Ununennium
| Theoretical element | ||||||
|---|---|---|---|---|---|---|
| Ununennium | ||||||
| Pronunciation | /ˌuːn.uːnˈɛniəm/ | |||||
| Alternative names | element 119, eka-francium | |||||
| Ununennium in the periodic table | ||||||
| ||||||
| Atomic number (Z) | 119 | |||||
| Group | group 1: hydrogen and alkali metals | |||||
| Period | period 8 (theoretical, extended table) | |||||
| Block | s-block | |||||
| Electron configuration | [Og] 8s1 (predicted)[1] | |||||
| Electrons per shell | 2, 8, 18, 32, 32, 18, 8, 1 (predicted) | |||||
| Physical properties | ||||||
| Phase at STP | unknown phase (could be solid or liquid)[1] | |||||
| Melting point | 273–303 K (0–30 °C, 32–86 °F) (predicted)[1] | |||||
| Boiling point | 903 K (630 °C, 1166 °F) (predicted)[2] | |||||
| Density (near r.t.) | 3 g/cm3 (predicted)[1] | |||||
| Heat of fusion | 2.01–2.05 kJ/mol (extrapolated)[3] | |||||
| Atomic properties | ||||||
| Oxidation states | common: (none) (+1), (+3), (+5)[1][4] | |||||
| Electronegativity | Pauling scale: 0.86 (predicted)[5] | |||||
| Ionization energies |
| |||||
| Atomic radius | empirical: 240 pm (predicted)[1] | |||||
| Covalent radius | 263–281 pm (extrapolated)[3] | |||||
| Other properties | ||||||
| Crystal structure | body-centered cubic (bcc) (extrapolated)[7] | |||||
| CAS Number | 54846-86-5 | |||||
| History | ||||||
| Naming | IUPAC systematic element name | |||||
| Isotopes of ununennium | ||||||
| Experiments and theoretical calculations | ||||||
Ununennium, also known as eka-francium or element 119, is the hypothetical chemical element with symbol Uue and atomic number 119. Ununennium and Uue are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to be an s-block element, an alkali metal, and the first element in the eighth period. It is the lightest element that has not yet been synthesized.
An attempt to synthesise ununennium is ongoing at RIKEN in Japan; another is planned for 2020–21 at the Joint Institute for Nuclear Research at Dubna, Russia. Theoretical and experimental evidence has shown that the synthesis of ununennium will likely be far more difficult than that of the previous elements, and it may even be the penultimate element that can be synthesized with current technology.
Ununennium's position as the seventh alkali metal suggests that it would have similar properties to its lighter congeners: lithium, sodium, potassium, rubidium, caesium, and francium. However, relativistic effects may cause some of its properties to differ from those expected from a straight application of periodic trends. For example, ununennium is expected to be less reactive than caesium and francium and to be closer in behavior to potassium or rubidium, and while it should show the characteristic +1 oxidation state of the alkali metals, it is also predicted to show the +3 oxidation state, which is unknown in any other alkali metal.
Introduction
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, or superheavies for short, are the chemical elements with atomic number greater than 104.[8] The superheavy elements are those beyond the actinides in the periodic table; the last actinide is lawrencium (atomic number 103). By definition, superheavy elements are also transuranium elements, i.e., having atomic numbers greater than that of uranium (92). Depending on the definition of group 3 adopted by authors, lawrencium may also be included to complete the 6d series.[9][10][11][12]
Glenn T. Seaborg first proposed the actinide concept, which led to the acceptance of the actinide series. He also proposed a transactinide series ranging from element 104 to 121 and a superactinide series approximately spanning elements 122 to 153 (though more recent work suggests the end of the superactinide series to occur at element 157 instead). The transactinide seaborgium was named in his honor.[13][14]
Superheavies are radioactive and have only been obtained synthetically in laboratories. No macroscopic sample of any of these elements has ever been produced. Superheavies are all named after physicists and chemists or important locations involved in the synthesis of the elements.
IUPAC defines an element to exist if its lifetime is longer than 10−14 seconds, which is the time it takes for the atom to form an electron cloud.[15]
The known superheavies form part of the 6d and 7p series in the periodic table. Except for rutherfordium and dubnium (and lawrencium if it is included), even the longest-lived known isotopes of superheavies have half-lives of minutes or less. The element naming controversy involved elements 102–109. Some of these elements thus used systematic names for many years after their discovery was confirmed. (Usually the systematic names are replaced with permanent names proposed by the discoverers relatively soon after a discovery has been confirmed.)
Introduction
Synthesis of superheavy nuclei
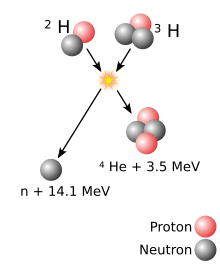
A superheavy[a] atomic nucleus is created in a nuclear reaction that combines two other nuclei of unequal size[b] into one; roughly, the more unequal the two nuclei in terms of mass, the greater the possibility that the two react.[21] The material made of the heavier nuclei is made into a target, which is then bombarded by the beam of lighter nuclei. Two nuclei can only fuse into one if they approach each other closely enough; normally, nuclei (all positively charged) repel each other due to electrostatic repulsion. The strong interaction can overcome this repulsion but only within a very short distance from a nucleus; beam nuclei are thus greatly accelerated in order to make such repulsion insignificant compared to the velocity of the beam nucleus.[22] The energy applied to the beam nuclei to accelerate them can cause them to reach speeds as high as one-tenth of the speed of light. However, if too much energy is applied, the beam nucleus can fall apart.[22]
Coming close enough alone is not enough for two nuclei to fuse: when two nuclei approach each other, they usually remain together for about 10−20 seconds and then part ways (not necessarily in the same composition as before the reaction) rather than form a single nucleus.[22][23] This happens because during the attempted formation of a single nucleus, electrostatic repulsion tears apart the nucleus that is being formed.[22] Each pair of a target and a beam is characterized by its cross section—the probability that fusion will occur if two nuclei approach one another expressed in terms of the transverse area that the incident particle must hit in order for the fusion to occur.[c] This fusion may occur as a result of the quantum effect in which nuclei can tunnel through electrostatic repulsion. If the two nuclei can stay close past that phase, multiple nuclear interactions result in redistribution of energy and an energy equilibrium.[22]
| External videos | |
|---|---|
The resulting merger is an excited state[26]—termed a compound nucleus—and thus it is very unstable.[22] To reach a more stable state, the temporary merger may fission without formation of a more stable nucleus.[27] Alternatively, the compound nucleus may eject a few neutrons, which would carry away the excitation energy; if the latter is not sufficient for a neutron expulsion, the merger would produce a gamma ray. This happens in about 10−16 seconds after the initial nuclear collision and results in creation of a more stable nucleus.[27] The definition by the IUPAC/IUPAP Joint Working Party (JWP) states that a chemical element can only be recognized as discovered if a nucleus of it has not decayed within 10−14 seconds. This value was chosen as an estimate of how long it takes a nucleus to acquire electrons and thus display its chemical properties.[28][d]
Decay and detection
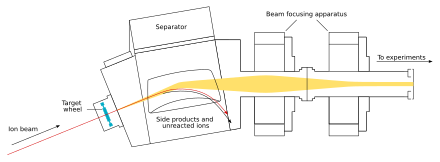
The beam passes through the target and reaches the next chamber, the separator; if a new nucleus is produced, it is carried with this beam.[30] In the separator, the newly produced nucleus is separated from other nuclides (that of the original beam and any other reaction products)[e] and transferred to a surface-barrier detector, which stops the nucleus. The exact location of the upcoming impact on the detector is marked; also marked are its energy and the time of the arrival.[30] The transfer takes about 10−6 seconds; in order to be detected, the nucleus must survive this long.[33] The nucleus is recorded again once its decay is registered, and the location, the energy, and the time of the decay are measured.[30]
Stability of a nucleus is provided by the strong interaction. However, its range is very short; as nuclei become larger, its influence on the outermost nucleons (protons and neutrons) weakens. At the same time, the nucleus is torn apart by electrostatic repulsion between protons, and its range is not limited.[34] Total binding energy provided by the strong interaction increases linearly with the number of nucleons, whereas electrostatic repulsion increases with the square of the atomic number, i.e. the latter grows faster and becomes increasingly important for heavy and superheavy nuclei.[35][36] Superheavy nuclei are thus theoretically predicted[37] and have so far been observed[38] to predominantly decay via decay modes that are caused by such repulsion: alpha decay and spontaneous fission.[f] Almost all alpha emitters have over 210 nucleons,[40] and the lightest nuclide primarily undergoing spontaneous fission has 238.[41] In both decay modes, nuclei are inhibited from decaying by corresponding energy barriers for each mode, but they can be tunneled through.[35][36]
Alpha particles are commonly produced in radioactive decays because the mass of an alpha particle per nucleon is small enough to leave some energy for the alpha particle to be used as kinetic energy to leave the nucleus.[43] Spontaneous fission is caused by electrostatic repulsion tearing the nucleus apart and produces various nuclei in different instances of identical nuclei fissioning.[36] As the atomic number increases, spontaneous fission rapidly becomes more important: spontaneous fission partial half-lives decrease by 23 orders of magnitude from uranium (element 92) to nobelium (element 102),[44] and by 30 orders of magnitude from thorium (element 90) to fermium (element 100).[45] The earlier liquid drop model thus suggested that spontaneous fission would occur nearly instantly due to disappearance of the fission barrier for nuclei with about 280 nucleons.[36][46] The later nuclear shell model suggested that nuclei with about 300 nucleons would form an island of stability in which nuclei will be more resistant to spontaneous fission and will primarily undergo alpha decay with longer half-lives.[36][46] Subsequent discoveries suggested that the predicted island might be further than originally anticipated; they also showed that nuclei intermediate between the long-lived actinides and the predicted island are deformed, and gain additional stability from shell effects.[47] Experiments on lighter superheavy nuclei,[48] as well as those closer to the expected island,[44] have shown greater than previously anticipated stability against spontaneous fission, showing the importance of shell effects on nuclei.[g]
