Diabetes medication: Difference between revisions
added new DPP 4 Inhibitor, added info about metformin |
No edit summary |
||
| Line 13: | Line 13: | ||
Insulin is usually given [[Subcutaneous tissue|subcutaneously]], either by injections or by an [[insulin pump]]. Research is underway of other routes of administration. In acute care settings, insulin may also be given intravenously. There are several types of insulin, characterized by the rate which they are metabolized by the body. |
Insulin is usually given [[Subcutaneous tissue|subcutaneously]], either by injections or by an [[insulin pump]]. Research is underway of other routes of administration. In acute care settings, insulin may also be given intravenously. There are several types of insulin, characterized by the rate which they are metabolized by the body. |
||
==Testosterone== |
|||
Testosterone deficiency ([[hypogonadism]]) can easily results in diabetes mellitus, therefore [[testosterone replacement therapy]] is proven to be very effective against diabetes mellitus type 2 because it reduces [[insulin resistance]].<ref name=pubmed19020265>{{cite journal |pmid=19020265 |year=2008 |author1=Rice |first2=RE |first3=RK |first4=S |first5=L |first6=JB |first7=J |title=Men's health, low testosterone, and diabetes: individualized treatment and a multidisciplinary approach. |volume=34 Suppl 5 |pages=97S–112S; quiz 113S–4S |doi=10.1177/0145721708327143 |journal=The Diabetes educator}}</ref><ref name=pmid19538523>{{cite journal |pmid=19538523 |year=2009 |author1=Corona |first2=E |first3=G |first4=M |title=Following the common association between testosterone deficiency and diabetes mellitus, can testosterone be regarded as a new therapy for diabetes? |volume=32 |issue=5 |pages=431–41 |doi=10.1111/j.1365-2605.2009.00965.x |journal=International journal of andrology}}</ref> |
|||
==Secretagogues== |
==Secretagogues== |
||
| Line 62: | Line 66: | ||
* [[buformin]]: also withdrawn due to lactic acidosis risk.<ref name=Verdonck>{{cite journal |pmid=7202882 |doi=10.1007/BF01789112 |year=1981 |author1=Verdonck |first2=B |first3=AN |first4=G |first5=RA |title=Buformin concentrations in a case of fatal lactic acidosis. |volume=20 |issue=1 |pages=45–6 |journal=Diabetologia}}</ref> |
* [[buformin]]: also withdrawn due to lactic acidosis risk.<ref name=Verdonck>{{cite journal |pmid=7202882 |doi=10.1007/BF01789112 |year=1981 |author1=Verdonck |first2=B |first3=AN |first4=G |first5=RA |title=Buformin concentrations in a case of fatal lactic acidosis. |volume=20 |issue=1 |pages=45–6 |journal=Diabetologia}}</ref> |
||
Metformin is usually the first-line medication used for treatment of type-2 diabetes |
Metformin is usually the first-line medication used for treatment of type-2 diabetes. Initial dosing is 500 mg once daily, then if need be increased to 500 mg twice daily up to 1000 mg twice daily. It is also available in combination with other oral diabetic medications. |
||
There is an extended release formulation available, but it is typically reserved for patients experiencing [[gastrointestinal|GI]] side effects. |
There is an extended release formulation available, but it is typically reserved for patients experiencing [[gastrointestinal|GI]] side effects. |
||
| Line 155: | Line 159: | ||
* [[vildagliptin]] (Galvus) EU Approved 2008. |
* [[vildagliptin]] (Galvus) EU Approved 2008. |
||
* [[sitagliptin]] (Januvia) FDA approved Oct 2006. |
* [[sitagliptin]] (Januvia) FDA approved Oct 2006. |
||
* [[saxagliptin]] (Onglyza) FDA Approved July 2009. |
|||
===Amylin analogues=== |
===Amylin analogues=== |
||
Revision as of 15:18, 4 December 2009
Anti-diabetic drugs treat diabetes mellitus by lowering glucose levels in the blood. With the exceptions of insulin, exenatide, and pramlintide, all are administered orally and are thus also called oral hypoglycemic agents or oral antihyperglycemic agents. There are different classes of anti-diabetic drugs, and their selection depends on the nature of the diabetes, age and situation of the person, as well as other factors.
