Depleted uranium: Difference between revisions
RVV, Hi James, isnt this a violation of your arbcom ban? |
|||
| Line 140: | Line 140: | ||
==Health concerns== |
==Health concerns== |
||
''The [[radiological]] dangers of pure depleted uranium are relatively low, lower (60%) than those of naturally-occurring uranium due to the removal of the more radioactive isotopes, as well as due to its long [[half-life]] (4.46 billion years). Depleted uranium differs from natural uranium in its [[Isotopic signature|isotopic composition]], but its biochemistry is for the most part the same |
''The [[radiological]] dangers of pure depleted uranium are relatively low, lower (60%) than those of naturally-occurring uranium due to the removal of the more radioactive isotopes, as well as due to its long [[half-life]] (4.46 billion years). Depleted uranium differs from natural uranium in its [[Isotopic signature|isotopic composition]], but its biochemistry is for the most part the same.'' |
||
:''For further details see [[Actinides in the environment]]''. |
:''For further details see [[Actinides in the environment]]''. |
||
Uranium is pyrophoric when finely divided. It will corrode under the influence of air and water producing insoluble uranium(IV) and soluble uranium(VI) salts, such as [[uranyl]] compounds, which are [[toxic]]. Uranium accumulates in several organs, such as the liver, spleen, and kidneys. The [[World Health Organization]] has established a daily "tolerated intake" of soluble uranium salts for the general public of 0.5 μg/kg body weight (or 35 μg for a 70 kg adult. |
Uranium is pyrophoric when finely divided. It will corrode under the influence of air and water producing insoluble uranium(IV) and soluble uranium(VI) salts, such as [[uranyl]] compounds, which are [[toxic]]. Uranium accumulates in several organs, such as the liver, spleen, and kidneys. The [[World Health Organization]] has established a daily "tolerated intake" of soluble uranium salts for the general public of 0.5 μg/kg body weight (or 35 μg for a 70 kg adult.) |
||
| ⚫ | The chemical toxicity of uranium salts is greater than their radiological toxicity. Its radiological hazards are dependent on the purity of the uranium, and there has been some concern that depleted uranium produced as a by-product of [[nuclear reprocessing]] may be contaminated with more dangerous [[isotope]]s: this should not be a concern for depleted uranium produced as tailings from initial [[uranium enrichment]]. |
||
The pyrophoric characteristic of DU is the primary basis of debate about DU effects. When DU strikes a hard surface at high velocity, it is essentially vaporized into a burning cloud of DU. 80% of a DU penetrator can be consumed in this explosion, ultimately becoming a very fine, smoke colored dust. In this form it is classified as a ceramic aerosol (much like a type of glass), and much of it is thus insoluble, meaning for instance, a body might not be able to rid itself of internal DU oxide. DU oxide dust is commonly seen deposited all over the inside of destroyed armored vehicles where it is considered a substantial hazard by the military, both for soldiers, children who may later play and adult scavangers of the hulks. It is fine enough to be blown around and inhaled, especially in arid climates, and it has low level radioactivity essentially forever. DU is also used as tank armor. It can wind up as intact penetrators buried in soil. In these solid forms it naturally oxidizes into a bright yellow scale. |
|||
| ⚫ | The possible dangers of exposure to depleted uranium have received renewed attention as a result of the use of DU munitions in the [[Gulf War]]. Some observers believe that exposure to uranium (or any of its compounds) is the cause of, or a contributing factor to, [[Gulf War syndrome]].{{fact}} Many scientists do not believe this, however.{{fact}} The long-term effects on populations living in the areas in which DU munitions were used have also caused some concern. |
||
| ⚫ | The chemical toxicity of uranium salts is |
||
| ⚫ | The possible dangers of exposure to depleted uranium have received renewed attention as a result of the use of DU munitions in the [[Gulf War]]. Some observers believe that exposure to uranium (or any of its compounds) is the cause of, or a contributing factor to, [[Gulf War syndrome]].{{fact}} |
||
The notorious Rand report, and a more recent similar US government funded report, neither of which are peer-reviewed, both publish a great deal of statistical analysis, and applications of the theoretical effects of inhaled or ingested quantities of DU, but inappropriately and misleadingly applied as a whole body, exterior radiation source, with almost zero corresponding field studies of people known to have been exposed. Also, quite conspicuously missing from both studies is any mention or consideration of the possibility of localized accumulation, especially in the lungs. Postmortem pathology of lung tissue would quickly solve many questions, and one can only wonder why the studies, both costing many millions of dollars, never sought this readily available data. |