Alpha decays are registered by the emitted alpha particles, and the decay products are easy to determine before the actual decay; if such a decay or a series of consecutive decays produces a known nucleus, the original product of a reaction can be easily determined.[h] (That all decays within a decay chain were indeed related to each other is established by the location of these decays, which must be in the same place.)[30] The known nucleus can be recognized by the specific characteristics of decay it undergoes such as decay energy (or more specifically, the kinetic energy of the emitted particle).[i] Spontaneous fission, however, produces various nuclei as products, so the original nuclide cannot be determined from its daughters.[j]
The information available to physicists aiming to synthesize a superheavy element is thus the information collected at the detectors: location, energy, and time of arrival of a particle to the detector, and those of its decay. The physicists analyze this data and seek to conclude that it was indeed caused by a new element and could not have been caused by a different nuclide than the one claimed. Often, provided data is insufficient for a conclusion that a new element was definitely created and there is no other explanation for the observed effects; errors in interpreting data have been made.[k]
History
Early predictions
This section needs expansion. You can help by adding to it. (November 2019) |
The heaviest element known at the end of the 19th century was uranium, with an atomic mass of about 240 (now known to be 238) amu. Accordingly, it was placed in the last row of the periodic table; this fueled speculation about the possible existence of elements heavier than uranium and why A = 240 seemed to be the limit. Following the discovery of the noble gases, beginning with argon in 1895, the possibility of heavier members of the group was considered. Danish chemist Julius Thomsen proposed in 1895 the existence of a sixth noble gas with Z = 86, A = 212 and a seventh with Z = 118, A = 292, the last closing a 32-element period containing thorium and uranium.[59] In 1913, Swedish physicist Johannes Rydberg extended Thomsen's extrapolation of the periodic table to include even heavier elements with atomic numbers up to 460, but he did not believe that these superheavy elements existed or occurred in nature.[60]
In 1914, German physicist Richard Swinne proposed that elements heavier than uranium, such as those around Z = 108, could be found in cosmic rays. He suggested that these elements may not necessarily have decreasing half-lives with increasing atomic number, leading to speculation about the possibility of some longer-lived elements at Z = 98–102 and Z = 108–110 (though separated by short-lived elements). Swinne published these predictions in 1926, believing that such elements might exist in Earth's core, iron meteorites, or the ice caps of Greenland where they had been locked up from their supposed cosmic origin.[61]
Discoveries
This section needs expansion. You can help by adding to it. (November 2019) |
Work performed from 1961 to 2013 at four labs – Lawrence Berkeley National Laboratory in the US, the Joint Institute for Nuclear Research in the USSR (later Russia), the GSI Helmholtz Centre for Heavy Ion Research in Germany, and Riken in Japan – identified and confirmed the elements lawrencium to oganesson according to the criteria of the IUPAC–IUPAP Transfermium Working Groups and subsequent Joint Working Parties. These discoveries complete the seventh row of the periodic table. The next two elements, ununennium (Z = 119) and unbinilium (Z = 120), have not yet been synthesized. They would begin an eighth period.
List of elements
- 103 Lawrencium, Lr, for Ernest Lawrence; sometimes but not always included[9][10]
- 104 Rutherfordium, Rf, for Ernest Rutherford
- 105 Dubnium, Db, for the town of Dubna, near Moscow
- 106 Seaborgium, Sg, for Glenn T. Seaborg
- 107 Bohrium, Bh, for Niels Bohr
- 108 Hassium, Hs, for Hassia (Hesse), location of Darmstadt
- 109 Meitnerium, Mt, for Lise Meitner
- 110 Darmstadtium, Ds, for Darmstadt)
- 111 Roentgenium, Rg, for Wilhelm Röntgen
- 112 Copernicium, Cn, for Nicolaus Copernicus
- 113 Nihonium, Nh, for Nihon (Japan), location of the Riken institute
- 114 Flerovium, Fl, for Russian physicist Georgy Flyorov
- 115 Moscovium, Mc, for Moscow
- 116 Livermorium, Lv, for Lawrence Livermore National Laboratory
- 117 Tennessine, Ts, for Tennessee, location of Oak Ridge National Laboratory
- 118 Oganesson, Og, for Russian physicist Yuri Oganessian
Characteristics
Due to their short half-lives (for example, the most stable known isotope of seaborgium has a half-life of 14 minutes, and half-lives decrease with increasing atomic number) and the low yield of the nuclear reactions that produce them, new methods have had to be created to determine their gas-phase and solution chemistry based on very small samples of a few atoms each. Relativistic effects become very important in this region of the periodic table, causing the filled 7s orbitals, empty 7p orbitals, and filling 6d orbitals to all contract inward toward the atomic nucleus. This causes a relativistic stabilization of the 7s electrons and makes the 7p orbitals accessible in low excitation states.[14]
Elements 103 to 112, lawrencium to copernicium, form the 6d series of transition elements. Experimental evidence shows that elements 103–108 behave as expected for their position in the periodic table, as heavier homologs of lutetium through osmium. They are expected to have ionic radii between those of their 5d transition metal homologs and their actinide pseudohomologs: for example, Rf4+ is calculated to have ionic radius 76 pm, between the values for Hf4+ (71 pm) and Th4+ (94 pm). Their ions should also be less polarizable than those of their 5d homologs. Relativistic effects are expected to reach a maximum at the end of this series, at roentgenium (element 111) and copernicium (element 112). Nevertheless, many important properties of the transactinides are still not yet known experimentally, though theoretical calculations have been performed.[14]
Elements 113 to 118, nihonium to oganesson, should form a 7p series, completing the seventh period in the periodic table. Their chemistry will be greatly influenced by the very strong relativistic stabilization of the 7s electrons and a strong spin–orbit coupling effect "tearing" the 7p subshell apart into two sections, one more stabilized (7p1/2, holding two electrons) and one more destabilized (7p3/2, holding four electrons). Lower oxidation states should be stabilized here, continuing group trends, as both the 7s and 7p1/2 electrons exhibit the inert-pair effect. These elements are expected to largely continue to follow group trends, though with relativistic effects playing an increasingly larger role. In particular, the large 7p splitting results in an effective shell closure at flerovium (element 114) and a hence much higher than expected chemical activity for oganesson (element 118).[14]
Element 118 is the last element that has been synthesized. The next two elements, 119 and 120, should form an 8s series and be an alkali and alkaline earth metal respectively. The 8s electrons are expected to be relativistically stabilized, so that the trend toward higher reactivity down these groups will reverse and the elements will behave more like their period 5 homologs, rubidium and strontium. The 7p3/2 orbital is still relativistically destabilized, potentially giving these elements larger ionic radii and perhaps even being able to participate chemically. In this region, the 8p electrons are also relativistically stabilized, resulting in a ground-state 8s28p1 valence electron configuration for element 121. Large changes are expected to occur in the subshell structure in going from element 120 to element 121: for example, the radius of the 5g orbitals should drop drastically, from 25 Bohr units in element 120 in the excited [Og] 5g1 8s1 configuration to 0.8 Bohr units in element 121 in the excited [Og] 5g1 7d1 8s1 configuration, in a phenomenon called "radial collapse". Element 122 should add either a further 7d or a further 8p electron to element 121's electron configuration. Elements 121 and 122 should be similar to actinium and thorium respectively.[14]
At element 121, the superactinide series is expected to begin, when the 8s electrons and the filling 8p1/2, 7d3/2, 6f5/2, and 5g7/2 subshells determine the chemistry of these elements. Complete and accurate calculations are not available for elements beyond 123 because of the extreme complexity of the situation:[62] the 5g, 6f, and 7d orbitals should have about the same energy level, and in the region of element 160 the 9s, 8p3/2, and 9p1/2 orbitals should also be about equal in energy. This will cause the electron shells to mix so that the block concept no longer applies very well, and will also result in novel chemical properties that will make positioning these elements in a periodic table very difficult.[14]
Beyond superheavy elements
It has been suggested that elements beyond Z = 126 be called beyond superheavy elements.[63] Other sources refer to elements around Z = 164 as hyperheavy elements.[64]
See also
- Bose–Einstein condensate (also known as Superatom)
- Island of stability
Notes
- ^ In nuclear physics, an element is called heavy if its atomic number is high; lead (element 82) is one example of such a heavy element. The term "superheavy elements" typically refers to elements with atomic number greater than 103 (although there are other definitions, such as atomic number greater than 100[16] or 112;[17] sometimes, the term is presented an equivalent to the term "transactinide", which puts an upper limit before the beginning of the hypothetical superactinide series).[18] Terms "heavy isotopes" (of a given element) and "heavy nuclei" mean what could be understood in the common language—isotopes of high mass (for the given element) and nuclei of high mass, respectively.
