Hepatitis B virus
| Hepatitis B virus | |
|---|---|

| |
| Transmission electron microscopy micrograph showing Hepatitis B virus virions | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Pararnavirae |
| Phylum: | Artverviricota |
| Class: | Revtraviricetes |
| Order: | Blubervirales |
| Family: | Hepadnaviridae |
| Genus: | Orthohepadnavirus |
| Species: | Hepatitis B virus
|
Hepatitis B virus (HBV), is a partially double-stranded DNA virus,[1] a species of the genus Orthohepadnavirus and a member of the Hepadnaviridae family of viruses.[2] This virus causes the disease hepatitis B.[3]
Disease
In addition to causing hepatitis, infection with HBV can lead to cirrhosis and hepatocellular carcinoma.[4]
It has also been suggested that it may increase the risk of pancreatic cancer.[3]
Roles in disease
Viral infection by Hepatitis B virus (HBV) causes many hepatocyte changes due to the direct action of a protein encoded by the virus, HBx, and to indirect changes due to a large increase in intracellular reactive oxygen species (ROS) after infection. HBx appears to dysregulate a number of cellular pathways. HBx causes dysregulation in part by binding to genomic DNA, changing expression patterns of miRNAs, affecting histone methyltransferases, binding to SIRT1 protein to activate transcription, and cooperating with histone methylases and demethylases to change cell expression patterns.[5] HBx is partly responsible for the approximate 10,000-fold increase in intracellular ROS upon chronic HBV infection.[6] Increased ROS can be caused, in part, by localization of HBx to the mitochondria where HBx decreases the mitochondrial membrane potential.[7] In addition, another HBV protein, HBsAg, also increases ROS through interactions with the endoplasmic reticulum.[7]
The increase in reactive oxygen species (ROS) after HBV infection causes inflammation, which leads to a further increase in ROS.[6] ROS cause more than 20 types of DNA damage.[8] Oxidative DNA damage is mutagenic.[9] In addition, repair of the DNA damage can cause epigenetic alterations at the site of the damage during repair of the DNA.[10] Epigenetic alterations and mutations may cause defects in the cellular machinery that then contribute to liver disease. By the time accumulating epigenetic and mutational changes eventually cause progression to cancer, epigenetic alterations appear to have a larger role in this carcinogenesis than mutations. Only one or two genes, TP53[11] and perhaps ARID1A,[12] are mutated in more than 20% of liver cancers while 41 genes each have hypermethylated promoters (repressing gene expression) in more than 20% of liver cancers, with seven of these genes being hypermethylated in more than 75% of liver cancers.[11] In addition to alterations at the sites of DNA repair, epigenetic alterations are also caused by HBx recruiting the DNA methyltransferase enzymes, DNMT1 and/or DNMT3A, to specific gene loci to alter their methylation levels and gene expression.[13] HBx also alters histone acetylation that can affect gene expression.[13]
Several thousand protein-coding genes appear to have HBx-binding sites.[5][14] In addition to protein coding genes, about 15 microRNAs and 16 Long non-coding RNAs are also affected by the binding of HBx to their promoters.[14] Each altered microRNA can affect the expression of several hundred messenger RNAs (see microRNA).
Classification
Hepatitis B virus is classified as the type species of the Orthohepadnavirus, which contains eight other species.[15] The genus is classified as part of the Hepadnaviridae family, which contains one other genus, Avihepadnavirus.[15] This family of viruses have not been assigned to a viral order.[16] Viruses similar to hepatitis B have been found in all apes (orangutans, gibbons, gorillas and chimpanzees), in Old World monkeys,[17] and in New World woolly monkeys (the woolly monkey hepatitis B virus), suggesting an ancient origin for this virus in primates.
The virus is divided into four major serotypes (adr, adw, ayr, ayw) based on antigenic epitopes present on its envelope proteins. These serotypes are based on a common determinant (a) and two mutually exclusive determinant pairs (d/y and w/r). The viral strains have also been divided into ten genotypes (A–J) and forty subgenotypes according to overall nucleotide sequence variation of the genome.[18] The genotypes have a distinct geographical distribution and are used in tracing the evolution and transmission of the virus. Differences between genotypes affect the disease severity, course and likelihood of complications, and response to treatment and possibly vaccination.[19][20] The serotypes and genotypes do not necessarily correspond.