Diabetes mellitus type 1 is a disease caused by the lack of insulin. Insulin must be used in Type I, which must be injected or inhaled.
Diabetes mellitus type 2 is a disease of insulin resistance by cells. Treatments include (1) agents which increase the amount of insulin secreted by the pancreas, (2) agents which increase the sensitivity of target organs to insulin, and (3) agents which decrease the rate at which glucose is absorbed from the gastrointestinal tract.
Several groups of drugs, mostly given by mouth, are effective in Type II, often in combination. The therapeutic combination in Type II may include insulin, not necessarily because oral agents have failed completely, but in search of a desired combination of effects. The great advantage of injected insulin in Type II is that a well-educated patient can adjust the dose, or even take additional doses, when blood glucose levels measured by the patient, usually with a simple meter, as needed by the measured amount of sugar in the blood.
Insulin
Insulin is usually given subcutaneously, either by injections or by an insulin pump. Research is underway of other routes of administration. In acute care settings, insulin may also be given intravenously. There are several types of insulin, characterized by the rate which they are metabolized by the body.
Testosterone
Testosterone deficiency (hypogonadism) can easily results in diabetes mellitus, therefore testosterone replacement therapy is proven to be very effective against diabetes mellitus type 2 because it reduces insulin resistance.[1][2]
Secretagogues
Sulfonylureas
Sulfonylureas were the first widely used oral hypoglycemic medications. They are insulin secretagogues, triggering insulin release by direct action on the KATP channel of the pancreatic beta cells. Eight types of these pills have been marketed in North America, but not all remain available. The "second-generation" drugs are now more commonly used. They are more effective than first-generation drugs and have fewer side effects. All may cause weight gain.
Sulfonylureas bind strongly to plasma proteins. Sulfonylureas are only useful in Type II diabetes, as they work by stimulating endogenous release of insulin. They work best with patients over 40 years old, who have had diabetes mellitus for under ten years. They can not be used with type I diabetes, or diabetes of pregnancy. They can be safely used with metformin or -glitazones. The primary side effect is hypoglycemia.
Typical reductions in A1C values for second generation sulfonylureas are 1.0-2.0%.
- First-generation agents
- tolbutamide (Orinase)
- acetohexamide (Dymelor)
- tolazamide (Tolinase)
- chlorpropamide (Diabinese)
- Second-generation agents
- glipizide (Glucotrol)
- glyburide (Diabeta, Micronase, Glynase)
- glimepiride (Amaryl)
- gliclazide (Diamicron)
Meglitinides
Meglitinides help the pancreas produce insulin and are often called "short-acting secretagogues." Their mode of action is original, affecting potassium channels.[3] By closing the potassium channels of the pancreatic beta cells, they open the calcium channels, hence enhancing insulin secretion.[4]
They are taken with or shortly before meals to boost the insulin response to each meal. If a meal is skipped, the medication is also skipped.
Typical reductions in A1C values are 0.5-1.0%.
- repaglinide (Prandin)
- nateglinide (Starlix)
Adverse reactions include weight gain and hypoglycemia.
Sensitizers
Biguanides
Biguanides reduce hepatic glucose output and increase uptake of glucose by the periphery, including skeletal muscle. Although it must be used with caution in patients with impaired liver or kidney function, metformin, a biguanide, has become the most commonly used agent for type 2 diabetes in children and teenagers. Amongst common diabetic drugs, metformin is the only widely used oral drug that does not cause weight gain.
Typical reductions in A1C values for metformin is 1.5-2.0%.
- metformin (Glucophage). Metformin may be the best choice for patients who also have heart failure.[5] Should be temporarily discontinued before any radiographic procedure involving intravenous iodinated contrast as patients are at an increased risk of lactic acidosis.
- phenformin (DBI): used from 1960s through 1980s, withdrawn due to lactic acidosis risk.[6]
- buformin: also withdrawn due to lactic acidosis risk.[7]
Metformin is usually the first-line medication used for treatment of type-2 diabetes. Initial dosing is 500 mg once daily, then if need be increased to 500 mg twice daily up to 1000 mg twice daily. It is also available in combination with other oral diabetic medications.
There is an extended release formulation available, but it is typically reserved for patients experiencing GI side effects.