- ^ In 2009, a team at the JINR led by Oganessian published results of their attempt to create hassium in a symmetric 136Xe + 136Xe reaction. They failed to observe a single atom in such a reaction, putting the upper limit on the cross section, the measure of probability of a nuclear reaction, as 2.5 pb.[19] In comparison, the reaction that resulted in hassium discovery, 208Pb + 58Fe, had a cross section of ~20 pb (more specifically, 19+19
-11 pb), as estimated by the discoverers.[20] - ^ The amount of energy applied to the beam particle to accelerate it can also influence the value of cross section. For example, in the 28
14Si
+ 1
0n
→ 28
13Al
+ 1
1p
reaction, cross section changes smoothly from 370 mb at 12.3 MeV to 160 mb at 18.3 MeV, with a broad peak at 13.5 MeV with the maximum value of 380 mb.[24] - ^ This figure also marks the generally accepted upper limit for lifetime of a compound nucleus.[29]
- ^ This separation is based on that the resulting nuclei move past the target more slowly then the unreacted beam nuclei. The separator contains electric and magnetic fields whose effects on a moving particle cancel out for a specific velocity of a particle.[31] Such separation can also be aided by a time-of-flight measurement and a recoil energy measurement; a combination of the two may allow to estimate the mass of a nucleus.[32]
- ^ Not all decay modes are caused by electrostatic repulsion. For example, beta decay is caused by the weak interaction.[39]
- ^ It was already known by the 1960s that ground states of nuclei differed in energy and shape as well as that certain magic numbers of nucleons corresponded to greater stability of a nucleus. However, it was assumed that there was no nuclear structure in superheavy nuclei as they were too deformed to form one.[44]
- ^ Since mass of a nucleus is not measured directly but is rather calculated from that of another nucleus, such measurement is called indirect. Direct measurements are also possible, but for the most part they have remained unavailable for superheavy nuclei.[49] The first direct measurement of mass of a superheavy nucleus was reported in 2018 at LBNL.[50] Mass was determined from the location of a nucleus after the transfer (the location helps determine its trajectory, which is linked to the mass-to-charge ratio of the nucleus, since the transfer was done in presence of a magnet).[51]
- ^ If the decay occurred in a vacuum, then since total momentum of an isolated system before and after the decay must be preserved, the daughter nucleus would also receive a small velocity. The ratio of the two velocities, and accordingly the ratio of the kinetic energies, would thus be inverse to the ratio of the two masses. The decay energy equals the sum of the known kinetic energy of the alpha particle and that of the daughter nucleus (an exact fraction of the former).[40] The calculations hold for an experiment as well, but the difference is that the nucleus does not move after the decay because it is tied to the detector.
- ^ Spontaneous fission was discovered by Soviet physicist Georgy Flerov,[52] a leading scientist at JINR, and thus it was a "hobbyhorse" for the facility.[53] In contrast, the LBL scientists believed fission information was not sufficient for a claim of synthesis of an element. They believed spontaneous fission had not been studied enough to use it for identification of a new element, since there was a difficulty of establishing that a compound nucleus had only ejected neutrons and not charged particles like protons or alpha particles.[29] They thus preferred to link new isotopes to the already known ones by successive alpha decays.[52]
- ^ For instance, element 102 was mistakenly identified in 1957 at the Nobel Institute of Physics in Stockholm, Stockholm County, Sweden.[54] There were no earlier definitive claims of creation of this element, and the element was assigned a name by its Swedish, American, and British discoverers, nobelium. It was later shown that the identification was incorrect.[55] The following year, RL was unable to reproduce the Swedish results and announced instead their synthesis of the element; that claim was also disproved later.[55] JINR insisted that they were the first to create the element and suggested a name of their own for the new element, joliotium;[56] the Soviet name was also not accepted (JINR later referred to the naming of the element 102 as "hasty").[57] This name was proposed to IUPAC in a written response to their ruling on priority of discovery claims of elements, signed 29 September 1992.[57] The name "nobelium" remained unchanged on account of its widespread usage.[58]
References
- ^ a b c d e f Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. ISBN 978-1-4020-3555-5.
- ^ Fricke, B.; Waber, J. T. (1971). "Theoretical Predictions of the Chemistry of Superheavy Elements" (PDF). Actinides Reviews. 1: 433–485. Retrieved 7 August 2013.
- ^ a b Bonchev, Danail; Kamenska, Verginia (1981). "Predicting the Properties of the 113–120 Transactinide Elements". Journal of Physical Chemistry. 85 (9). American Chemical Society: 1177–1186. doi:10.1021/j150609a021.
- ^ Cao, Chang-Su; Hu, Han-Shi; Schwarz, W. H. Eugen; Li, Jun (2022). "Periodic Law of Chemistry Overturns for Superheavy Elements". ChemRxiv (preprint). doi:10.26434/chemrxiv-2022-l798p. Retrieved 16 November 2022.
- ^ Pershina, V.; Borschevsky, A.; Anton, J. (20 February 2012). "Fully relativistic study of intermetallic dimers of group-1 elements K through element 119 and prediction of their adsorption on noble metal surfaces". Chemical Physics. 395. Elsevier: 87–94. Bibcode:2012CP....395...87P. doi:10.1016/j.chemphys.2011.04.017. This article gives the Mulliken electronegativity as 2.72, which has been converted to the Pauling scale via χP = 1.35χM1/2 − 1.37.
- ^ Fricke, Burkhard (1975). "Superheavy elements: a prediction of their chemical and physical properties". Recent Impact of Physics on Inorganic Chemistry. Structure and Bonding. 21: 89–144. doi:10.1007/BFb0116498. ISBN 978-3-540-07109-9. Retrieved 4 October 2013.
- ^ Seaborg, Glenn T. (1969). "Prospects for further considerable extension of the periodic table" (PDF). Journal of Chemical Education. 46 (10): 626–634. Bibcode:1969JChEd..46..626S. doi:10.1021/ed046p626. Retrieved 22 February 2018.
- ^ "Superheavy Element Discovery | Glenn T. Seaborg Institute". seaborg.llnl.gov. Retrieved 2024-09-02.
- ^ a b Neve, Francesco (2022). "Chemistry of superheavy transition metals". Journal of Coordination Chemistry. 75 (17–18): 2287–2307. doi:10.1080/00958972.2022.2084394. S2CID 254097024.
- ^ a b Mingos, Michael (1998). Essential Trends in Inorganic Chemistry. Oxford University Press. p. 387. ISBN 978-0-19-850109-1.
- ^ "A New Era of Discovery: the 2023 Long Range Plan for Nuclear Science" (PDF). U.S. Department of Energy. October 2023. Archived from the original (PDF) on 2023-10-05. Retrieved 20 October 2023 – via OSTI.
Superheavy elements (Z > 102) are teetering at the limits of mass and charge.
- ^ Kragh, Helge (2017). "The search for superheavy elements: Historical and philosophical perspectives". arXiv:1708.04064 [physics.hist-ph].
- ^ IUPAC Provisional Recommendations for the Nomenclature of Inorganic Chemistry (2004) (online draft of an updated version of the "Red Book" IR 3-6) Archived October 27, 2006, at the Wayback Machine
- ^ a b c d e f Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean, eds. (2006). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer. ISBN 978-1-4020-3555-5.
- ^ "Kernchemie". www.kernchemie.de.
- ^ Krämer, K. (2016). "Explainer: superheavy elements". Chemistry World. Retrieved 2020-03-15.
- ^ "Discovery of Elements 113 and 115". Lawrence Livermore National Laboratory. Archived from the original on 2015-09-11. Retrieved 2020-03-15.
- ^ Eliav, E.; Kaldor, U.; Borschevsky, A. (2018). "Electronic Structure of the Transactinide Atoms". In Scott, R. A. (ed.). Encyclopedia of Inorganic and Bioinorganic Chemistry. John Wiley & Sons. pp. 1–16. doi:10.1002/9781119951438.eibc2632. ISBN 978-1-119-95143-8. S2CID 127060181.
- ^ Oganessian, Yu. Ts.; Dmitriev, S. N.; Yeremin, A. V.; et al. (2009). "Attempt to produce the isotopes of element 108 in the fusion reaction 136Xe + 136Xe". Physical Review C. 79 (2): 024608. doi:10.1103/PhysRevC.79.024608. ISSN 0556-2813.