|||
The rational that DU is safe is based on whole body exposure levels from sources outside the body, where alpha and beta radiation (the types of the vast majority of radiation from U-238, or "DU") is mostly harmless. This is based on the penetration ability of these types of radiation. Alpha radiation is fully stopped by a piece of paper. However, these standard notions of whole body radiation tolerance has been used to carefully conceal the issue of long term intra-body exposure, such as in the lungs, where fine, inhaled, insoluble DU stays essentially for life, irreparably and continuously damaging adjacent tissue (even if only a 1/1000th inch radius), possibly creating a continuous source of mutated scar cells, mutated immune response cells, trace other radioactive byproducts, all combined with long term localized toxicological effects. This would be easy to prove or disprove via postmortem pathology of lungs and other organs of exposed people. Fine, alpha emitting DU would still be present and would be easy to detect. Analysis of the tiny area of adjacent tissues would solve many doubts. The question is doubly valid considering the US Veterans Bureau has seemed to actually refuse to track data about DU exposed veterans, and also because of the DoD's own training manuals which specifically warn against long term contact with DU ordnance outside the body. Why should it be any less dangerous inside the body, considering the exposure period is life long? These are currently valid, unanswered questions. The US government bore the responsibility of addressing these reasonable concerns beyond all doubts before deploying DU, essentially nuclear waste, in the field. The US government and military is clearly negligent in deploying DU prior to demonstrating it to be completely free of lasting, damaging aftereffects in the environment, effecting soldiers and civilians alike essentially forever. |
|||
* Exposure to DU can cause [[kidney]] damage in humans. |
* Exposure to DU can cause [[kidney]] damage in humans. |
||
Revision as of 15:29, 24 July 2006

Depleted uranium (DU) is uranium that has a reduced proportion of the isotope Uranium-235. It is mostly made up of Uranium-238. The names Q-metal, depletalloy, and D-38, once applied to depleted uranium, have fallen into disuse.
Sources
Depleted uranium is a byproduct of the enriching of natural uranium for use in nuclear reactors. When most of the fissile radioactive isotopes of uranium are removed from natural uranium, the residue is called depleted uranium. A less common source of the material is reprocessed spent reactor fuel. The origin can be distinguished by the content of uranium-236,[1] produced by neutron capture from uranium-235 in nuclear reactors.
As a toxic and radioactive waste product that requires long term storage as low level nuclear waste, depleted uranium is costly to keep but relatively inexpensive to obtain. Generally the only real costs are those associated with conversion of UF6 to metal. Its extremely high density, only slightly less than that of tungsten and its low cost make it attractive for a variety of uses. However, the material is prone to corrosion and small particles are pyrophoric. [2]
History
Depleted uranium was first stored in stockpiles in the 1940s when the U.S. and USSR began their nuclear weapons and nuclear power programs. While it is possible to design civilian power reactors with unenriched fuel, only about 10% of reactors ever built utilize that technology, and both nuclear weapons production and naval reactors require the concentrated isotope. Originally, DU was conserved in the hope that more efficient enrichment techniques would allow further extraction of the fissile isotope; however, those hopes did not materialize.
In the 1970s, The Pentagon reported that the Soviet military had developed armor plating for Warsaw Pact tanks that NATO ammunition couldn't penetrate. The Pentagon began searching for material to make harder bullets. After testing various metals, ordinance researchers settled on depleted uranium. DU was useful in ammunition not only because of its unique physical properties and effectiveness, but also because it was cheap and readily available. Tungsten, the only other candidate, had to be sourced from China. With DU stockpiles estimated to be more than 500,000 tons, the financial burden of housing this amount of low-level radioactive waste was very apparent. It was therefore more economical to use depleted uranium rather than storing it. Thus, from the late 1970s, the U.S., the Soviet Union, Britain and France, began converting their stockpiles of depleted uranium into Kinetic energy penetrators.