Thiazolidinediones
Thiazolidinediones (TZDs), also known as "glitazones," bind to PPARγ, a type of nuclear regulatory proteins involved in transcription of genes regulating glucose and fat metabolism. These PPARs act on Peroxysome Proliferator Responsive Elements (PPRE [1]). The PPREs influence insulin sensitive genes, which enhance production of mRNAs of insulin dependent enzymes. The final result is better use of glucose by the cells.
Typical reductions in A1C values are 1.5-2.0%.
- rosiglitazone (Avandia)
- pioglitazone (Actos)
- troglitazone (Rezulin): used in 1990s, withdrawn due to hepatitis and liver damage risk.[8]
As a result of multiple retrospective studies, there is a concern about rosiglitazone's safety, although it is established that the group, as a whole, has beneficial effects on diabetes. The greatest concern is an increase in the number of severe cardiac events in patients taking it. The ADOPT study showed that initial therapy with drugs of this type may prevent the progression of disease,[9] as did the DREAM trial.[10]
Concerns about the safety of rosiglitazone arose when a retrospective meta-analysis was published in the New England Journal of Medicine.[11] There have been a significant number of publications since then, and a Food and Drug Administration panel[12] voted, with some controversy, 20:3 that available studies "supported a signal of harm," but voted 22:1 to keep the drug on the market. The meta-analysis was not supported by an interim analysis of the trial designed to evaluate the issue, and several other reports have failed to conclude the controversy. This weak evidence for adverse effects has reduced the use of rosiglitazone, despite its important and sustained effects on glycemic control.[13] Safety studies are continuing.
In contrast, at least one large prospective study, PROactive 05, has shown that pioglitazone may decrease the overall incidence of cardiac events in people with type II diabetes who have already had a heart attack.[14]
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors are "diabetes pills" but not technically hypoglycemic agents because they do not have a direct effect on insulin secretion or sensitivity. These agents slow the digestion of starch in the small intestine, so that glucose from the starch of a meal enters the bloodstream more slowly, and can be matched more effectively by an impaired insulin response or sensitivity. These agents are effective by themselves only in the earliest stages of impaired glucose tolerance, but can be helpful in combination with other agents in type 2 diabetes.
Typical reductions in A1C values are 0.5-1.0%.
These medications are rarely used in the United States because of the severity of their side effects (flatulence and bloating). They are more commonly prescribed in Europe. They do have the potential to cause weight loss by lowering the amount of sugar metabolized.
Research has shown the culinary mushroom Maitake (Grifola frondosa) has a hypoglycemic effect,[15][16][17][18][19][20] possibly due to the fact the mushroom naturally acts as an alpha-glucosidase inhibitor.[21]
Peptide analogs
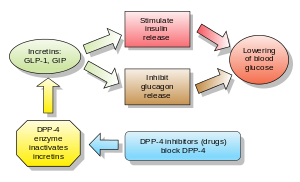
Incretin mimetics
Incretins are insulin secretagogues. The two main candidate molecules that fulfill criteria for being an incretin are Glucagon-like peptide-1 (GLP-1) and Gastric inhibitory peptide (aka glucose-dependent Insulinotropic peptide or GIP). Both GLP-1 and GIP are rapidly inactivated by the enzyme dipeptidyl peptidase-4 (DPP-4).
Glucagon-like peptide (GLP) analogs and agonists
GLP agonists bind to a membrane GLP receptor.[4] As a consequence of this, insulin release from the pancreatic beta cells is increased. Endogenous GLP has a half life of only a few minutes; thus an analogue of GLP would not be practical.
- Exenatide (also Exendin-4, marketed as Byetta) is the first GLP-1 agonist approved for the treatment of type 2 diabetes. Exenatide is not an analogue of GLP, but rather a GLP agonist.[22][23] Exenatide has only 53% homology with GLP, which increases its resistance to degradation by DPP-4 and extends its half-life.[24] Typical reductions in A1C values are 0.5-1.0%.
- Liraglutide, a once daily human analogue (97% homology), is being developed by Novo Nordisk. As of 2007[update], it is in phase III clinical trials.[25]
- Taspoglatide is presently in Phase III Clinical Trials with Hoffman-La Roche.
These agents may also cause a decrease in gastric motility, responsible for the common side effect of nausea, and is probably the mechanism by which weight loss occurs.