- ^ Münzenberg, G.; Armbruster, P.; Folger, H.; et al. (1984). "The identification of element 108" (PDF). Zeitschrift für Physik A. 317 (2): 235–236. Bibcode:1984ZPhyA.317..235M. doi:10.1007/BF01421260. S2CID 123288075. Archived from the original (PDF) on 7 June 2015. Retrieved 20 October 2012.
- ^ Subramanian, S. (28 August 2019). "Making New Elements Doesn't Pay. Just Ask This Berkeley Scientist". Bloomberg Businessweek. Retrieved 2020-01-18.
- ^ a b c d e f Ivanov, D. (2019). "Сверхтяжелые шаги в неизвестное" [Superheavy steps into the unknown]. nplus1.ru (in Russian). Retrieved 2020-02-02.
- ^ Hinde, D. (2017). "Something new and superheavy at the periodic table". The Conversation. Retrieved 2020-01-30.
- ^ Kern, B. D.; Thompson, W. E.; Ferguson, J. M. (1959). "Cross sections for some (n, p) and (n, α) reactions". Nuclear Physics. 10: 226–234. Bibcode:1959NucPh..10..226K. doi:10.1016/0029-5582(59)90211-1.
- ^ Wakhle, A.; Simenel, C.; Hinde, D. J.; et al. (2015). Simenel, C.; Gomes, P. R. S.; Hinde, D. J.; et al. (eds.). "Comparing Experimental and Theoretical Quasifission Mass Angle Distributions". European Physical Journal Web of Conferences. 86: 00061. Bibcode:2015EPJWC..8600061W. doi:10.1051/epjconf/20158600061. hdl:1885/148847. ISSN 2100-014X.
- ^ "Nuclear Reactions" (PDF). pp. 7–8. Retrieved 2020-01-27. Published as Loveland, W. D.; Morrissey, D. J.; Seaborg, G. T. (2005). "Nuclear Reactions". Modern Nuclear Chemistry. John Wiley & Sons, Inc. pp. 249–297. doi:10.1002/0471768626.ch10. ISBN 978-0-471-76862-3.
- ^ a b Krása, A. (2010). "Neutron Sources for ADS". Faculty of Nuclear Sciences and Physical Engineering. Czech Technical University in Prague: 4–8. S2CID 28796927.
- ^ Wapstra, A. H. (1991). "Criteria that must be satisfied for the discovery of a new chemical element to be recognized" (PDF). Pure and Applied Chemistry. 63 (6): 883. doi:10.1351/pac199163060879. ISSN 1365-3075. S2CID 95737691.
- ^ a b Hyde, E. K.; Hoffman, D. C.; Keller, O. L. (1987). "A History and Analysis of the Discovery of Elements 104 and 105". Radiochimica Acta. 42 (2): 67–68. doi:10.1524/ract.1987.42.2.57. ISSN 2193-3405. S2CID 99193729.
- ^ a b c d Chemistry World (2016). "How to Make Superheavy Elements and Finish the Periodic Table [Video]". Scientific American. Retrieved 2020-01-27.
- ^ Hoffman, Ghiorso & Seaborg 2000, p. 334.
- ^ Hoffman, Ghiorso & Seaborg 2000, p. 335.
- ^ Zagrebaev, Karpov & Greiner 2013, p. 3.
- ^ Beiser 2003, p. 432.
- ^ a b Pauli, N. (2019). "Alpha decay" (PDF). Introductory Nuclear, Atomic and Molecular Physics (Nuclear Physics Part). Université libre de Bruxelles. Retrieved 2020-02-16.
- ^ a b c d e Pauli, N. (2019). "Nuclear fission" (PDF). Introductory Nuclear, Atomic and Molecular Physics (Nuclear Physics Part). Université libre de Bruxelles. Retrieved 2020-02-16.
- ^ Staszczak, A.; Baran, A.; Nazarewicz, W. (2013). "Spontaneous fission modes and lifetimes of superheavy elements in the nuclear density functional theory". Physical Review C. 87 (2): 024320–1. arXiv:1208.1215. Bibcode:2013PhRvC..87b4320S. doi:10.1103/physrevc.87.024320. ISSN 0556-2813.
- ^ Audi et al. 2017, pp. 030001-129–030001-138.
- ^ Beiser 2003, p. 439.
- ^ a b Beiser 2003, p. 433.
- ^ Audi et al. 2017, p. 030001-125.
- ^ Aksenov, N. V.; Steinegger, P.; Abdullin, F. Sh.; et al. (2017). "On the volatility of nihonium (Nh, Z = 113)". The European Physical Journal A. 53 (7): 158. Bibcode:2017EPJA...53..158A. doi:10.1140/epja/i2017-12348-8. ISSN 1434-6001. S2CID 125849923.
- ^ Beiser 2003, p. 432–433.
- ^ a b c Oganessian, Yu. (2012). "Nuclei in the "Island of Stability" of Superheavy Elements". Journal of Physics: Conference Series. 337 (1): 012005-1–012005-6. Bibcode:2012JPhCS.337a2005O. doi:10.1088/1742-6596/337/1/012005. ISSN 1742-6596.
- ^ Moller, P.; Nix, J. R. (1994). Fission properties of the heaviest elements (PDF). Dai 2 Kai Hadoron Tataikei no Simulation Symposium, Tokai-mura, Ibaraki, Japan. University of North Texas. Retrieved 2020-02-16.
- ^ a b Oganessian, Yu. Ts. (2004). "Superheavy elements". Physics World. 17 (7): 25–29. doi:10.1088/2058-7058/17/7/31. Retrieved 2020-02-16.
- ^ Schädel, M. (2015). "Chemistry of the superheavy elements". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 373 (2037): 20140191. Bibcode:2015RSPTA.37340191S. doi:10.1098/rsta.2014.0191. ISSN 1364-503X. PMID 25666065.
- ^ Hulet, E. K. (1989). Biomodal spontaneous fission. 50th Anniversary of Nuclear Fission, Leningrad, USSR. Bibcode:1989nufi.rept...16H.
- ^ Oganessian, Yu. Ts.; Rykaczewski, K. P. (2015). "A beachhead on the island of stability". Physics Today. 68 (8): 32–38. Bibcode:2015PhT....68h..32O. doi:10.1063/PT.3.2880. ISSN 0031-9228. OSTI 1337838. S2CID 119531411.
- ^ Grant, A. (2018). "Weighing the heaviest elements". Physics Today. doi:10.1063/PT.6.1.20181113a. S2CID 239775403.
- ^ Howes, L. (2019). "Exploring the superheavy elements at the end of the periodic table". Chemical & Engineering News. Retrieved 2020-01-27.
- ^ a b Robinson, A. E. (2019). "The Transfermium Wars: Scientific Brawling and Name-Calling during the Cold War". Distillations. Retrieved 2020-02-22.
- ^ "Популярная библиотека химических элементов. Сиборгий (экавольфрам)" [Popular library of chemical elements. Seaborgium (eka-tungsten)]. n-t.ru (in Russian). Retrieved 2020-01-07. Reprinted from "Экавольфрам" [Eka-tungsten]. Популярная библиотека химических элементов. Серебро – Нильсборий и далее [Popular library of chemical elements. Silver through nielsbohrium and beyond] (in Russian). Nauka. 1977.
- ^ "Nobelium - Element information, properties and uses | Periodic Table". Royal Society of Chemistry. Retrieved 2020-03-01.
- ^ a b Kragh 2018, pp. 38–39.
- ^ Kragh 2018, p. 40.
- ^ a b Ghiorso, A.; Seaborg, G. T.; Oganessian, Yu. Ts.; et al. (1993). "Responses on the report 'Discovery of the Transfermium elements' followed by reply to the responses by Transfermium Working Group" (PDF). Pure and Applied Chemistry. 65 (8): 1815–1824. doi:10.1351/pac199365081815. S2CID 95069384. Archived (PDF) from the original on 25 November 2013. Retrieved 7 September 2016.
- ^ Commission on Nomenclature of Inorganic Chemistry (1997). "Names and symbols of transfermium elements (IUPAC Recommendations 1997)" (PDF). Pure and Applied Chemistry. 69 (12): 2471–2474. doi:10.1351/pac199769122471.
- ^ Kragh 2018, p. 6
- ^ Kragh 2018, p. 7
- ^ Kragh 2018, p. 10
- ^ van der Schoor, K. (2016). Electronic structure of element 123 (PDF) (Thesis). Rijksuniversiteit Groningen.
- ^ Hofmann, Sigurd (2019). "Synthesis and properties of isotopes of the transactinides". Radiochimica Acta. 107 (9–11): 879–915. doi:10.1515/ract-2019-3104. S2CID 203848120.
- ^ Laforge, Evan; Price, Will; Rafelski, Johann (2023). "Superheavy elements and ultradense matter". The European Physical Journal Plus. 138 (9): 812. arXiv:2306.11989. Bibcode:2023EPJP..138..812L. doi:10.1140/epjp/s13360-023-04454-8.