Photographic evidence of destroyed equipment suggests that DU was first used during the 1973 Arab-Israeli war. Various written reports cite information that was obtained as a consequence of that use.[1]
Production and availability
Natural uranium metal contains about 0.71% U-235, 99.28% U-238, and about 0.0054% U-234. In order to produce enriched uranium, the process of isotope separation removes a substantial portion of the U-235 for use in nuclear power, weapons, or other uses. The remainder, depleted uranium, contains only 0.2% to 0.4% U-235. Because natural uranium begins with such a low percentage of U-235, the enrichment process produces large quantities of depleted uranium. For example, producing 1 kg of 5% enriched uranium requires 11.8 kg of natural uranium, and leaves about 10.8 kg of depleted uranium with only 0.3% U-235 remaining.
The Nuclear Regulatory Commission (NRC) defines depleted uranium as uranium with a percentage of the 235U isotope that is less than 0.711 percent by weight (See 10 CFR 40.4.) The military specifications designate that the DU used by DoD contain less than 0.3 percent 235U (AEPI, 1995). In actuality, DoD uses only DU that contains approximately 0.2 percent 235U (AEPI, 1995).
- World Depleted Uranium Inventory
Military applications
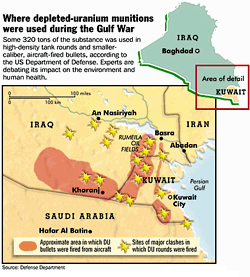
Depleted uranium is very dense; at 19050 kg/m³, it is 70% denser than lead. Thus a given weight of it has a smaller diameter than an equivalent lead projectile, with less aerodynamic drag and deeper penetration due to a higher pressure at point of impact. DU projectile ordnance is often incendiary because of its pyrophoric property. DU munitions, in the form of ordnance, tank, and naval artillery rounds, are deployed by the armed forces of several countries.
It had been widely assumed that the type used by the US in its weapons was the uncontaminated variety, until 2001 when UN scientists found evidence of contaminated DU in the field[2]. The U.S. Army admitted the problem the following day, and began to correct the issue. [3] [4] [5] [6]
Most military use of depleted uranium has been as 30 mm and smaller ordnance, primarily the 30mm PGU-14/B armour-piercing incendiary round from the GAU-8 Avenger cannon of the A-10 Thunderbolt II [7] used by the Air Force. 25 mm DU rounds have been used in the M242 gun mounted on the U.S. Army's Bradley Fighting Vehicle and LAV-AT. The U.S. Marine Corps uses DU in the 25 mm PGU-20 round fired by the GAU-12 Equalizer cannon of the AV-8B Harrier, and also in the 20 mm M197 gun mounted on AH-1 helicopter gunships. The US Navy's Phalanx CIWS's M61 Vulcan gatling gun used 20mm armor-piercing penetrator rounds with discarding plastic sabots which were made using depleted uranium, later changed to tungsten.
Armor plate
Because of its high density, depleted uranium can also be used in tank armor, sandwiched between sheets of steel armor plate. For instance, some late-production M1A1HA and M1A2 Abrams tanks built after 1998 have DU reinforcement as part of its armor plating in the front of the hull and the front of the turret and there is a program to upgrade the rest.
Projectile munitions

Another use of depleted uranium is in kinetic energy penetrators anti-armor role. Kinetic energy penetrator rounds consist of a long, relatively thin penetrator surrounded by discarding sabot. Two materials lend themselves to penetrator construction: tungsten and depleted uranium, the latter in designated alloys known as staballoys. Staballoys are metal alloys of depleted uranium with a very small proportion of other metals, usually titanium or molybdenum. One formulation has a composition of 99.25% by weight of depleted uranium and 0.75% by weight of titanium. Another variant can have 3.5% by weight of titanium. Staballoys are about twice as dense as lead and are designed for use in kinetic energy penetrator armor-piercing munitions. The US Army uses DU in an alloy with around 3.5% titanium.
Staballoys, along with lower raw material costs, have the advantage of being easy to melt and cast into shape; a difficult and expensive process for tungsten. Depleted uranium is favoured for the penetrator because it is self-sharpening and pyrophoric. On impact with a hard target, such as an armoured vehicle, the nose of the rod fractures in such a way that it remains sharp. The impact and subsequent release of heat energy causes it to disintegrate to dust and burn when it reaches air because of its pyrophoric properties (compare to ferrocerium). After a disintegrated DU penetrator reaches the interior of an armored vehicle, it explodes, often igniting ammunition and fuel, incinerating the crew, and causing the vehicle to explode. DU is used by the U.S. Army in 120 mm or 105 mm cannons employed on the M1 Abrams and M60A3 tanks. The Russian military has used DU munitions in tank main gun ammunition since the late 1970s, mostly for the 115 mm guns in the T-62 tank and the 125 mm guns in the T-64, T-72, T-80, and T-90 tanks.