Genotype D has 10 subgenotypes.[21][18]
Unclassified species
A number of as yet unclassified Hepatitis B-like species have been isolated from bats.[22]
Morphology
Structure
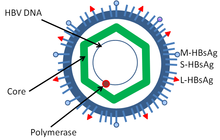
Hepatitis B virus is a member of the Hepadnavirus family.[23] The virus particle, called Dane particle[24] (virion), consists of an outer lipid envelope and an icosahedral nucleocapsid core composed of protein. The nucleocapsid encloses the viral DNA and a DNA polymerase that has reverse transcriptase activity similar to retroviruses.[25] The outer envelope contains embedded proteins which are involved in viral binding of, and entry into, susceptible cells. The virus is one of the smallest enveloped animal viruses with a virion diameter of 42 nm, but pleomorphic forms exist, including filamentous and spherical bodies lacking a core. These particles are not infectious and are composed of the lipid and protein that forms part of the surface of the virion, which is called the surface antigen (HBsAg), and is produced in excess during the life cycle of the virus.[26]
Components
It consists of:
- HBsAg - Hepatitis B surface antigen (HBsAg) was the first hepatitis B virus protein to be discovered.[27] It consists of small (S), medium (M) and large (L) protein.[28]
- HBcAg (HBeAg is a splice variant) - HBcAg is the main structural protein of HBV icosahedral nucleocapsid and it has function in replication of the virus.[29] Capsid formation of HBV antigen (HBcAg) is the main factor for infection of the cell.[30] HBcAg contributes to HBV clearance in vivo, but it is unknown whether HBcAg has to be in the capsid form to contribute to viral clearance.[31]
- Hepatitis B virus DNA polymerase
- HBx. Hepatitis B virus protein HBx is small,[32] 154 amino acid long, nonstructural and has an important role in HBV-associated liver disease and in HBV replication in HepG2 cells. Many activities have been linked to expression of HBx. However, the molecular mechanisms many of these activities are unknown.[33] This protein is multifunctional and it activates cellular signaling pathways and is essential for viral infection.[34]
Hepatitis D virus requires HBV envelope particles to become virulent.[35]
Evolution
The early evolution of the Hepatitis B, like that of all viruses, is difficult to establish.
The divergence of orthohepadnavirus and avihepadnavirus occurred ~125,000 years ago (95% interval 78,297–313,500).[36] Both the Avihepadnavirus and Orthohepadna viruses began to diversify about 25,000 years ago.[36] The branching at this time lead to the emergence of the Orthohepadna genotypes A–H. Human strains have a most recent common ancestor dating back to 7,000 (95% interval: 5,287–9,270) to 10,000 (95% interval: 6,305–16,681) years ago.
The Avihepadnavirus lack a X protein but a vestigial X reading frame is present in the genome of duck hepadnavirus.[37] The X protein may have evolved from a DNA glycosylase.
The rate of nonsynonymous mutations in this virus has been estimated to be about 2×10−5 amino acid replacements per site per year.[38] The mean number of nucleotide substitutions/site/year is ~7.9×10−5.
A second estimate of the origin of this virus suggests a most recent common ancestor of the human strains evolved ~1500 years ago.[39] The most recent common ancestor of the avian strains was placed at 6000 years ago. The mutation rate was estimated to be ~10−6 substitutions/site/year.
Another analysis with a larger data set suggests that Hepatitis B infected humans 33,600 years ago (95% higher posterior density 22,000-47,100 years ago.[40] The estimated substitution rate was 2.2 × 10−6 substitutions/site/year. A significant increase in the population was noted within the last 5,000 years. Cross species infection to orangutans and gibbons occurred within the last 6,100 years.
Examination of sequences in the zebra finch have pushed the origin of this genus back at least to 40 million years ago and possibly to 80 million years ago.[41] Chimpanzee, gorilla, orangutan, and gibbons species cluster with human isolates. Non primate species included the woodchuck hepatitis virus, the ground squirrel hepatitis virus and arctic squirrel hepatitis virus. A number of bat infecting species have also been described. It has been proposed that a New World bat species may be the origin of the primate species.[42]
A study of isolates from the circumpolar Arctic human population has proposed that the ancestor of the subgenotype B5 (the endemic type found in this population) that the ancestral virus originated in Asia about 2000 years ago (95% HPD 900 BC - 830 AD).[43] Coalescence occurred about 1000 AD. This subgenotype spread from Asia initially to Greenland and then spread westward within the last 400 years.