Bibliography
- Audi, G.; Kondev, F. G.; Wang, M.; et al. (2017). "The NUBASE2016 evaluation of nuclear properties". Chinese Physics C. 41 (3). 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
pp. 030001-1–030001-17, pp. 030001-18–030001-138, Table I. The NUBASE2016 table of nuclear and decay properties - Beiser, A. (2003). Concepts of modern physics (6th ed.). McGraw-Hill. ISBN 978-0-07-244848-1. OCLC 48965418.
- Hoffman, D. C.; Ghiorso, A.; Seaborg, G. T. (2000). The Transuranium People: The Inside Story. World Scientific. ISBN 978-1-78-326244-1.
- Kragh, H. (2018). From Transuranic to Superheavy Elements: A Story of Dispute and Creation. Springer. ISBN 978-3-319-75813-8.
- Zagrebaev, V.; Karpov, A.; Greiner, W. (2013). "Future of superheavy element research: Which nuclei could be synthesized within the next few years?". Journal of Physics: Conference Series. 420 (1). 012001. arXiv:1207.5700. Bibcode:2013JPhCS.420a2001Z. doi:10.1088/1742-6596/420/1/012001. ISSN 1742-6588.
History
Superheavy elements are produced by nuclear fusion. These fusion reactions can be divided into "hot" and "cold" fusion,[a] depending on the excitation energy of the compound nucleus produced. In hot fusion reactions, very light, high-energy projectiles are accelerated toward very heavy targets (actinides), giving rise to compound nuclei at high excitation energy (~40–50 MeV) that may fission, or alternatively evaporate several (3 to 5) neutrons.[2] In cold fusion reactions (which use heavier projectiles, typically from the fourth period, and lighter targets, usually lead and bismuth), the fused nuclei produced have a relatively low excitation energy (~10–20 MeV), which decreases the probability that these products will undergo fission reactions. As the fused nuclei cool to the ground state, they require emission of only one or two neutrons. However, hot fusion reactions tend to produce more neutron-rich products because the actinides have the highest neutron-to-proton ratios of any elements that can presently be made in macroscopic quantities.[3]
Ununennium and unbinilium (elements 119 and 120) are the elements with the lowest atomic numbers that have not yet been synthesized. Attempts to synthesize them would push the limits of current technology, due to the decreasing cross sections of the production reactions and their probably short half-lives,[4] expected to be on the order of microseconds.[5][6] Elements beyond unbiunium (element 121) would likely be too short-lived to be detected with current technology: they would decay within a microsecond, before reaching the detectors. The possibility of detection of elements 121 through 124 depends greatly on the theoretical model being used, as their half-lives are predicted to be very close to the one-microsecond border.[4] Previously, important help (characterized as "silver bullets") in the synthesis of superheavy elements came from the deformed nuclear shells around hassium-270 which increased the stability of surrounding nuclei, and the existence of the quasi-stable neutron-rich isotope calcium-48 which could be used as a projectile to produce more neutron-rich isotopes of superheavy elements.[7] The more neutron-rich a superheavy nuclide is, the closer it is expected to be to the sought-after island of stability.[b] Even so, the synthesized isotopes still have fewer neutrons than those expected to be in the island of stability.[10] Furthermore, using calcium-48 to synthesize ununennium would require a target of einsteinium-253 or -254, which are very difficult to produce in sufficiently large quantities (only micrograms are presently available; in comparison, milligrams of berkelium and californium are available). More practical production of further superheavy elements would require projectiles heavier than 48Ca.[7]
Synthesis attempts
Past
The synthesis of ununennium was first attempted in 1985 by bombarding a target of einsteinium-254 with calcium-48 ions at the superHILAC accelerator at Berkeley, California:
- 254
99Es
+ 48
20Ca
→ 302
119Uue
* → no atoms
No atoms were identified, leading to a limiting cross section of 300 nb.[11] Later calculations suggest that the cross section of the 3n reaction (which would result in 299Uue and three neutrons as products) would actually be six hundred thousand times lower than this upper bound, at 0.5 pb.[12]
As ununennium is the lightest undiscovered element, it has been the target of synthesis experiments by German, Russian, and Japanese teams in recent years. The Russian team at the Joint Institute for Nuclear Research in Dubna, Russia planned to conduct an experiment before 2012, and no results were released, strongly implying that either the experiment was not done or no ununennium atoms were identified.[citation needed] From April to September 2012, an attempt to synthesize the isotopes 295Uue and 296Uue was made by bombarding a target of berkelium-249 with titanium-50 at the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, Germany.[13][14] Based on the theoretically predicted cross section, it was expected that an ununennium atom would be synthesized within five months of the beginning of the experiment.[4]
- 249
97Bk
+ 50
22Ti
→ 299
119Uue
* → 296
119Uue
+ 3 1
0
n - 249
97Bk
+ 50
22Ti
→ 299
119Uue
* → 295
119Uue
+ 4 1
0
n
The experiment was originally planned to continue to November 2012,[15] but was stopped early to make use of the 249Bk target to confirm the synthesis of tennessine (thus changing the projectiles to 48Ca).[16] This reaction between 249Bk and 50Ti was predicted to be the most favorable practical reaction for formation of ununennium,[14] as it is rather asymmetrical,[4] though also somewhat cold.[16] (The reaction between 254Es and 48Ca would be superior, but preparing milligram quantities of 254Es for a target is difficult.)[4] Nevertheless, the necessary change from the "silver bullet" 48Ca to 50Ti divides the expected yield of ununennium by about twenty, as the yield is strongly dependent on the asymmetry of the fusion reaction.[4]
Due to the predicted short half-lives, the GSI team used new "fast" electronics capable of registering decay events within microseconds.[14] No ununennium atoms were identified, implying a limiting cross section of 65 fb.[16][17] The predicted actual cross section is around 40 fb, which is at the limits of current technology.[4] (The record lowest cross section of an experimentally successful reaction is 30 fb for the reaction between 209Bi and 70Zn producing nihonium.)[4]
Present
The team at RIKEN in Wakō, Japan began bombarding curium-248 targets with a vanadium-51 beam in June 2018[18] to search for element 119. Curium was chosen as a target, rather than heavier berkelium or californium, as these heavier targets are difficult to prepare.[19] The reduced asymmetry of the reaction is expected to approximately halve the cross section, requiring a sensitivity "on the order of at least 30 fb".[17] The 248Cm targets were provided by Oak Ridge National Laboratory, which had provided the necessary 249Bk target from the synthesis of tennessine (element 117) at Dubna. The RIKEN experiment began by being conducted at a cyclotron while it upgrades its linear accelerators, which resume operation in 2020. Bombardment may be continued with both machines until the first event is observed; the experiment is currently running intermittently for at least 100 days per year.[19][20] Hideto En'yo, director of the RIKEN Nishina Centre, predicted that elements 119 and 120 would probably be discovered by 2022.[21] The RIKEN team's efforts are being bankrolled by the Emperor of Japan.[22]
- 248
96Cm
+ 51
23V
→ 299
119Uue
* → 296
119Uue
+ 3 1
0
n - 248
96Cm
+ 51
23V
→ 299
119Uue
* → 295
119Uue
+ 4 1
0
n
The produced isotopes of ununennium are expected to undergo two alpha decays to known isotopes of moscovium (288Mc and 287Mc respectively), which would anchor them to a known sequence of five further alpha decays and corroborate their production. The predicted cross section for these reactions is about 10 fb.[19]
Planned
Following the claimed synthesis of 293Og in 1999 at the Lawrence Berkeley National Laboratory from 208Pb and 86Kr, the analogous reactions 209Bi + 86Kr and 208Pb + 87Rb were proposed for the synthesis of element 119 and its then-unknown alpha decay daughters, elements 117, 115, and 113.[23] The retraction of these results in 2001[24] and more recent calculations on the cross sections for "cold" fusion reactions cast doubt on this possibility; for example, a maximum yield of 2 fb is predicted for the production of 294Uue in the former reaction.[25] Radioactive ion beams may provide an alternative method utilizing a lead or bismuth target, and may enable the production of more neutron-rich isotopes should they become available at required intensities.[25]
The team at the Joint Institute for Nuclear Research in Dubna, Russia planned to begin new experiments on the synthesis of ununennium using the 249Bk + 50Ti reaction using a new experimental complex.[26][27][28][29][30][31] As of November 2019, results were expected in mid-2021 at the earliest.[32]
The laboratories at RIKEN in Japan and at the JINR in Russia are best suited to these experiments as they are the only ones in the world where long beam times are accessible for reactions with such low predicted cross sections.[33]
Naming
Using Mendeleev's nomenclature for unnamed and undiscovered elements, ununennium should be known as eka-francium. Using the 1979 IUPAC recommendations, the element should be temporarily called ununennium (symbol Uue) until it is discovered, the discovery is confirmed, and a permanent name chosen.[34] Although widely used in the chemical community on all levels, from chemistry classrooms to advanced textbooks, the recommendations are mostly ignored among scientists who work theoretically or experimentally on superheavy elements, who call it "element 119", with the symbol E119, (119) or 119.[5]
Predicted properties
Nuclear stability and isotopes


The stability of nuclei decreases greatly with the increase in atomic number after curium, element 96, whose half-life is four orders of magnitude longer than that of any currently known higher-numbered element. All isotopes with an atomic number above 101 undergo radioactive decay with half-lives of less than 30 hours. No elements with atomic numbers above 82 (after lead) have stable isotopes.[36] Nevertheless, for reasons not yet well understood, there is a slight increase of nuclear stability around atomic numbers 110–114, which leads to the appearance of what is known in nuclear physics as the "island of stability". This concept, proposed by University of California professor Glenn Seaborg, explains why superheavy elements last longer than predicted.[37]