The DU content in various munitions is 180 g in 20 mm projectiles, 200 g in 25 mm ones, 280g in 30 mm, 3.5 kg in 105 mm, and 4.5 kg in 120 mm penetrators. It is used in the form of Staballoy. The US Navy used DU in its 20 mm Phalanx CIWS guns, but switched in the late 1990s to armor-piercing tungsten for this application, because of the fire risk associated with stray pyrophoric rounds. DU was used during the mid-1990s in the U.S. to make 9mm and similar caliber armor piercing bullets, grenades, cluster bombs, and mines, but those applications have been discontinued, according to Alliant Techsystems. Whether or not other nations still make such use of DU is difficult to determine.
It is thought that between 17 and 20 states, approximately, have weapons incorporating depleted uranium in their arsenals. They include the USA, the UK, France, Russia, Greece, Turkey, Israel, Saudi Arabia, Bahrain, Egypt, Kuwait, Pakistan, Thailand, Iraq and Taiwan. DU munitions are manufactured in 18 countries. While only the US and the UK have acknowledged using DU weapons, its use by other states cannot be excluded.The International Legality of the Use of Depleted Uranium Weapons: A Precautionary Approach, Avril McDonald, Jann K. Kleffner and Brigit Toebes, eds. (TMC Asser Press Fall-2003).
Legal status of weapons
In 1996 the International Court of Justice (ICJ) gave an advisory opinion on the "legality of the threat or use of nuclear weapons".[3] This made it clear, in paragraph 54, 55 and 56, that international law on poisonous weapon, – the Second Hague Declaration of 29 July 1899, Hague Convention IV of 18 October 1907 and the Geneva Protocol of 17 June 1925 – did not cover nuclear weapons, because their prime or exclusive use was not to poison or asphyxiate. This ICJ opinion was about nuclear weapons, but the sentence "The terms have been understood, in the practice of States, in their ordinary sense as covering weapons whose prime, or even exclusive, effect is to poison or asphyxiate." also removes depleted uranium weaponry from coverage by the same treaties as their primary use is not to poison or asphyxiate, but to destroy materiel and kill soldiers through kinetic energy.
In 1996 and 1997, the Sub-Commission on Prevention of Discrimination and Protection of Minorities of the United Nations Human Rights Commission[4], passed two motions [5] the first in 1996[6] and the second in 1997[7]. They listed weapons of mass destruction, or weapons with indiscriminate effect, or of a nature to cause superfluous injury or unnecessary suffering and urged all states to curb the production and the spread of such weapons. Included in the list was weaponry containing depleted uranium. The committee authorized a working paper, in the context of human rights and humanitarian norms, of the weapons. The requested UN working paper was delivered in 2002[8] by Y.K.J. Yeung Sik Yuen in accordance with Sub-Commission on Promotion and Protection of Human Rights resolution 2001/36. He argues that the use of DU in weapons, along with the other weapons listed by the Sub‑Commission, may breach one or more of the following treaties: The Universal Declaration of Human Rights; the Charter of the United Nations; the Genocide Convention; the United Nations Convention Against Torture; the Geneva Conventions including Protocol I; the Convention on Conventional Weapons of 1980; and the Chemical Weapons Convention. Yeung Sik Yuen writes in Paragraph 133 under the title "Legal compliance of weapons containing DU as a new weapon":
- Annex II to the Convention on the Physical Protection of Nuclear Material 1980 (which became operative on 8 February 1997) classifies DU as a category II nuclear material. Storage and transport rules are set down for that category which indicates that DU is considered sufficiently “hot” and dangerous to warrant these protections. But since weapons containing DU are relatively new weapons no treaty exists yet to regulate, limit or prohibit its use. The legality or illegality of DU weapons must therefore be tested by recourse to the general rules governing the use of weapons under humanitarian and human rights law which have already been analysed in Part I of this paper, and more particularly at paragraph 35 which states that parties to Protocol I to the Geneva Conventions of 1949 have an obligation to ascertain that new weapons do not violate the laws and customs of war or any other international law. As mentioned, the ICJ considers this rule binding customary humanitarian law.
