus (in French). 151: 523–525. Retrieved 1 August 2009.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ Ronneau, C.; Bitchaeva, O. (1997). Biotechnology for waste management and site restoration: Technological, educational, business, political aspects. Scientific Affairs Division, North Atlantic Treaty Organization. p. 206. ISBN 978-0-7923-4769-9.
- ^ Frame, Paul W. "How the Curie Came to Be". Retrieved 30 April 2008.
- ^ a b Terrill Jr, J. G.; Ingraham Sc, 2nd; Moeller, D. W. (1954). "Radium in the healing arts and in industry: Radiation exposure in the United States". Public Health Reports. 69 (3): 255–62. doi:10.2307/4588736. PMC 2024184. PMID 13134440.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ Frame, Paul. Radioluminescent Paint, Oak Ridge Associated Universities. Retrieved September 17, 2007.
- ^ a b c d e f g h Radium: Radiation Protection – US EPA
- ^ "Environmental history timeline – Radium Girls". Retrieved 29 December 2014.
- ^ Rowland, R. E. (1995) Radium in humans: a review of U.S. studies. Argonne National Laboratory. p. 22
- ^ Tykva, Richard; Berg, Dieter (2004). Man-made and natural radioactivity in environmental pollution and radiochronology. Springer. p. 78. ISBN 1-4020-1860-6.
- ^ Lavrukhina, A. K.; Pozdnyakov, A. A. (1966). Аналитическая химия технеция, прометия, астатина и франция [Analytical Chemistry of Technetium, Promethium, Astatine, and Francium] (in Russian). Nauka. p. 118.
- ^ Nuclide safety data sheet: Hydrogen-3. ehso.emory.edu
- ^ Zerriffi, Hisham (January 1996). "Tritium: The environmental, health, budgetary, and strategic effects of the Department of Energy's decision to produce tritium". Institute for Energy and Environmental Research. Retrieved 15 September 2010.
- ^ a b c Emsley, John (2003). Nature's building blocks: an A-Z guide to the elements. Oxford University Press. pp. 351–. ISBN 978-0-19-850340-8.
- ^ a b radium. Encyclopædia Britannica
- ^ Luminous Radium Paint. vintagewatchstraps.com
- ^ "French Web site featuring products (medicines, mineral water, even underwear) containing radium". Retrieved 1 August 2009.
- ^ Cherbonnier, Alice (1 October 1997). "Nasal Radium Irradiation of Children Has Health Fallout". Baltimore Chronicle. Retrieved 1 August 2009.
- ^ Hayter, Charles (2005). "The Politics of Radon Therapy in the 1930s". An Element of Hope: Radium and the Response to Cancer in Canada, 1900–1940. McGill-Queen's Press. ISBN 978-0-7735-2869-7.
- ^ Harvie, David I. (1999). "The radium century". Endeavour. 23 (3): 100–5. doi:10.1016/S0160-9327(99)01201-6. PMID 10589294.
- ^ Hamilton, Vivien (2016). "The Secrets of Life: Historian Luis Campos resurrects radium's role in early genetics research". Distillations. 2 (2): 44–45. Retrieved 22 March 2018.
- ^ "The Four Founding Physicians". Retrieved 10 April 2013.
- ^ Dastur, Adi E.; Tank, P. D. (2011). "Howard Atwood Kelly: much beyond the stitch". The Journal of Obstetrics and Gynecology of India. 60 (5): 392–394. doi:10.1007/s13224-010-0064-6. PMC 3394615.
- ^ a b Aronowitz, Jesse N.; Robison, Roger F. (2010). "Howard Kelly establishes gynecologic brachytherapy in the United States". Brachytherapy. 9 (2): 178–184. doi:10.1016/j.brachy.2009.10.001. PMID 20022564.
- ^ Rebecca Skloot (2 February 2010). The Immortal Life of Henrietta Lacks. Random House Digital, Inc. ISBN 978-0-307-58938-5. Retrieved 8 April 2013.
- ^ a b Ceranski, Beate (2008). "Tauschwirtschaft, Reputationsökonomie, Bürokratie". NTM Zeitschrift für Geschichte der Wissenschaften, Technik und Medizin. 16 (4): 413–443. doi:10.1007/s00048-008-0308-z.
- ^ "Lateral Science". lateralscience.blogspot.se. November 2012
- ^ Just, Evan; Swain, Philip W.; Kerr, William A. (1952). "Peacetíme Impact of Atomíc Energy". Financial Analysts Journal. 8 (1): 85–93. doi:10.2469/faj.v8.n1.85. JSTOR 40796935.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ Kuebel, A. (1940). "Extraction of radium from Canadian pitchblende". Journal of Chemical Education. 17 (9): 417. Bibcode:1940JChEd..17..417K. doi:10.1021/ed017p417.