The oldest evidence of hepatitis B infection dates to Bronze Age.[44][45] The evidence was obtained from 4,500-year-old human remains.[45] According to the 2018 study, the viral genomes obtained by shotgun sequencing became the oldest ever recovered from vertebrate samples.[45] It was also found that some ancient hepatitis viral strains still infect humans, while other became extinct.[45] This disproved the belief that hepatitis B originated in the New World and spread to Europe around 16th century.[45]
Genome
Size
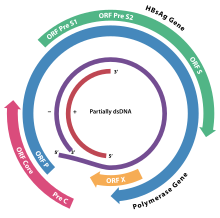
The genome of HBV is made of circular DNA, but it is unusual because the DNA is not fully double-stranded. One end of the full length strand is linked to the viral DNA polymerase. The genome is 3020–3320 nucleotides long (for the full length strand) and 1700–2800 nucleotides long (for the short length strand).[46]
Encoding
The negative-sense, (non-coding) strand is complementary to the viral mRNA. The viral DNA is found in the nucleus soon after infection of the cell. The partially double-stranded DNA is rendered fully double-stranded by completion of the (+) sense strand by cellular DNA polymerases (viral DNA polymerase is used for a later stage) and removal of the viral polymerase protein (P) from the (-) sense strand and a short sequence of RNA from the (+) sense strand. Non-coding bases are removed from the ends of the (-)sense strand and the ends are rejoined.
The viral genes are transcribed by the cellular RNA polymerase II in the cell nucleus from a covalently closed circular DNA (cccDNA) template. Two enhancers designated enhancer I (EnhI) and enhancer II (EnhII) have been identified in the HBV genome. Both enhancers exhibit greater activity in cells of hepatic origin, and together they drive and regulate the expression of the complete viral transcripts.[47][48][49] There are four known genes encoded by the genome called C, P, S, and X. The core protein is coded for by gene C (HBcAg), and its start codon is preceded by an upstream in-frame AUG start codon from which the pre-core protein is produced. HBeAg is produced by proteolytic processing of the pre-core protein. The DNA polymerase is encoded by gene P. Gene S is the gene that codes for the surface antigen (HBsAg). The HBsAg gene is one long open reading frame but contains three in frame "start" (ATG) codons that divide the gene into three sections, pre-S1, pre-S2, and S. Because of the multiple start codons, polypeptides of three different sizes called large, middle, and small (pre-S1 + pre-S2 + S, pre-S2 + S, or S) are produced.[50] The function of the protein coded for by gene X is not fully understood,[51] but some evidence suggests that it may function as a transcriptional transactivator.
Several non-coding RNA elements have been identified in the HBV genome. These include: HBV PREalpha, HBV PREbeta and HBV RNA encapsidation signal epsilon.[52][53]
Genotypes
Genotypes differ by at least 8% of the sequence and have distinct geographical distributions and this has been associated with anthropological history. Within genotypes subtypes have been described: these differ by 4–8% of the genome.
There are eight known genotypes labeled A through H.[19]
A possible new "I" genotype has been described,[54] but acceptance of this notation is not universal.[55]
Two further genotypes have since been recognised.[56] The current (2014) listing now runs A though to J. Several subtypes are also recognised.
There are at least 24 subtypes.
Different genotypes may respond to treatment in different ways.[57][58]
- Individual genotypes
Type F which diverges from the other genomes by 14% is the most divergent type known. Type A is prevalent in Europe, Africa and South-east Asia, including the Philippines. Type B and C are predominant in Asia; type D is common in the Mediterranean area, the Middle East and India; type E is localized in sub-Saharan Africa; type F (or H) is restricted to Central and South America. Type G has been found in France and Germany. Genotypes A, D and F are predominant in Brazil and all genotypes occur in the United States with frequencies dependent on ethnicity.
The E and F strains appear to have originated in aboriginal populations of Africa and the New World, respectively.
Type A has two subtypes: Aa (A1) in Africa/Asia and the Philippines and Ae (A2) in Europe/United States.
Type B has two distinct geographical distributions: Bj/B1 ('j'—Japan) and Ba/B2 ('a'—Asia). Type Ba has been further subdivided into four clades (B2–B4).