In 2001, Carla del Ponte, the chief prosecutor for the International Criminal Tribunal for the Former Yugoslavia, said that NATO's use of depleted uranium in former Yugoslavia could be investigated as a possible war crime[9]. Louise Arbour, del Ponte's predecessor as chief prosecutor, had created a small, internal committee, made up of staff lawyers, to assess the allegation. Their findings, that were accepted and endorsed by del Ponte,[10] concluded that:
- There is no specific treaty ban on the use of DU projectiles. There is a developing scientific debate and concern expressed regarding the impact of the use of such projectiles and it is possible that, in future, there will be a consensus view in international legal circles that use of such projectiles violate general principles of the law applicable to use of weapons in armed conflict. No such consensus exists at present. (Emphasis added)[11]
Civilian applications
Civilian applications for depleted uranium are fairly limited and are typically unrelated to its radioactive properties. It primarily finds application as ballast because of its high density. Such applications include sailboat keels, as counterweights and sinker bars in oil drills, gyroscope rotors, and in other places where there is a need to place a weight that occupies as little space as possible. Other relatively minor consumer product uses have included: incorporation into dental porcelain used for false teeth to simulate the fluorescence of natural teeth; and in uranium-bearing reagents used in chemistry laboratories.
Uranium was widely used as a coloring matter for porcelain and glass in the 19th century. The practice was believed to be a matter of history, however in 1999 concentrations of 10% depleted uranium were found in "jaune no.17" a yellow enamel powder that was being produced in France by Cristallerie de Saint-Paul, a manufacturer of enamel pigments. The depleted uranium used in the powder was sold by Cogéma's Pierrelatte facility. Cogema has since confirmed that it has made a decision to stop the sale of depleted uranium to producers of enamel and glass. [8]
DU is also used for shielding for radiation sources used in medical and industrial radiography.
U.S. Nuclear Regulatory Commission regulations at 10 CFR 40.25 establish mandatory licensing for the use of depleted uranium contained in industrial products or devices for mass-volume applications. Other jurisdictions have similar regulations
Trim weights in aircraft
Aircraft may also contain depleted uranium trim weights (a Boeing 747 may contain 400 to 1,500 kg). This application of DU is controversial. If an aircraft crashes there is concern that the uranium would enter the environment: the metal can oxidise to a fine powder in a fire. While arguably other hazardous materials released from a burning commercial aircraft overshadow the contributions made by DU, its use has been phased out in many newer aircraft, Both Boeing and McDonnell-Douglas discontinued using DU counterweights in the 1980s.
Uranium hexafluoride
About 95% of the depleted uranium produced till now is stored as uranium hexafluoride, (D)UF6, in steel cylinders in open air yards close to enrichment plants. Each cylinder contains up to 12.7 tonnes (or 14 US tons) of UF6. In the U.S. alone, 560,000 tonnes of depleted UF6 had accumulated by 1993. In 2005, 686,500 tonnes in 57,122 storage cylinders were located near Portsmouth, Ohio, Oak Ridge, Tennessee, and Paducah, Kentucky. [9], [10] The long-term storage of DUF6 presents environmental, health, and safety risks because of its chemical instability. When UF6 is exposed to moist air, it reacts with the water in the air to produce UO2F2 (uranyl fluoride) and HF (hydrogen fluoride) both of which are highly soluble and toxic. Storage cylinders must be regularly inspected for signs of corrosion and leaks. The estimated life time of the steel cylinders is measured in decades. [11]

There have been several accidents involving uranium hexafluoride in the United States. [12] The U.S. government has been converting DUF6 to solid uranium oxides for disposal. [13] Such disposal of the entire DUF6 inventory could cost anywhere from 15 to 450 million dollars. [14]
Health concerns
The radiological dangers of pure depleted uranium are relatively low, lower (60%) than those of naturally-occurring uranium due to the removal of the more radioactive isotopes, as well as due to its long half-life (4.46 billion years). Depleted uranium differs from natural uranium in its isotopic composition, but its biochemistry is for the most part the same.
- For further details see Actinides in the environment.
Uranium is pyrophoric when finely divided. It will corrode under the influence of air and water producing insoluble uranium(IV) and soluble uranium(VI) salts, such as uranyl compounds, which are toxic. Uranium accumulates in several organs, such as the liver, spleen, and kidneys. The World Health Organization has established a daily "tolerated intake" of soluble uranium salts for the general public of 0.5 μg/kg body weight (or 35 μg for a 70 kg adult.)