- ^ Emsley, John (2011). Nature's building blocks: an A-Z guide to the elements. Oxford University Press. p. 437. ISBN 9780199605637.
- ^ Viol, C. H. (1919). "Radium Production". Science. 49 (1262): 227–8. Bibcode:1919Sci....49..227V. doi:10.1126/science.49.1262.227. PMID 17809659.
- ^ Committee On Radiation Source Use And Replacement, National Research Council (U.S.); Nuclear And Radiation Studies Board, National Research Council (U.S.) (January 2008). Radiation source use and replacement: Abbreviated version. p. 24. ISBN 978-0-309-11014-3.
- ^ Bentel, Gunilla Carleson (1996). Radiation therapy planning. p. 8. ISBN 978-0-07-005115-7.
- ^ "FDA OKs pinpoint prostate cancer radiation drug Xofigo from Bayer, Algeta". Archived from the original on 15 May 2013.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "FDA Approves Xofigo for Advanced Prostate Cancer". cancer.org. (2013-05-15)
- ^ Maffioli, L.; Florimonte, L.; Costa, D. C.; Correia Castanheira, J.; Grana, C.; Luster, M.; Bodei, L.; Chinol, M. (2015). "New radiopharmaceutical agents for the treatment of castration-resistant prostate cancer". Q J Nucl Med Mol Imaging. 59: 420–38. PMID 26222274.
- ^ Stoll, Wolfgang (2005). "Thorium and Thorium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. p. 717. doi:10.1002/14356007.a27_001. ISBN 978-3-527-31097-5.
- ^ l'Annunziata, Michael F. (2007). "Alpha particle induced nuclear reactions". Radioactivity: Introduction and history. Elsevier. pp. 260–261. ISBN 978-0-444-52715-8.
- ^ Holden, N. E.; Reciniello, R. N.; Hu, J. P.; Rorer, David C. (2004). "Radiation dosimetry of a graphite moderated radium-beryllium source" (PDF). Health physics. 86 (5 Suppl): S110–2. Bibcode:2003rdtc.conf..484H. doi:10.1142/9789812705563_0060. PMID 15069300.
- ^ Красивая версия "самоубийства" Литвиненко вследствие криворукости (in Russian). stringer.ru (2006-11-26).
- ^ Weisgall, Jonathan M. (1994). Operation crossroads: the atomic tests at Bikini Atoll. Naval Institute Press. p. 238. ISBN 978-1-55750-919-2. Retrieved 20 August 2011.
- ^ Fry, Shirley A. (1998). "Supplement: Madame Curie's Discovery of Radium (1898): A Commemoration by Women in Radiation Sciences". Radiation Research. 150 (5): S21–S29. doi:10.2307/3579805. PMID 9806606.
- ^ Redniss, Lauren (2011). Radioactive: Marie & Pierre Curie: A Tale Of Love And Fallout. New York, NY: HarperCollins. p. 70. ISBN 978-0-06-135132-7.
Bibliography
- Kirby, H. W.; Salutsky, Murrell L. (1964). The Radiochemistry of Radium (PDF). National Academies Press.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Keller, Cornelius; Wolf, Walter; Shani, Jashovam. "Radionuclides, 2. Radioactive Elements and Artificial Radionuclides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o22_o15. ISBN 978-3527306732.
Further reading
- Albert Stwertka (1998). Guide to the Elements – Revised Edition. Oxford University Press. ISBN 0-19-508083-1.
- Denise Grady (6 October 1998). "A Glow in the Dark, and a Lesson in Scientific Peril". The New York Times. Retrieved 25 December 2007.
- Nanny Fröman (1 December 1996). "Marie and Pierre Curie and the Discovery of Polonium and Radium". Nobel Foundation. Retrieved 25 December 2007.
- Macklis, R. M. (1993). "The great radium scandal". Scientific American. 269 (2): 94–99. Bibcode:1993SciAm.269b..94M. doi:10.1038/scientificamerican0893-94. PMID 8351514.
- Clark, Claudia (1987). Radium Girls: Women and Industrial Health Reform, 1910–1935. University of North Carolina Press. ISBN 0-8078-4640-6.
- Curie, Marie (1921). . Poughkeepsie: Vassar College.
External links
- "Lateral Science: The Discovery of Radium". 8 July 2012. Archived from the original on 9 March 2016. Retrieved 13 May 2017.
- Photos of Radium Water Bath in Oklahoma
- NLM Hazardous Substances Databank – Radium, Radioactive
- Annotated bibliography for radium from the Alsos Digital Library for Nuclear Issues
- The Poisoner Next Door – Japan Today, 10/20/2001
- Radium at The Periodic Table of Videos (University of Nottingham)
- Radioactivity.eu.com (Created and maintained by physicists)