Type C has two geographically subtypes: Cs (C1) in South-east Asia and Ce (C2) in East Asia. The C subtypes have been divided into five clades (C1–C5). A sixth clade (C6) has been described in the Philippines but only in one isolate to date.[59] Type C1 is associated with Vietnam, Myanmar and Thailand; type C2 with Japan, Korea and China; type C3 with New Caledonia and Polynesia; C4 with Australia; and C5 with the Philippines. A further subtype has been described in Papua, Indonesia.[60]
Type D has been divided into 7 subtypes (D1–D7).
Type F has been subdivided into 4 subtypes (F1–F4). F1 has been further divided into 1a and 1b. In Venezuela subtypes F1, F2, and F3 are found in East and West Amerindians. Among South Amerindians only F3 was found. Subtypes Ia, III, and IV exhibit a restricted geographic distribution (Central America, the North and the South of South America respectively) while clades Ib and II are found in all the Americas except in the Northern South America and North America respectively.
Life cycle
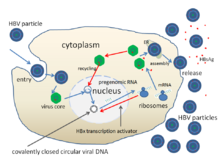
The life cycle of Hepatitis B virus is complex. Hepatitis B is one of a few known non-retroviral viruses which use reverse transcription as a part of its replication process.
- Attachment
- The virus gains entry into the cell by binding to receptors on the surface of the cell and entering it by endocytosis mediated by either clathrin or caveolin-1.[61] HBV initially binds to heparin sulfate proteoglycan. The pre-S1 segment of the HBV L protein then binds tightly to the cell surface receptor sodium taurocolate cotransporting polypeptide (NTCP), encoded by the SLC10A1gene.[62] NTCP is mostly found in the sinusoidal membrane of liver cells. The presence of NTCP in liver cells correlates with the tissue specificity of HBV infection.[61]
- Penetration
- Following endocytosis, the virus membrane fuses with the host cell's membrane, releasing the nucleocapsid into the cytoplasm.[63]
- Uncoating
- Because the virus multiplies via RNA made by a host enzyme, the viral genomic DNA has to be transferred to the cell nucleus. It is thought the capsid is transported on the microtubules to the nuclear pore. The core proteins dissociate from the partially double stranded viral DNA, which is then made fully double stranded (by host DNA polymerases) and transformed into covalently closed circular DNA (cccDNA) that serves as a template for transcription of four viral mRNAs.
- Replication
- The largest mRNA, (which is longer than the viral genome), is used to make the new copies of the genome and to make the capsid core protein and the viral RNA-dependent-DNA-polymerase.
- Assembly
- These four viral transcripts undergo additional processing and go on to form progeny virions which are released from the cell or returned to the nucleus and re-cycled to produce even more copies.[50][64]
- Release
- The long mRNA is then transported back to the cytoplasm where the virion P protein synthesizes DNA via its reverse transcriptase activity.
Transactivated genes
HBV has the ability to transactivate FAM46A.[65]
See also
- Hepatitis B vaccine
- Nucleoside analogues
- Oncovirus (cancer virus)
References
- ^ Ryu, Wang-Shick (2017). Molecular Virology of Human Pathogenic Viruses. Academic Press. pp. 247–260. ISBN 978-0-12-800838-6.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Hunt, Richard (21 November 2007). "Hepatitis viruses". University of Southern California, Department of Pathology and Microbiology. Retrieved 13 March 2008.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Hassan MM, Li D, El-Deeb AS, Wolff RA, Bondy ML, Davila M, Abbruzzese JL (October 2008). "Association between hepatitis B virus and pancreatic cancer". Journal of Clinical Oncology. 26 (28): 4557–62. doi:10.1200/JCO.2008.17.3526. PMC 2562875. PMID 18824707.
- ^ Schwalbe M, Ohlenschläger O, Marchanka A, Ramachandran R, Häfner S, Heise T, Görlach M (March 2008). "Solution structure of stem-loop alpha of the hepatitis B virus post-transcriptional regulatory element". Nucleic Acids Research. 36 (5): 1681–9. doi:10.1093/nar/gkn006. PMC 2275152. PMID 18263618.
- ^ a b Balakrishnan L, Milavetz B (November 2017). "Epigenetic Regulation of Viral Biological Processes". Viruses. 9 (11): 346. doi:10.3390/v9110346. PMC 5707553. PMID 29149060.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Ivanov AV, Valuev-Elliston VT, Tyurina DA, Ivanova ON, Kochetkov SN, Bartosch B, Isaguliants MG (January 2017). "Oxidative stress, a trigger of hepatitis C and B virus-induced liver carcinogenesis". Oncotarget. 8 (3): 3895–3932. doi:10.18632/oncotarget.13904. PMC 5354803. PMID 27965466.