The chemical toxicity of uranium salts is greater than their radiological toxicity. Its radiological hazards are dependent on the purity of the uranium, and there has been some concern that depleted uranium produced as a by-product of nuclear reprocessing may be contaminated with more dangerous isotopes: this should not be a concern for depleted uranium produced as tailings from initial uranium enrichment.
The possible dangers of exposure to depleted uranium have received renewed attention as a result of the use of DU munitions in the Gulf War. Some observers believe that exposure to uranium (or any of its compounds) is the cause of, or a contributing factor to, Gulf War syndrome.[citation needed] Many scientists do not believe this, however.[citation needed] The long-term effects on populations living in the areas in which DU munitions were used have also caused some concern.
- Exposure to DU can cause kidney damage in humans.
- DU was shown to have cytotoxic, genotoxic and carcinogenic effects in animal studies (PMID 7694141, PMID 16283518)
- Epidemiological evidence suggests that uranium causes reproductive effects in humans. (PMID 16124873)
- It has been shown in rodents and frogs that water soluble forms of uranium are teratogenic (PMID 16124873, PMID 11738513, PMID 12539863)
- Evidence of human health effects caused by DU is inconclusive, due largely to the fact that the health status of only a few dozen people with verified exposures has been assessed. [citation needed]
- After DU munitions have been used in combat, the presence of DU and DU compounds in soil and water, or on equipment and in buildings, may – depending on a variety of factors – present short- and long-term hazards to the health of local populations. [citation needed]
Further reading
Scientific bodies
- Canadian Uranium Medical Research Centre
- German World Uranium Weapons Conference
- U.K. Depleted Uranium Oversight Board
United Nations
- "Depleted Uranium: Sources, Exposure and Health Effects," World Health Organization, Ionizing Radiation Unit, 2001 (see Chapter 8, "The Chemical Toxicity of Uranium," in particular.)
- "Human rights and weapons of mass destruction, or with indiscriminate effect, or of a nature to cause superfluous injury or unnecessary suffering"
(The UN 2002 report) - The "Report of the World Health Organization Depleted Uranium Mission to Kosovo" (Draft text 28 January 2001). Undertaken at the request of the Special Representative of the Secretary-General and Head of the United Nations Interim Administration Mission in Kosovo (UNMIK), from 22 January to 31 January 2001.
Scientific reports
- [15] RAND review of scientific literature relating to depleted uranium as it pertains to Gulf War illnesses.
- U.S. Center for Disease Control's Toxicological Profile for Uranium (includes discussion of teratogenic and immunotoxic effects)
- Wilson, W.B. (1961) "High-Pressure High-Temperature Investigation of the Uranium-Oxygen System," Journal Of Inorganic and Nuclear Chemistry, 19, 212-222, p. 213.
- Arfsten, D.P. et al. (2001) "A review of the effects of uranium and depleted uranium exposure on reproduction and fetal development," Toxicology and Industrial Health, vol. 17, pp. 180-91.
- Hindin, R. et al. (2005) "Teratogenicity of depleted uranium aerosols: A review from an epidemiological perspective," Environmental Health, vol. 4, pp. 17.
- Domingo, J.L. (2001) "Reproductive and developmental toxicity of natural and depleted uranium: a review" Reproductive Toxicology, 15, 603-609.
- Durakovic A. (1999) "Medical effects of internal contamination with uranium," Croatian Medical Journal, vol. 40, pp. 49-66.
- Briner, W. and J. Murray (2005) "Effects of short-term and long-term depleted uranium exposure on open-field behavior and brain lipid oxidation in rats," Neurotoxicology and Teratology, vol. 27, pp. 135-44.
- Monleau, M. et al. (2005) "Bioaccumulation and behavioural effects of depleted uranium in rats exposed to repeated inhalations," Neuroscience Letters, vol. 390, pp. 31-6.
- Lestaevel, P. et al. (2005) "The brain is a target organ after acute exposure to depleted uranium" Toxicology, 212, 219-226.
- Depleted Uranium article from the Royal Society
- An Analysis of Uranium Dispersal and Health Effects Using a Gulf War Case Study by Sandia National Laboratories
- Depleted Uranium Human Health Fact Sheet by Argonne National Laboratory Environmental Assessment Division
- Uranium Human Health Fact Sheet
- Hindin R, Brugge D, Panikkar B., Teratogenicity of depleted uranium aerosols: a review from an epidemiological perspective, PMID 16124873
- "Animal studies firmly support the possibility that DU is a teratogen. While the detailed pathways by which environmental DU can be internalized and reach reproductive cells are not yet fully elucidated, again, the evidence supports plausibility."