Gastric inhibitory peptide (GIP) analogs
- None are FDA approved
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors increase blood concentration of the incretin GLP-1 (glucagon-like peptide-1) by inhibiting its degradation by dipeptidyl peptidase-4 (DPP-4).
Typical reductions in A1C values are 0.5-1.0%.
Examples are:
- vildagliptin (Galvus) EU Approved 2008.
- sitagliptin (Januvia) FDA approved Oct 2006.
Amylin analogues
Amylin agonist analogues slow gastric emptying and suppress glucagon. They have all the incretins actions except stimulation of insulin secretion. As of 2007[update], pramlintide is the only clinically available amylin analogue. Like insulin, it is administered by subcutaneous injection. The most frequent and severe adverse effect of pramlintide is nausea, which occurs mostly at the beginning of treatment and gradually reduces. Typical reductions in A1C values are 0.5-1.0%.
Experimental agents
Many other potential drugs are currently in investigation by pharmaceutical companies. Some of these are simply newer members of one of the above classes, but some work by novel mechanisms. For example, at least one compound that enhances the sensitivity of glucokinase to rising glucose is in the stage of animal research. Others are undergoing phase I/II studies.
- PPARα/γ ligands (muraglitazar and tesaglitazar - development stopped due to adverse risk profile, aleglitazar - under clinical development)
- SGLT (sodium-dependent glucose transporter 1) inhibitors increase urinary glucose.
- FBPase (fructose 1,6-bisphosphatase) inhibitors decrease gluconeogenesis in the liver.
Herbal extracts
A recent review article presents the profiles of plants with hypoglycaemic properties, reported in the literature from 1990 to 2000 and states that "Medical plants play an important role in the management of diabetes mellitus especially in developing countries where resources are meager."[26]
The first registered use of anti-diabetic drugs was as herbal extracts used by Indians in the Amazon Basin for the treatment of type 2 diabetes, and today promoted as vegetable insulin although not formally an insulin analog.[27] The major recent development was done in Brazil around Myrcia sphaerocarpa and other Myrcia species.[28] The usual treatment is with concentrated (root) Myrcia extracts, commercialized as "Pedra hume de kaá". Phytochemical analysis of the Myrcia extracts reported kinds of flavanone glucosides (myrciacitrins) and acetophenone glucosides (myrciaphenones), and inhibitory activities on aldose reductase and alpha-glucosidase.[29]
Walnut leaf can significantly reduce fasting blood glucose levels in rats with alloxan-induced diabetes, and rats thus treates show some evidence of regeneration of the beta cells.[30] Garlic also significantly reduces fasting blood glucose levels in rats with alloxan-induced diabetes.[31]
At least two studies have shown that cinnamon can act significantly reducing some effects of diabetes. One study on people used fine ground cassia (Cinnamomum aromaticum) for oral consumption. Another study used an extract (MHCP) on laboratory rats. The study on people published in 2003 conducted in the Department of Human Nutrition, NWFP Agricultural University, Peshawar, Pakistan concluded "that the inclusion of cinnamon in the diet of people with type 2 diabetes will reduce risk factors associated with diabetes and cardiovascular diseases."[32] The study on laboratory rats at Department of Biochemistry, Biophysics and Molecular Biology, Iowa State University published in 2001 used purified hydroxychalcone (MHCP) from cinnamon. Part of the study's conclusion stated that "the MHCP is fully capable of mimicking insulin" and recommended further studies.[33][34] The Food and Drug Administration has not yet evaluated the use of cinnamon for the management of diabetes. It should be noted that the spice sold as cinnamon is often obtained from C. verum (true cinnamon), not C. aromaticum (cassia).
Research has shown the Maitake mushroom (Grifola frondosa) has a hypoglycemic effect, and may be beneficial for the management of diabetes.[15][16][17][18][19][20] The reason Maitake lowers blood sugar is due to the fact the mushroom naturally acts as an alpha glucosidase inhibitor.[2] Other mushrooms like Reishi,[35][36] Agaricus blazei,[37][38][39][40] Agrocybe cylindracea[41] and Cordyceps[42][43][44][45][46] have been noted to lower blood sugar levels to a certain extent, although the mechanism is currently unknown.