- ^ a b Higgs MR, Chouteau P, Lerat H (May 2014). "'Liver let die': oxidative DNA damage and hepatotropic viruses" (PDF). The Journal of General Virology. 95 (Pt 5): 991–1004. doi:10.1099/vir.0.059485-0. PMID 24496828.
- ^ Yu Y, Cui Y, Niedernhofer LJ, Wang Y (December 2016). "Occurrence, Biological Consequences, and Human Health Relevance of Oxidative Stress-Induced DNA Damage". Chemical Research in Toxicology. 29 (12): 2008–2039. doi:10.1021/acs.chemrestox.6b00265. PMC 5614522. PMID 27989142.
- ^ Dizdaroglu M (December 2012). "Oxidatively induced DNA damage: mechanisms, repair and disease". Cancer Letters. 327 (1–2): 26–47. doi:10.1016/j.canlet.2012.01.016. PMID 22293091.
- ^ Nishida N, Kudo M (2013). "Oxidative stress and epigenetic instability in human hepatocarcinogenesis". Digestive Diseases. 31 (5–6): 447–53. doi:10.1159/000355243. PMID 24281019.
- ^ a b Ozen C, Yildiz G, Dagcan AT, Cevik D, Ors A, Keles U, et al. (May 2013). "Genetics and epigenetics of liver cancer". New Biotechnology. 30 (4): 381–4. doi:10.1016/j.nbt.2013.01.007. hdl:11693/20956. PMID 23392071.
- ^ Shibata T, Aburatani H (June 2014). "Exploration of liver cancer genomes". Nature Reviews. Gastroenterology & Hepatology. 11 (6): 340–9. doi:10.1038/nrgastro.2014.6. PMID 24473361.
- ^ a b Tian Y, Yang W, Song J, Wu Y, Ni B (August 2013). "Hepatitis B virus X protein-induced aberrant epigenetic modifications contributing to human hepatocellular carcinoma pathogenesis". Molecular and Cellular Biology. 33 (15): 2810–6. doi:10.1128/MCB.00205-13. PMC 3719687. PMID 23716588.
- ^ a b Guerrieri F, Belloni L, D'Andrea D, Pediconi N, Le Pera L, Testoni B, et al. (February 2017). "Genome-wide identification of direct HBx genomic targets". BMC Genomics. 18 (1): 184. doi:10.1186/s12864-017-3561-5. PMC 5316204. PMID 28212627.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b "Virus Taxonomy: 2018b Release". International Committee on Taxonomy of Viruses (ICTV). February 2019. Retrieved 14 March 2019.
- ^ Mason, W.S.; et al. (8 July 2008). "00.030. Hepadnaviridae". ICTVdB Index of Viruses. International Committee on Taxonomy of Viruses. Retrieved 13 March 2009.
- ^ Dupinay T, et al. (November 2013). "Discovery of naturally occurring transmissible chronic hepatitis B virus infection among Macaca fascicularis from Mauritius Island". Hepatology. Vol. 58, no. 5. pp. 1610–1620. doi:10.1002/hep.26428. PMID 23536484.
- ^ a b Hundie GB, Stalin Raj V, Gebre Michael D, Pas SD, Koopmans MP, Osterhaus AD, et al. (February 2017). "A novel hepatitis B virus subgenotype D10 circulating in Ethiopia". Journal of Viral Hepatitis. 24 (2): 163–173. doi:10.1111/jvh.12631. PMID 27808472.
- ^ a b Kramvis A, Kew M, François G (March 2005). "Hepatitis B virus genotypes". Vaccine. 23 (19): 2409–23. doi:10.1016/j.vaccine.2004.10.045. PMID 15752827.
- ^ Magnius LO, Norder H (1995). "Subtypes, genotypes and molecular epidemiology of the hepatitis B virus as reflected by sequence variability of the S-gene". Intervirology. 38 (1–2): 24–34. doi:10.1159/000150411. PMID 8666521.