- Lin RH, Wu LJ, Lee CH, Lin-Shiau SY, Cytogenetic toxicity of uranyl nitrate in Chinese hamster ovary cells, PMID 7694141
- Miller AC, Bonait-Pellie C, Merlot RF, Michel J, Stewart M, Lison PD., Leukemic transformation of hematopoietic cells in mice internally exposed to depleted uranium, PMID 16283518
- S.E. Mitchell, C.A. Caldwell, G. Gonzales, W.R. Gould and R. Arimoto, Journal of Toxicology and Environmental Health-Part A- Current Issues, 2005, 68, 951-965. (Frogs)
- M.L. Albina, M. Belles, M. Gomez, D.J. Sanchez and J.L Domingo, Experimental Biology and Medicine, 2003, 228, 1072-1077. (mice)
- A.U. Arfsten, K.R. Still and G.D. Ritchie, Toxicology and industrial Health, 2001, 17, 180-191. (Review)
Other publications
- Depleted uranium: the health debate Extract from article discussing the medical effects of depleted uranium
- "After the Dust Settles" (Bulletin of the Atomic Scientists report from 1999)
- Better World Links on Depleted Uranium Weapons 500+ links
- 'Blowin' in the Wind' information about a film about depleted uranium by David Bradbury
- Campaign Against Depleted Uranium
- Depleted UF6 Management Information Network – on U.S. Department of Energy's inventory of depleted uranium hexafluoride.
- Guardian Unlimited's Special Report on Depleted Uranium
- International Atomic Energy Agency Depleted Uranium FAQ
- International Coalition to Ban Uranium Weapons
- My Life Living With Depleted Uranium
- Proposal for Research on Depleted Uranium (U.K. Ministry of Defence)
- Radioactive Wounds of War
- U.S. Soldiers Contaminated With Depleted Uranium Speak Out – Democracy Now!, April 5, 2004
- Uranium and Weapons – Uranium Medical Research Centre
- Annotated bibliography for depleted uranium from the Alsos Digital Library
- Nuclear Files.org information and articles relating to depleted uranium
- Have DU Will Travel series of sixteen articles in the Crawford, Texas Lone Star Iconoclast
- DU-Watch
- International Atomic Energy Agency Depleted uranium FAQ
- The Depleted UF6 Management Information Network
- Cost-Effectiveness of Utilizing Surplus Depleted Uranium (DU)
- NATO Background Information on a current topic Depleted uranium
- Properties, use and health effects of depleted uranium (DU): A general overview. Journal of Environmental Radioactivity
- Depleted Uranium Quotations and links
- Avril McDonald The International Legality of Depleted Uranium on the website of the International Coalition to Ban Uranium Weapons
Footnotes
- ^ Doug Rokke Depleted Uranium: Uses and Hazards (PDF) an updated version of the paper presented in the British House of Commons on December 16, 1999
- ^ Katherine Rizzo Plutonium traces found in munitions tracked to processing plants Associated Press 25 January 2001
- ^ legality of the threat or use of nuclear weapons
- ^ Citizen Inspectors Foiled in Search for DU Weapons
- ^ Depleted Uranium UN Resolutions
- ^ Sub-Commission resolution 1996/16
- ^ Sub-Commission resolution 1997/36
- ^ E/CN.4/Sub.2/2002/38 Human rights and weapons of mass destruction, or with indiscriminate effect, or of a nature to cause superfluous injury or unnecessary suffering (backup) "In its decision 2001/36 of 16 August 2001, the Sub‑Commission, recalling its resolutions 1997/36 and 1997/37 of 28 August 1997, authorized Mr. Y.K.J. Yeung Sik Yuen to prepare, without financial implications, in the context of human rights and humanitarian norms, the working paper originally assigned to Ms. Forero Ucros."
- ^ The Associated Press & Reuters contributed to this report: Use of DU weapons could be war crime CNN January 14, 2001
- ^ Joe Sills et al Environmental Crimes in Military Actions and the International Criminal. Court(ICC)-United Nations Perspectives (PDF) (HTML) of American Council for the UN University, April 2002. Page 28
- ^ The Final Report to the Prosecutor by the Committee Established to Review the NATO Bombing Campaign Against the Federal Republic of Yugoslavia: Use of Depleted Uranium Projectiles