The alpha-decay half-lives predicted for 291–307Uue are on the order of microseconds. The longest alpha-decay half-life predicted is ~485 microseconds for the isotope 294Uue.[38][39][40] When factoring in all decay modes, the predicted half-lives drop further to only tens of microseconds.[5][6] Some heavier isotopes may be more stable; Fricke and Waber predicted 315Uue to be the most stable ununennium isotope in 1971.[41] This has consequences for the synthesis of ununennium, as isotopes with half-lives below one microsecond would decay before reaching the detector, and the heavier isotopes cannot be synthesised by the collision of any known usable target and projectile nuclei.[5][6] Nevertheless, new theoretical models show that the expected gap in energy between the proton orbitals 2f7/2 (filled at element 114) and 2f5/2 (filled at element 120) is smaller than expected, so that element 114 no longer appears to be a stable spherical closed nuclear shell, and this energy gap may increase the stability of elements 119 and 120. The next doubly magic nucleus is now expected to be around the spherical 306Ubb (element 122), but the expected low half-life and low production cross section of this nuclide makes its synthesis challenging.[35]
Atomic and physical
Being the first period 8 element, ununennium is predicted to be an alkali metal, taking its place in the periodic table below lithium, sodium, potassium, rubidium, caesium, and francium. Each of these elements has one valence electron in the outermost s-orbital (valence electron configuration ns1), which is easily lost in chemical reactions to form the +1 oxidation state: thus the alkali metals are very reactive elements. Ununennium is predicted to continue the trend and have a valence electron configuration of 8s1. It is therefore expected to behave much like its lighter congeners; however, it is also predicted to differ from the lighter alkali metals in some properties.[5]
The main reason for the predicted differences between ununennium and the other alkali metals is the spin–orbit (SO) interaction—the mutual interaction between the electrons' motion and spin. The SO interaction is especially strong for the superheavy elements because their electrons move faster—at velocities comparable to the speed of light—than those in lighter atoms.[42] In ununennium atoms, it lowers the 7p and 8s electron energy levels, stabilizing the corresponding electrons, but two of the 7p electron energy levels are more stabilized than the other four.[43] The effect is called subshell splitting, as it splits the 7p subshell into more-stabilized and the less-stabilized parts. Computational chemists understand the split as a change of the second (azimuthal) quantum number l from 1 to 1/2 and 3/2 for the more-stabilized and less-stabilized parts of the 7p subshell, respectively.[42][c] Thus, the outer 8s electron of ununennium is stabilized and becomes harder to remove than expected, while the 7p3/2 electrons are correspondingly destabilized, perhaps allowing them to participate in chemical reactions.[5] This stabilization of the outermost s-orbital (already significant in francium) is the key factor affecting ununennium's chemistry, and causes all the trends for atomic and molecular properties of alkali metals to reverse direction after caesium.[44]
 |
 |
 |
Due to the stabilization of its outer 8s electron, ununennium's first ionization energy—the energy required to remove an electron from a neutral atom—is predicted to be 4.53 eV, higher than those of the known alkali metals from potassium onward. This effect is so large that unbiunium (element 121) is predicted to have a lower ionization energy of 4.45 eV, so that the alkali metal in period 8 would not have the lowest ionization energy in the period, as is true for all previous periods.[5] Ununennium's electron affinity is expected to be far greater than that of caesium and francium; indeed, ununennium is expected to have an electron affinity higher than all the alkali metals lighter than it at about 0.662 eV, close to that of cobalt (0.662 eV) and chromium (0.676 eV).[46] Relativistic effects also cause a very large drop in the polarizability of ununennium[5] to 169.7 a.u.[47] Indeed, the static dipole polarisability (αD) of ununennium, a quantity for which the impacts of relativity are proportional to the square of the element's atomic number, has been calculated to be small and similar to that of sodium.[48]
The electron of the hydrogen-like ununennium atom—oxidized so it has only one electron, Uue118+—is predicted to move so quickly that its mass is 1.99 times that of a non-moving electron, a feature coming from the relativistic effects. For comparison, the figure for hydrogen-like francium is 1.29 and the figure for hydrogen-like caesium is 1.091.[42] According to simple extrapolations of relativity laws, that indirectly indicates the contraction of the atomic radius[42] to around 240 pm,[5] very close to that of rubidium (247 pm); the metallic radius is also correspondingly lowered to 260 pm.[5] The ionic radius of Uue+ is expected to be 180 pm.[5]
Ununennium is predicted to have a melting point between 0 °C and 30 °C: thus it may be a liquid at room temperature.[49] It is not known whether this continues the trend of decreasing melting points down the group, as caesium's melting point is 28.5 °C and francium's is estimated to be around 8.0 °C.[50] The boiling point of ununennium is expected to be around 630 °C, similar to that of francium, estimated to be around 620 °C; this is lower than caesium's boiling point of 671 °C.[41][50] The density of ununennium has been variously predicted to be between 3 and 4 g/cm3, continuing the trend of increasing density down the group: the density of francium is estimated at about 2.48 g/cm3, and that of caesium is known to be 1.93 g/cm3.[41][51][50]
Chemical
Bond lengths and bond-dissociation energies of alkali metal dimers. Data for Fr2 and Uue2 is predicted.[52] Compound Bond length (Å) Bond-dissociation energy (kJ/mol) Li2 2.673 101.9 Na2 3.079 72.04 K2 3.924 53.25 Rb2 4.210 47.77 Cs2 4.648 43.66 Fr2 ~ 4.61 ~ 42.1 Uue2 ~ 4.27 ~ 53.4
The chemistry of ununennium is predicted to be similar to that of the alkali metals,[5] but it would probably behave more like potassium[53] or rubidium[5] than caesium or francium. This is due to relativistic effects, as in their absence periodic trends would predict ununennium to be even more reactive than caesium and francium. This lowered reactivity is due to the relativistic stabilization of ununennium's valence electron, increasing ununennium's first ionization energy and decreasing the metallic and ionic radii;[53] this effect is already seen for francium.[5]
The chemistry of ununennium in the +1 oxidation state should be more similar to the chemistry of rubidium than to that of francium. On the other hand, the ionic radius of the Uue+ ion is predicted to be larger than that of Rb+, because the 7p orbitals are destabilized and are thus larger than the p-orbitals of the lower shells. Ununennium may also show the +3 oxidation state,[5] which is not seen in any other alkali metal,[54] in addition to the +1 oxidation state that is characteristic of the other alkali metals and is also the main oxidation state of all the known alkali metals: this is because of the destabilization and expansion of the 7p3/2 spinor, causing its outermost electrons to have a lower ionization energy than what would otherwise be expected.[5][54] Many ununennium compounds are expected to have a large covalent character, due to the involvement of the 7p3/2 electrons in the bonding: this effect is also seen to a lesser extent in francium, which shows some 6p3/2 contribution to the bonding in francium superoxide (FrO2).[42] Thus, instead of ununennium being the most electropositive element, as a simple extrapolation would seem to indicate, caesium instead retains this position, with ununennium's electronegativity most likely being close to sodium's (0.93 on the Pauling scale).[44] The standard reduction potential of the Uue+/Uue couple is predicted to be −2.9 V, the same as that of the Fr+/Fr couple and just over that of the K+/K couple at −2.931 V.[49]
Bond lengths and bond-dissociation energies of MAu (M = an alkali metal). All data is predicted, except for the bond-dissociation energies of KAu, RbAu, and CsAu.[44] Compound Bond length (Å) Bond-dissociation energy (kJ/mol) KAu 2.856 2.75 RbAu 2.967 2.48 CsAu 3.050 2.53 FrAu 3.097 2.75 UueAu 3.074 2.44
In the gas phase, and at very low temperatures in the condensed phase, the alkali metals form covalently bonded diatomic molecules. The metal–metal bond lengths in these M2 molecules increase down the group from Li2 to Cs2, but then decrease after that to Uue2, due to the aforementioned relativistic effects that stabilize the 8s orbital. The opposite trend is shown for the metal–metal bond-dissociation energies. The Uue–Uue bond should be slightly stronger than the K–K bond.[44][52] From these M2 dissociation energies, the enthalpy of sublimation (ΔHsub) of ununennium is predicted to be 94 kJ/mol (the value for francium should be around 77 kJ/mol).[44]
The UueF molecule is expected to have a significant covalent character owing to the high electron affinity of ununennium. The bonding in UueF is predominantly between a 7p orbital on ununennium and a 2p orbital on fluorine, with lesser contributions from the 2s orbital of fluorine and the 8s, 6dz2, and the two other 7p orbitals of ununennium. This is very different from the behaviour of s-block elements, as well as gold and mercury, in which the s-orbitals (sometimes mixed with d-orbitals) are the ones participating in the bonding. The Uue–F bond is relativistically expanded due to the splitting of the 7p orbital into 7p1/2 and 7p3/2 spinors, forcing the bonding electrons into the largest orbital measured by radial extent: a similar expansion in bond length is found in the hydrides AtH and TsH.[55] The Uue–Au bond should be the weakest of all bonds between gold and an alkali metal, but should still be stable. This gives extrapolated medium-sized adsorption enthalpies (−ΔHads) of 106 kJ/mol on gold (the francium value should be 136 kJ/mol), 76 kJ/mol on platinum, and 63 kJ/mol on silver, the smallest of all the alkali metals, that demonstrate that it would be feasible to study the chromatographic adsorption of ununennium onto surfaces made of noble metals.[44] The enthalpy of adsorption of ununennium on a Teflon surface is predicted to be 17.6 kJ/mol, which would be the lowest among the alkali metals: this information would be very useful for future chemistry experiments on ununennium.[47] The ΔHsub and −ΔHads values are not proportionally related for the alkali metals, as they change in opposite directions as atomic number increases.[44]
See also
Notes
- ^ Despite the name, "cold fusion" in the context of superheavy element synthesis is a distinct concept from the idea that nuclear fusion can be achieved in room temperature conditions (see cold fusion).[1]
- ^ Stable isotopes of the lightest elements usually have a neutron–proton ratio close or equal to one (for example, the only stable isotope of aluminium has 13 protons and 14 neutrons,[8] making a neutron–proton ratio of 1.077). However, isotopes of heavier elements have higher neutron–proton ratios, increasing with the number of protons (iodine's only stable isotope has 53 protons and 74 neutrons, neutron–proton ratio of 1.396; gold's only stable isotope has 79 protons and 118 neutrons, neutron–proton ratio of 1.494; plutonium's most stable isotope has 94 protons and 150 neutrons, neutron–proton ratio of 1.596).[8] The trend is expected to continue to the superheavy elements,[9] making it difficult to synthesize their most stable isotopes, because the neutron–proton ratios of the elements they are synthesized from are lower than the expected ratios of the most stable isotopes of the superheavy elements.