- ^ Ghosh S, Banerjee P, Deny P, Mondal RK, Nandi M, Roychoudhury A, et al. (March 2013). "New HBV subgenotype D9, a novel D/C recombinant, identified in patients with chronic HBeAg-negative infection in Eastern India". Journal of Viral Hepatitis. 20 (3): 209–18. doi:10.1111/j.1365-2893.2012.01655.x. PMID 23383660.
- ^ Drexler JF, Geipel A, König A, Corman VM, van Riel D, Leijten LM, et al. (October 2013). "Bats carry pathogenic hepadnaviruses antigenically related to hepatitis B virus and capable of infecting human hepatocytes". Proceedings of the National Academy of Sciences of the United States of America. 110 (40): 16151–6. Bibcode:2013PNAS..11016151D. doi:10.1073/pnas.1308049110. PMC 3791787. PMID 24043818.
- ^ Zuckerman AJ (1996). "Chapter 70: Hepatitis Viruses". In Baron S; et al. (eds.). Baron's Medical Microbiology (4th ed.). Univ of Texas Medical Branch. ISBN 978-0-9631172-1-2. Retrieved 11 April 2018.
- ^ "WHO | Hepatitis B". www.who.int. Archived from the original on 10 July 2015. Retrieved 12 July 2015.
- ^ Locarnini S (2004). "Molecular virology of hepatitis B virus". Seminars in Liver Disease. 24 Suppl 1 (Suppl 1): 3–10. CiteSeerX 10.1.1.618.7033. doi:10.1055/s-2004-828672. PMID 15192795.
- ^ Howard CR (July 1986). "The biology of hepadnaviruses". The Journal of General Virology. 67 (7): 1215–35. doi:10.1099/0022-1317-67-7-1215. PMID 3014045.
- ^ Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, et al. (April 2010). "Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective". Journal of Hepatology. 52 (4): 514–22. doi:10.1016/j.jhep.2010.01.014. PMID 20207438.
- ^ Seeger C, Mason WS (March 2000). "Hepatitis B virus biology". Microbiology and Molecular Biology Reviews. 64 (1): 51–68. doi:10.1128/mmbr.64.1.51-68.2000. PMC 98986. PMID 10704474.
- ^ Lin YJ, Wu HL, Chen DS, Chen PJ (September 2012). "Hepatitis B virus nucleocapsid but not free core antigen controls viral clearance in mice". Journal of Virology. 86 (17): 9266–73. doi:10.1128/JVI.00608-12. PMC 3416136. PMID 22718814.
- ^ Lin YJ, Huang LR, Yang HC, Tzeng HT, Hsu PN, Wu HL, et al. (May 2010). "Hepatitis B virus core antigen determines viral persistence in a C57BL/6 mouse model". Proceedings of the National Academy of Sciences of the United States of America. 107 (20): 9340–5. doi:10.1073/pnas.1004762107. PMC 2889105. PMID 20439715.
- ^ Bourne CR, Katen SP, Fulz MR, Packianathan C, Zlotnick A (March 2009). "A mutant hepatitis B virus core protein mimics inhibitors of icosahedral capsid self-assembly". Biochemistry. 48 (8): 1736–42. doi:10.1021/bi801814y. PMC 2880625. PMID 19196007.
- ^ Tang H, Oishi N, Kaneko S, Murakami S (October 2006). "Molecular functions and biological roles of hepatitis B virus x protein". Cancer Science. 97 (10): 977–83. doi:10.1111/j.1349-7006.2006.00299.x. PMID 16984372.
- ^ McClain SL, Clippinger AJ, Lizzano R, Bouchard MJ (November 2007). "Hepatitis B virus replication is associated with an HBx-dependent mitochondrion-regulated increase in cytosolic calcium levels". Journal of Virology. 81 (21): 12061–5. doi:10.1128/JVI.00740-07. PMC 2168786. PMID 17699583.
- ^ Bouchard MJ, Puro RJ, Wang L, Schneider RJ (July 2003). "Activation and inhibition of cellular calcium and tyrosine kinase signaling pathways identify targets of the HBx protein involved in hepatitis B virus replication". Journal of Virology. 77 (14): 7713–9. doi:10.1128/JVI.77.14.7713-7719.2003. PMC 161925. PMID 12829810.
- ^ Chai N, Chang HE, Nicolas E, Han Z, Jarnik M, Taylor J (August 2008). "Properties of subviral particles of hepatitis B virus". Journal of Virology. 82 (16): 7812–7. doi:10.1128/JVI.00561-08. PMC 2519590. PMID 18524834.