Notes
- ^ Rice (2008). "Men's health, low testosterone, and diabetes: individualized treatment and a multidisciplinary approach". The Diabetes educator. 34 Suppl 5: 97S–112S, quiz 113S–4S. doi:10.1177/0145721708327143. PMID 19020265.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help);|first6=missing|last6=(help);|first7=missing|last7=(help) - ^ Corona (2009). "Following the common association between testosterone deficiency and diabetes mellitus, can testosterone be regarded as a new therapy for diabetes?". International journal of andrology. 32 (5): 431–41. doi:10.1111/j.1365-2605.2009.00965.x. PMID 19538523.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help) - ^ Rendell (2004). "Advances in diabetes for the millennium: drug therapy of type 2 diabetes". MedGenMed : Medscape general medicine. 6 (3 Suppl): 9. PMC 1474831. PMID 15647714.
- ^ a b "Helping the pancreas produce insulin". HealthValue. Retrieved 2007-09-21.
- ^ Eurich (2007). "Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review". BMJ (Clinical research ed.). 335 (7618): 497. doi:10.1136/bmj.39314.620174.80. PMC 1971204. PMID 17761999.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help);|first6=missing|last6=(help);|first7=missing|last7=(help) - ^ Fimognari (2006). "Phenformin-induced lactic acidosis in an older diabetic patient: a recurrent drama (phenformin and lactic acidosis)". Diabetes care. 29 (4): 950–1. doi:10.2337/diacare.29.04.06.dc06-0012. PMID 16567854.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help) - ^ Verdonck (1981). "Buformin concentrations in a case of fatal lactic acidosis". Diabetologia. 20 (1): 45–6. doi:10.1007/BF01789112. PMID 7202882.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help) - ^ Hinterthuer, Adam (2008). "Retired Drugs: Failed Blockbusters, Homicidal Tampering, Fatal Oversights". Wired News. Retrieved 2009-06-21.
- ^ Haffner, Steven M. (2007). "Expert Column - A Diabetes Outcome Progression Trial (ADOPT)". Medscape. Retrieved 2007-09-21.
- ^ Gagnon, Louise (2007). "DREAM: Rosiglitazone Effective in Preventing Diabetes". Medscape. Retrieved 2007-09-21.
- ^ Nissen (2007). "Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes". The New England journal of medicine. 356 (24): 2457–71. doi:10.1056/NEJMoa072761. PMID 17517853.
{{cite journal}}:|first2=missing|last2=(help); Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help) - ^ Wood, Shelley (2007-07-31). "FDA Advisory Panels Acknowledge Signal of Risk With Rosiglitazone, but Stop Short of Recommending Its Withdrawal". Heartwire. Retrieved 2007-09-21.
- ^ Ajjan (2008). "The cardiovascular safety of rosiglitazone". Expert opinion on drug safety. 7 (4): 367–76. doi:10.1517/14740338.7.4.367. PMID 18613801.
{{cite journal}}:|first2=missing|last2=(help) - ^ Erdmann (2007). "The effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction: results from the PROactive (PROactive 05) Study". Journal of the American College of Cardiology. 49 (17): 1772–80. doi:10.1016/j.jacc.2006.12.048. PMID 17466227.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help);|first6=missing|last6=(help);|first7=missing|last7=(help) - ^ a b Konno (2001). "A possible hypoglycaemic effect of maitake mushroom on Type 2 diabetic patients". Diabetic medicine : a journal of the British Diabetic Association. 18 (12): 1010. doi:10.1046/j.1464-5491.2001.00532-5.x. PMID 11903406.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help);|first6=missing|last6=(help) - ^ a b Hong (2007). "Anti-diabetic effect of an alpha-glucan from fruit body of maitake (Grifola frondosa) on KK-Ay mice". The Journal of pharmacy and pharmacology. 59 (4): 575–82. doi:10.1211/jpp.59.4.0013. PMID 17430642.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help) - ^ a b Kubo (1994). "Anti-diabetic activity present in the fruit body of Grifola frondosa (Maitake). I.". Biological & pharmaceutical bulletin. 17 (8): 1106–10. PMID 7820117.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help) - ^ a b cite journal |doi=10.1142/S0192415X0800576X |pmid=18457360}}
- ^ a b Manohar (2002). "Effects of a water-soluble extract of maitake mushroom on circulating glucose/insulin concentrations in KK mice". Diabetes, obesity & metabolism. 4 (1): 43–8. doi:10.1046/j.1463-1326.2002.00180.x. PMID 11874441.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help) - ^ a b Horio (2001). "Maitake (Grifola frondosa) improve glucose tolerance of experimental diabetic rats". Journal of nutritional science and vitaminology. 47 (1): 57–63. PMID 11349892.