- ^ The quantum number corresponds to the letter in the electron orbital name: 0 to s, 1 to p, 2 to d, etc. See azimuthal quantum number for more information.
References
- ^ Fleischmann, Martin; Pons, Stanley (1989). "Electrochemically induced nuclear fusion of deuterium". Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 261 (2): 301–308. doi:10.1016/0022-0728(89)80006-3.
- ^ Barber, Robert C.; Gäggeler, Heinz W.; Karol, Paul J.; Nakahara, Hiromichi; Vardaci, Emanuele; Vogt, Erich (2009). "Discovery of the element with atomic number 112 (IUPAC Technical Report)". Pure and Applied Chemistry. 81 (7): 1331. doi:10.1351/PAC-REP-08-03-05.
- ^ Armbruster, Peter & Munzenberg, Gottfried (1989). "Creating superheavy elements". Scientific American. 34: 36–42.
- ^ a b c d e f g h i Zagrebaev, Valeriy; Karpov, Alexander; Greiner, Walter (2013). "Future of superheavy element research: Which nuclei could be synthesized within the next few years?" (PDF). Journal of Physics. 420 (1): 012001. arXiv:1207.5700. Bibcode:2013JPhCS.420a2001Z. doi:10.1088/1742-6596/420/1/012001. S2CID 55434734.
- ^ a b c d e f g h i j k l m n o p q r s Cite error: The named reference
Hairewas invoked but never defined (see the help page). - ^ a b c Hofmann, Sigurd (2013). Overview and Perspectives of SHE Research at GSI SHIP. p. 23–32. doi:10.1007/978-3-319-00047-3.
- ^ a b Folden III, C. M.; Mayorov, D. A.; Werke, T. A.; Alfonso, M. C.; Bennett, M. E.; DeVanzo, M. J. (2013). "Prospects for the discovery of the next new element: Influence of projectiles with Z > 20". Journal of Physics: Conference Series. 420 (1): 012007. arXiv:1209.0498. Bibcode:2013JPhCS.420a2007F. doi:10.1088/1742-6596/420/1/012007. S2CID 119275964.
- ^ a b Audi, Georges; Bersillon, Olivier; Blachot, Jean; et al. (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729 (1): 3–128. Bibcode:2003NuPhA.729....3A. CiteSeerX 10.1.1.692.8504. doi:10.1016/j.nuclphysa.2003.11.001. Archived from the original (PDF) on 2011-07-20. Retrieved 2010-07-05.
- ^ Karpov, A. V.; Zagrebaev, V. I.; Palenzuela, Y. Martinez; Greiner, Walter (2013). "Superheavy Nuclei: Decay and Stability". Exciting Interdisciplinary Physics. p. 69. doi:10.1007/978-3-319-00047-3_6. ISBN 978-3-319-00046-6. S2CID 55180285.
- ^ "Universal nuclide chart". Nucleonica. Institute for Transuranium Elements. 2007–2012. Retrieved 2012-07-03. (registration required)
- ^ Lougheed, R.; Landrum, J.; Hulet, E.; Wild, J.; Dougan, R.; Dougan, A.; Gäggeler, H.; Schädel, M.; Moody, K. (1985). "Search for superheavy elements using 48Ca + 254Esg reaction". Physical Review C. 32 (5): 1760–1763. Bibcode:1985PhRvC..32.1760L. doi:10.1103/PhysRevC.32.1760. PMID 9953034.
- ^ Feng, Z; Jin, G.; Li, J.; Scheid, W. (2009). "Production of heavy and superheavy nuclei in massive fusion reactions". Nuclear Physics A. 816 (1): 33. arXiv:0803.1117. Bibcode:2009NuPhA.816...33F. doi:10.1016/j.nuclphysa.2008.11.003. S2CID 18647291.
- ^ Modern alchemy: Turning a line, The Economist, May 12, 2012.
- ^ a b c Superheavy Element Search Campaign at TASCA. J. Khuyagbaatar
- ^ "Search for element 119: Christoph E. Düllmann for the TASCA E119 collaboration" (PDF). Archived from the original (PDF) on 2016-03-04. Retrieved 2015-09-15.
- ^ a b c Superheavy Element Research at TASCA. Alexander Yakushev
- ^ a b Khuyagbaatar, J.; Yakushev, A.; Düllmann, Ch. E.; Ackermann, D.; Andersson, L.-L.; et al. (December 2020). "Search for elements 119 and 120" (PDF). Physical Review C. 102 (6). doi:10.1103/PhysRevC.102.064602. Retrieved 25 January 2021.
- ^ Ball, P. (2019). "Extreme chemistry: experiments at the edge of the periodic table". Nature. 565 (7741): 552–555. Bibcode:2019Natur.565..552B. doi:10.1038/d41586-019-00285-9. ISSN 1476-4687. PMID 30700884.
- ^ a b c Sakai, Hideyuki (27 February 2019). "Search for a New Element at RIKEN Nishina Center" (PDF). infn.it. Retrieved 17 December 2019.
- ^ Ball, P. (2019). "Extreme chemistry: experiments at the edge of the periodic table" (PDF). Nature. 565 (7741): 552–555. Bibcode:2019Natur.565..552B. doi:10.1038/d41586-019-00285-9. PMID 30700884. S2CID 59524524.
- ^ "Hunt for element 119 set to begin". Chemistry World. 12 September 2017. Retrieved 9 January 2018.
- ^ Chapman, Kit; Turner, Kristy (13 February 2018). "The hunt is on". Education in Chemistry. Royal Society of Chemistry. Retrieved 28 June 2019.
The hunt for element 113 was almost abandoned because of lack of resources, but this time Japan's emperor is bankrolling Riken's efforts to extend the periodic table to its eighth row.
- ^ Hoffman, D.C; Ghiorso, A.; Seaborg, G.T. (2000). The Transuranium People: The Inside Story. Imperial College Press. ISBN 978-1-86094-087-3.
- ^ Public Affairs Department (21 July 2001). "Results of element 118 experiment retracted". Berkeley Lab. Archived from the original on 29 January 2008. Retrieved 18 January 2008.
- ^ a b Loveland, W. (2007). "Synthesis of transactinide nuclei using radioactive beams" (PDF). Physical Review C. 76 (1): 014612. Bibcode:2007PhRvC..76a4612L. doi:10.1103/PhysRevC.76.014612.
- ^ "Scientists will begin experiments on the synthesis of element 119 in 2019". www.jinr.ru. JINR. 28 September 2016. Retrieved 31 March 2017.