- ^ a b van Hemert FJ, van de Klundert MA, Lukashov VV, Kootstra NA, Berkhout B, Zaaijer HL (2011). "Protein X of hepatitis B virus: origin and structure similarity with the central domain of DNA glycosylase". PLOS ONE. 6 (8): e23392. Bibcode:2011PLoSO...623392V. doi:10.1371/journal.pone.0023392. PMC 3153941. PMID 21850270.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lin B, Anderson DA (2000). "A vestigial X open reading frame in duck hepatitis B virus". Intervirology. 43 (3): 185–90. doi:10.1159/000025037. PMID 11044813.
- ^ Osiowy C, Giles E, Tanaka Y, Mizokami M, Minuk GY (November 2006). "Molecular evolution of hepatitis B virus over 25 years". Journal of Virology. 80 (21): 10307–14. doi:10.1128/JVI.00996-06. PMC 1641782. PMID 17041211.
- ^ Zhou Y, Holmes EC (August 2007). "Bayesian estimates of the evolutionary rate and age of hepatitis B virus". Journal of Molecular Evolution. 65 (2): 197–205. Bibcode:2007JMolE..65..197Z. doi:10.1007/s00239-007-0054-1. PMID 17684696.
- ^ Paraskevis D, Magiorkinis G, Magiorkinis E, Ho SY, Belshaw R, Allain JP, Hatzakis A (March 2013). "Dating the origin and dispersal of hepatitis B virus infection in humans and primates". Hepatology. 57 (3): 908–16. doi:10.1002/hep.26079. PMID 22987324.
- ^ Littlejohn M, Locarnini S, Yuen L (January 2016). "Origins and Evolution of Hepatitis B Virus and Hepatitis D Virus". Cold Spring Harbor Perspectives in Medicine. 6 (1): a021360. doi:10.1101/cshperspect.a021360. PMC 4691804. PMID 26729756.
- ^ Rasche A, Souza BF, Drexler JF (February 2016). "Bat hepadnaviruses and the origins of primate hepatitis B viruses". Current Opinion in Virology. 16: 86–94. doi:10.1016/j.coviro.2016.01.015. PMID 26897577.
- ^ Bouckaert R, Simons BC, Krarup H, Friesen TM, Osiowy C (2017). "Tracing hepatitis B virus (HBV) genotype B5 (formerly B6) evolutionary history in the circumpolar Arctic through phylogeographic modelling". PeerJ. 5: e3757. doi:10.7717/peerj.3757. PMC 5581946. PMID 28875087.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Mühlemann B, Jones TC, Damgaard PB, Allentoft ME, Shevnina I, Logvin A, et al. (May 2018). "Ancient hepatitis B viruses from the Bronze Age to the Medieval period". Nature. 557 (7705): 418–423. Bibcode:2018Natur.557..418M. doi:10.1038/s41586-018-0097-z. PMID 29743673.
- ^ a b c d e Ben Guarino (9 May 2018). "New strains of hepatitis B virus discovered in ancient human remains". The Washington Post. Retrieved 9 January 2018.
- ^ Kay A, Zoulim F (August 2007). "Hepatitis B virus genetic variability and evolution". Virus Research. 127 (2): 164–76. doi:10.1016/j.virusres.2007.02.021. PMID 17383765.
- ^ Doitsh G, Shaul Y (February 2004). "Enhancer I predominance in hepatitis B virus gene expression". Molecular and Cellular Biology. 24 (4): 1799–808. doi:10.1128/mcb.24.4.1799-1808.2004. PMC 344184. PMID 14749394.
- ^ Antonucci TK, Rutter WJ (February 1989). "Hepatitis B virus (HBV) promoters are regulated by the HBV enhancer in a tissue-specific manner". Journal of Virology. 63 (2): 579–83. PMC 247726. PMID 2536093.
- ^ Huan B, Siddiqui A (1993). "Regulation of hepatitis B virus gene expression". Journal of Hepatology. 17 Suppl 3: S20-3. doi:10.1016/s0168-8278(05)80419-2. PMID 8509635.
- ^ a b Beck J, Nassal M (January 2007). "Hepatitis B virus replication". World Journal of Gastroenterology. 13 (1): 48–64. doi:10.3748/wjg.v13.i1.48. PMC 4065876. PMID 17206754.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Bouchard MJ, Schneider RJ (December 2004). "The enigmatic X gene of hepatitis B virus". Journal of Virology. 78 (23): 12725–34. doi:10.1128/JVI.78.23.12725-12734.2004. PMC 524990. PMID 15542625.