{{cite journal}}:|first2=missing|last2=(help) - ^ Matsuur (2002). "Alpha-glucosidase inhibitor from the seeds of balsam pear (Momordica charantia) and the fruit bodies of Grifola frondosa". Bioscience, biotechnology, and biochemistry. 66 (7): 1576–8. PMID 12224646.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help) - ^ Briones (2006). "Exenatide: a GLP-1 receptor agonist as novel therapy for Type 2 diabetes mellitus". Expert opinion on pharmacotherapy. 7 (8): 1055–64. doi:10.1517/14656566.7.8.1055. PMID 16722815.
{{cite journal}}:|first2=missing|last2=(help) - ^ Gallwitz (2006). "Exenatide in type 2 diabetes: treatment effects in clinical studies and animal study data". International journal of clinical practice. 60 (12): 1654–61. doi:10.1111/j.1742-1241.2006.01196.x. PMID 17109672.
- ^ Cvetković (2007). "Exenatide: a review of its use in patients with type 2 diabetes mellitus (as an adjunct to metformin and/or a sulfonylurea)". Drugs. 67 (6): 935–54. doi:10.2165/00003495-200767060-00008. PMID 17428109.
{{cite journal}}:|first2=missing|last2=(help) - ^ "Novo Nordisk A/S - R&D Pipeline: Liraglutide (NN2211)". Novo Nordisk. 2007. Retrieved 2007-09-30.
- ^ Bnouham M; et al. (2006). "Medicinal plants with potential antidiabetic activity - A review of ten years of herbal medicine research (1990-2000)" (PDF). Int J Diabetes & Metabolism. 14: 1–25.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Soumyanath, Amala(ed.) (2005-11-01). Traditional Medicines for Modern Times (1st ed.). Taylor & Francis. ISBN 0-415-33464-0.
{{cite book}}:|first=has generic name (help); Cite has empty unknown parameters:|accessyear=,|accessmonth=, and|coauthors=(help) - ^ McNeill, John H. (1999-02-01). Experimental Models of Diabetes (1st ed.). CRC Press. p. 208. ISBN 0-8493-1667-7.
{{cite book}}: Cite has empty unknown parameters:|accessyear=,|accessmonth=, and|coauthors=(help) - ^ Matsuda, Hisashi (2002). "Antidiabetic Principles of Natural Medicines. V. Aldose Reductase Inhibitors from Myrcia multiflora DC. (2): Structures of Myrciacitrins III, IV, and V". Chemical & Pharmaceutical Bulletin. 50: 429. doi:10.1248/cpb.50.429.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help) - ^ Jelodar G, Sirus Sh, Mohsen M (2007). "Effect of Walnut leaf, coriander and pomegranate on blood glucose and histopathology of pancreas of alloxan induced diabetic rats". African Journal of Traditional, Complimentary and Alternative Medicines. 4 (3): 299–305. Retrieved 2008-05-10.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jelodar (2005). "Effect of fenugreek, onion and garlic on blood glucose and histopathology of pancreas of alloxan-induced diabetic rats". Indian journal of medical sciences. 59 (2): 64–9. PMID 15738612.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help) - ^ Khan, A. (2003). "Cinnamon Improves Glucose and Lipids of People With Type 2 Diabetes". Diabetes Care. 26: 3215. doi:10.2337/diacare.26.12.3215.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help) - ^ Jarvill-Taylor, Karalee J. (2001). "A Hydroxychalcone Derived from Cinnamon Functions as a Mimetic for Insulin in 3T3-L1 Adipocytes". Journal of the American College of Nutrition. 20 (4): 327. PMID 11506060.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help) - ^ Richard A. Anderson, Ph.D., CNS, Cinnamon, Glucose Tolerance and Diabetes, www.ars.usda.gov, August 23 2005, retrieved August 4 2008
- ^ Zhang (2004). "Hypoglycemic effect of Ganoderma lucidum polysaccharides". Acta pharmacologica Sinica. 25 (2): 191–5. PMID 14769208.