"The discovery of elements 115, 117 and 118 is an accomplished fact; they were placed in the periodic table, though still unnamed and will be confirmed only at the end of the year. The D.I.Mendeleev Periodic Table is not infinite. In 2019, scientists will begin the synthesis of elements 119 and 120 which are the first in the 8th period," said S.N. Dmitriev.
- ^ Dmitriev, Sergey; Itkis, Mikhail; Oganessian, Yuri (2016). Status and perspectives of the Dubna superheavy element factory (PDF). Nobel Symposium NS160 – Chemistry and Physics of Heavy and Superheavy Elements. doi:10.1051/epjconf/201613108001.
- ^ "What it takes to make a new element". Chemistry World. Retrieved 2016-12-03.
- ^ Roberto, J. B. (31 March 2015). "Actinide Targets for Super-Heavy Element Research" (PDF). cyclotron.tamu.edu. Texas A & M University. Retrieved 28 April 2017.
- ^ Morita, Kōsuke (5 February 2016). "The Discovery of Element 113". YouTube. Retrieved 28 April 2017.
- ^ Morimoto, Kouji (2016). "The discovery of element 113 at RIKEN" (PDF). www.physics.adelaide.edu.au. 26th International Nuclear Physics Conference. Retrieved 14 May 2017.
- ^
"Yu. Oganessian commented on synthesis of new elements". JINR. 18 November 2019. Retrieved 16 June 2020.
Academician notes that the target will be completed until the end of 2020, and another 150 days will be spent on experiments with the target for the synthesis of element 119. Thus, results should be awaited not earlier than the middle of 2021.
- ^ Hagino, Kouichi; Hofmann, Sigurd; Miyatake, Hiroari; Nakahara, Hiromichi (2012). "平成23年度 研究業績レビュー(中間レビュー)の実施について" (PDF). www.riken.jp. RIKEN. Archived from the original (PDF) on 4 November 2018. Retrieved 5 May 2017.
- ^ Chatt, J. (1979). "Recommendations for the naming of elements of atomic numbers greater than 100". Pure and Applied Chemistry. 51 (2): 381–384. doi:10.1351/pac197951020381.
- ^ a b Kratz, J. V. (5 September 2011). The Impact of Superheavy Elements on the Chemical and Physical Sciences (PDF). 4th International Conference on the Chemistry and Physics of the Transactinide Elements. Retrieved 27 August 2013.
- ^ de Marcillac, Pierre; Coron, Noël; Dambier, Gérard; et al. (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201. S2CID 4415582.
- ^ Considine, Glenn D.; Kulik, Peter H. (2002). Van Nostrand's scientific encyclopedia (9th ed.). Wiley-Interscience. ISBN 978-0-471-33230-5. OCLC 223349096.
- ^ Chowdhury, P. Roy; Samanta, C. & Basu, D. N. (2007). "Predictions of alpha decay half lives of heavy and superheavy elements". Nucl. Phys. A. 789 (1–4): 142–154. arXiv:nucl-th/0703086. Bibcode:2007NuPhA.789..142S. CiteSeerX 10.1.1.264.8177. doi:10.1016/j.nuclphysa.2007.04.001. S2CID 7496348.
- ^ Chowdhury, P. Roy; Samanta, C. & Basu, D. N. (2008). "Search for long lived heaviest nuclei beyond the valley of stability". Phys. Rev. C. 77 (4): 044603. arXiv:0802.3837. Bibcode:2008PhRvC..77d4603C. doi:10.1103/PhysRevC.77.044603. S2CID 119207807.
- ^ Chowdhury, P. Roy; Samanta, C. & Basu, D. N. (2008). "Nuclear half-lives for α -radioactivity of elements with 100 ≤ Z ≤ 130". Atomic Data and Nuclear Data Tables. 94 (6): 781–806. arXiv:0802.4161. Bibcode:2008ADNDT..94..781C. doi:10.1016/j.adt.2008.01.003.
- ^ a b c Cite error: The named reference
Fricke1971was invoked but never defined (see the help page). - ^ a b c d e Thayer, John S. (2010). "Relativistic Effects and the Chemistry of the Heavier Main Group Elements". In Maria, Barysz; Ishikawa, Yasuyuki (eds.). Relativistic Methods for Chemists. Challenges and Advances in Computational Chemistry and Physics. Vol. 10. Springer Netherlands. pp. 63–7, 81, 84. doi:10.1007/978-1-4020-9975-5_2. ISBN 978-1-4020-9974-8.
- ^ Fægri Jr., Knut; Saue, Trond (2001). "Diatomic molecules between very heavy elements of group 13 and group 17: A study of relativistic effects on bonding". The Journal of Chemical Physics. 115 (6): 2456. Bibcode:2001JChPh.115.2456F. doi:10.1063/1.1385366.
- ^ a b c d e f g Cite error: The named reference
Pershinawas invoked but never defined (see the help page). - ^ a b c Pyykkö, Pekka (2011). "A suggested periodic table up to Z ≤ 172, based on Dirac–Fock calculations on atoms and ions". Physical Chemistry Chemical Physics. 13 (1): 161–8. Bibcode:2011PCCP...13..161P. doi:10.1039/c0cp01575j. PMID 20967377. S2CID 31590563.
- ^ a b Landau, Arie; Eliav, Ephraim; Ishikawa, Yasuyuki; Kador, Uzi (25 May 2001). "Benchmark calculations of electron affinities of the alkali atoms sodium to eka-francium (element 119)". Journal of Chemical Physics. 115 (6): 2389–92. Bibcode:2001JChPh.115.2389L. doi:10.1063/1.1386413. Retrieved 15 September 2015.
- ^ a b Borschevsky, A.; Pershina, V.; Eliav, E.; Kaldor, U. (22 March 2013). "Ab initio studies of atomic properties and experimental behavior of element 119 and its lighter homologs" (PDF). The Journal of Chemical Physics. 138 (12): 124302. Bibcode:2013JChPh.138l4302B. doi:10.1063/1.4795433. PMID 23556718.
- ^ Lim, Ivan S.; Pernpointner, Markus; Seth, Michael; Laerdahl, Jon K.; Schwerdtfeger, Peter; Neogrady, Pavel; Urban, Miroslav (1 October 1999). "Relativistic coupled-cluster static dipole polarizabilities of the alkali metals from Li to element 119". Physical Review A. 60 (4): 2822. Bibcode:1999PhRvA..60.2822L. doi:10.1103/PhysRevA.60.2822.
- ^ a b Cite error: The named reference
BFrickewas invoked but never defined (see the help page). - ^ a b c Lavrukhina, Avgusta Konstantinovna; Pozdnyakov, Aleksandr Aleksandrovich (1970). Analytical Chemistry of Technetium, Promethium, Astatine, and Francium. Translated by R. Kondor. Ann Arbor–Humphrey Science Publishers. p. 269. ISBN 978-0-250-39923-9.
- ^ Cite error: The named reference
B&Kwas invoked but never defined (see the help page). - ^ a b Jones, Cameron; Mountford, Philip; Stasch, Andreas; Blake, Matthew P. (22 June 2015). "s-block Metal-Metal Bonds". In Liddle, Stephen T. (ed.). Molecular Metal-Metal Bonds: Compounds, Synthesis, Properties. John Wiley and Sons. pp. 23–24. ISBN 9783527335411.
- ^ a b Seaborg (c. 2006). "transuranium element (chemical element)". Encyclopædia Britannica. Retrieved 2010-03-16.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- ^ Miranda, Patrícia S.; Mendes, Anna Paula S.; Gomes, Jose S.; Alves, Claudio N.; de Souza, Aguinaldo R.; Sambrano, Julio R.; Gargano, Ricardo; de Macedo, Luiz Guilherme M. (2012). "Ab Initio Correlated All Electron Dirac-Fock Calculations for Eka-Francium Fluoride (E119F)". Journal of the Brazilian Chemical Society. 23 (6): 1104–1113. doi:10.1590/S0103-50532012000600015. Retrieved 14 January 2018.
Bibliography
- Beiser, A. (2003). Concepts of modern physics (6th ed.). McGraw-Hill. ISBN 978-0-07-244848-1. OCLC 48965418.
{{cite book}}: CS1 maint: ref duplicates default (link) - Hoffman, D. C.; Ghiorso, A.; Seaborg, G. T. (2000). The Transuranium People: The Inside Story. World Scientific. ISBN 978-1-78-326244-1.
- Kragh, H. (2018). From Transuranic to Superheavy Elements: A Story of Dispute and Creation. Springer. ISBN 978-3-319-75813-8.
{{cite book}}: CS1 maint: ref duplicates default (link) - Zagrebaev, V.; Karpov, A.; Greiner, W. (2013). "Future of superheavy element research: Which nuclei could be synthesized within the next few years?". Journal of Physics: Conference Series. 420 (1): 012001. arXiv:1207.5700. Bibcode:2013JPhCS.420a2001Z. doi:10.1088/1742-6596/420/1/012001. ISSN 1742-6588.
External links
 The dictionary definition of ununennium at Wiktionary
The dictionary definition of ununennium at Wiktionary