- ^ Smith GJ, Donello JE, Lück R, Steger G, Hope TJ (November 1998). "The hepatitis B virus post-transcriptional regulatory element contains two conserved RNA stem-loops which are required for function". Nucleic Acids Research. 26 (21): 4818–27. doi:10.1093/nar/26.21.4818. PMC 147918. PMID 9776740.
- ^ Flodell S, Schleucher J, Cromsigt J, Ippel H, Kidd-Ljunggren K, Wijmenga S (November 2002). "The apical stem-loop of the hepatitis B virus encapsidation signal folds into a stable tri-loop with two underlying pyrimidine bulges". Nucleic Acids Research. 30 (21): 4803–11. doi:10.1093/nar/gkf603. PMC 135823. PMID 12409471.
- ^ Olinger CM, Jutavijittum P, Hübschen JM, Yousukh A, Samountry B, Thammavong T, et al. (November 2008). "Possible new hepatitis B virus genotype, southeast Asia". Emerging Infectious Diseases. 14 (11): 1777–80. doi:10.3201/eid1411.080437. PMC 2630741. PMID 18976569.
- ^ Kurbanov F, Tanaka Y, Kramvis A, Simmonds P, Mizokami M (August 2008). "When should "I" consider a new hepatitis B virus genotype?". Journal of Virology. 82 (16): 8241–2. doi:10.1128/JVI.00793-08. PMC 2519592. PMID 18663008.
- ^ Hernández S, Venegas M, Brahm J, Villanueva RA (October 2014). "Full-genome sequence of a hepatitis B virus genotype f1b clone from a chronically infected chilean patient". Genome Announcements. 2 (5): e01075–14. doi:10.1128/genomeA.01075-14. PMC 4208329. PMID 25342685.
- ^ Palumbo E (2007). "Hepatitis B genotypes and response to antiviral therapy: a review". American Journal of Therapeutics. 14 (3): 306–9. doi:10.1097/01.pap.0000249927.67907.eb. PMID 17515708.
- ^ Mahtab MA, Rahman S, Khan M, Karim F (October 2008). "Hepatitis B virus genotypes: an overview". Hepatobiliary & Pancreatic Diseases International. 7 (5): 457–64. PMID 18842489.
- ^ Cavinta L, Sun J, May A, Yin J, von Meltzer M, Radtke M, et al. (June 2009). "A new isolate of hepatitis B virus from the Philippines possibly representing a new subgenotype C6". Journal of Medical Virology. 81 (6): 983–7. doi:10.1002/jmv.21475. PMID 19382274.
- ^ Lusida MI, Nugrahaputra VE, Handajani R, Nagano-Fujii M, Sasayama M, Utsumi T, Hotta H (July 2008). "Novel subgenotypes of hepatitis B virus genotypes C and D in Papua, Indonesia". Journal of Clinical Microbiology. 46 (7): 2160–6. doi:10.1128/JCM.01681-07. PMC 2446895. PMID 18463220.
- ^ a b Zhang Z, Zehnder B, Damrau C, Urban S (July 2016). "Visualization of hepatitis B virus entry - novel tools and approaches to directly follow virus entry into hepatocytes". FEBS Letters. 590 (13): 1915–26. doi:10.1002/1873-3468.12202. PMID 27149321.
- ^ Yan H, Liu Y, Sui J, Li W (September 2015). "NTCP opens the door for hepatitis B virus infection". Antiviral Research. 121: 24–30. doi:10.1016/j.antiviral.2015.06.002. PMID 26071008.
- ^ Watashi K, Wakita T (August 2015). "Hepatitis B Virus and Hepatitis D Virus Entry, Species Specificity, and Tissue Tropism". Cold Spring Harbor Perspectives in Medicine. 5 (8): a021378. doi:10.1101/cshperspect.a021378. PMC 4526719. PMID 26238794.
- ^ Bruss V (January 2007). "Hepatitis B virus morphogenesis". World Journal of Gastroenterology. 13 (1): 65–73. doi:10.3748/wjg.v13.i1.65. PMC 4065877. PMID 17206755.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Fam46A (Protein Coding)". GeneCards. GeneCards. Retrieved 18 February 2015.