{{cite journal}}:|first2=missing|last2=(help) - ^ Yang (2007). "Hypoglycemic effects of Ganoderma applanatum and Collybia confluens exo-polymers in streptozotocin-induced diabetic rats". Phytotherapy research : PTR. 21 (11): 1066–9. doi:10.1002/ptr.2214. PMID 17600864.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help) - ^ Liu (2008). "Immunomodulating Activity of Agaricus brasiliensis KA21 in Mice and in Human Volunteers". Evidence-based complementary and alternative medicine : eCAM. 5 (2): 205–219. doi:10.1093/ecam/nem016. PMC 2396466. PMID 18604247.
{{cite journal}}:|first10=missing|last10=(help);|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help);|first6=missing|last6=(help);|first7=missing|last7=(help);|first8=missing|last8=(help);|first9=missing|last9=(help) - ^ Kim (2005). "Anti-diabetic activity of beta-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei". Biotechnology letters. 27 (7): 483–7. doi:10.1007/s10529-005-2225-8. PMID 15928854.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help) - ^ Hsu (2007). "The mushroom Agaricus Blazei Murill in combination with metformin and gliclazide improves insulin resistance in type 2 diabetes: a randomized, double-blinded, and placebo-controlled clinical trial". Journal of alternative and complementary medicine (New York, N.Y.). 13 (1): 97–102. doi:10.1089/acm.2006.6054. PMID 17309383.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help) - ^ Fortes (2009). "Immunological, hematological, and glycemia effects of dietary supplementation with Agaricus sylvaticus on patients' colorectal cancer". Experimental biology and medicine (Maywood, N.J.). 234 (1): 53–62. doi:10.3181/0806-RM-193. PMID 18997106.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help) - ^ Kiho (1994). "Structural features and hypoglycemic activities of two polysaccharides from a hot-water extract of Agrocybe cylindracea". Carbohydrate research. 251: 81–7. PMID 8149381.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help) - ^ Kiho (1993). "Polysaccharides in fungi. XXXII. Hypoglycemic activity and chemical properties of a polysaccharide from the cultural mycelium of Cordyceps sinensis". Biological & pharmaceutical bulletin. 16 (12): 1291–3. PMID 8130781.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help) - ^ Kiho (1996). "Polysaccharides in fungi. XXXVI. Hypoglycemic activity of a polysaccharide (CS-F30) from the cultural mycelium of Cordyceps sinensis and its effect on glucose metabolism in mouse liver". Biological & pharmaceutical bulletin. 19 (2): 294–6. PMID 8850325.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help) - ^ Zhao (2002). "CordyMax Cs-4 improves glucose metabolism and increases insulin sensitivity in normal rats". Journal of alternative and complementary medicine (New York, N.Y.). 8 (3): 309–14. doi:10.1089/10755530260127998. PMID 12165188.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help);|first6=missing|last6=(help);|first7=missing|last7=(help);|first8=missing|last8=(help) - ^ Lo (2004). "The anti-hyperglycemic activity of the fruiting body of Cordyceps in diabetic rats induced by nicotinamide and streptozotocin". Life sciences. 74 (23): 2897–908. doi:10.1016/j.lfs.2003.11.003. PMID 15050427.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help) - ^ Li (2006). "Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia". Phytomedicine : international journal of phytotherapy and phytopharmacology. 13 (6): 428–33. doi:10.1016/j.phymed.2005.02.002. PMID 16716913.
{{cite journal}}:|first2=missing|last2=(help);|first3=missing|last3=(help);|first4=missing|last4=(help);|first5=missing|last5=(help);|first6=missing|last6=(help);|first7=missing|last7=(help)
References
- Lebovitz, Harold E. (2004). Therapy For Diabetes Mellitus and Related Disorders (4th ed.). Alexandria, VA: American Diabetes Association. ISBN 1-58040-187-2.
- Adams, Michael Ian; Holland, Norman Norwood (2003). Core Concepts in Pharmacology. Englewood Cliffs, NJ: Prentice Hall. ISBN 0-13-089329-3.
{{cite book}}: CS1 maint: multiple names: authors list (link)
