Human papillomavirus infection
| Human papillomavirus infection | |
|---|---|
| Other names | Human papillomavirus |
| Specialty | Infectious diseases |
Human papillomavirus infection is an infection by human papillomavirus (HPV).[1] Most HPV infections cause no symptoms and resolve spontaneously.[2] In some, they persist and result in warts or precancerous lesions.[3] The precancerous lesions increase the risk of cancer of the cervix, vulva, vagina, penis, anus, mouth, or throat.[2][3] Nearly all cervical cancer is due to HPV with two types, HPV16 and HPV18, accounting for 70% of cases.[2][4] Between 60 and 90% of the other cancers are also linked to HPV.[4] HPV6 and HPV11 are common causes of genital warts and respiratory papillomatosis.[2]
HPV infection is caused by a human papillomavirus, a DNA virus from the papillomavirus family, of which over 150 types are known.[1][5] More than 40 types are transmitted through sexual contact and infect the anus and genitals.[5] Risk factors for persistent HPV infections include early age of first sexual intercourse, multiple partners, smoking, and poor immune function.[2] HPV is typically spread by sustained direct skin-to-skin contact with vaginal and anal sex being the most common methods.[5] Occasionally, it can spread from a mother to her baby during pregnancy. It does not spread via common items like toilet seats. People can become infected with more than one type of HPV.[6] HPV only affects humans.[1]
HPV vaccines can prevent the most common types of infection.[5] To be effective, they must be used before an infection occurs and are therefore recommended between the ages of nine and 13. Cervical cancer screening, such as with the Papanicolaou test (pap) or looking at the cervix after using acetic acid, can detect early cancer or abnormal cells that may develop into cancer. This allows for early treatment which results in better outcomes.[2] Screening has reduced both the number and deaths from cervical cancer in the developed world.[7] Warts can be removed by freezing.[1]
HPV is the most common sexually transmitted infection globally.[1] Most people are infected at some point in their lives.[5] In 2012, about 528,000 new cases and 266,000 deaths occurred from cervical cancer worldwide.[8] Around 85% of these occurred in the developing world.[2] In the United States, about 27,000 cases of cancer due to HPV occur each year. About 1% of sexually active adults have genital warts.[6] While cases of warts have been described since the time of ancient Greece, their viral nature was discovered in 1907.[9]
Signs and symptoms

Over 170 types of HPV have been identified, and they are designated by numbers.[11][12][13]
Some HPV types, such as HPV-5, may establish infections that persist for the lifetime of the individual without ever manifesting any clinical symptoms. HPV types 1 and 2 can cause common warts in some infected individuals.[citation needed] HPV types 6 and 11 can cause genital warts and respiratory papillomatosis.[2] HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82 are considered carcinogenic.[14]
This table lists common symptoms of HPV infection and associated strains of HPV:
| Disease | HPV type |
|---|---|
| Common warts | 2, 7, 22 |
| Plantar warts | 1, 2, 4, 63 |
| Flat warts | 3, 10, 8 |
| Anogenital warts | 6, 11, 42, 44 and others[15] |
| Anal dysplasia (lesions) | 6, 16, 18, 31, 53, 58[16] |
| Genital cancers | |
| Epidermodysplasia verruciformis | more than 15 types |
| Focal epithelial hyperplasia (mouth) | 13, 32 |
| Mouth papillomas | 6, 7, 11, 16, 32 |
| Oropharyngeal cancer | 16 |
| Verrucous cyst | 60 |
| Laryngeal papillomatosis | 6, 11 |
Warts

Skin infection ("cutaneous" infection) with HPV is very widespread.[18] Skin infections with HPV can cause noncancerous skin growths called warts (verrucae). Warts are caused by a rapid growth of cells on the outer layer of the skin.[19] While cases of warts have been described since the time of ancient Greece, their viral etiology was not known until 1907.[9]
Skin warts are most common in childhood and typically appear and regress spontaneously over the course of weeks to months. About 10% of adults also suffer from recurring skin warts.[citation needed] All HPVs are believed to be capable of establishing long-term "latent" infections in small numbers of stem cells present in the skin. Although these latent infections may never be fully eradicated, immunological control is thought to block the appearance of symptoms such as warts. Immunological control is HPV type-specific, meaning an individual may become resistant to one HPV type while remaining susceptible to other types. In one study, infection by HPV types 2, 27, and 57 was found in people with warts, while infection by HPV types 1, 2, 63, and 27 was found in people with clinically normal skin.[20]
Types of warts include:
- Common warts are usually found on the hands and feet, but can also occur in other areas, such as the elbows or knees. Common warts have a characteristic cauliflower-like surface and are typically slightly raised above the surrounding skin. Cutaneous HPV types can cause genital warts but are not associated with the development of cancer.
- Plantar warts are found on the soles of the feet; they grow inward, generally causing pain when walking.
- Subungual or periungual warts form under the fingernail (subungual), around the fingernail, or on the cuticle (periungual). They are more difficult to treat than warts in other locations.[21]
- Flat warts are most commonly found on the arms, face, or forehead. Like common warts, flat warts occur most frequently in children and teens. In people with normal immune function, flat warts are not associated with the development of cancer.[22]
Genital warts are quite contagious, while common, flat, and plantar warts are much less likely to spread from person to person.
Genital warts
HPV infection of the skin in the genital area is the most common sexually transmitted infection worldwide.[8] Such infections are associated with genital or anal warts (medically known as condylomata acuminata or venereal warts), and these warts are the most easily recognized sign of genital HPV infection.
The strains of HPV that can cause genital warts are usually different from those that cause warts on other parts of the body, such as the hands or feet, or even the inner thighs. A wide variety of HPV types can cause genital warts, but types 6 and 11 together account for about 90% of all cases.[23][24] However, in total more than 40 types of HPV are transmitted through sexual contact and can infect the skin of the anus and genitals.[5] Such infections may cause genital warts, although they may also remain asymptomatic.
The great majority of genital HPV infections never cause any overt symptoms and are cleared by the immune system in a matter of months. Moreover, people may transmit the virus to others even if they do not display overt symptoms of infection. Most people acquire genital HPV infections at some point in their lives, and about 10% of women are currently infected.[8] A large increase in the incidence of genital HPV infection occurs at the age when individuals begin to engage in sexual activity. As with cutaneous HPVs, immunity to genital HPV is believed to be specific to a specific strain of HPV.
Respiratory papillomatosis
In addition to genital warts, infection by HPV types 6 and 11 can cause a rare condition known as recurrent respiratory papillomatosis, in which warts form on the larynx[25] or other areas of the respiratory tract.[26][27] These warts can recur frequently, may interfere with breathing, and in extremely rare cases can progress to cancer. For these reasons, repeated surgery to remove the warts may be advisable.[26][28]
Cancer

About a dozen HPV types (including types 16, 18, 31, and 45) are called "high-risk" types because persistent infection has been linked to cancers such as cancer of the vulva, vagina, cervix, penis, and anus. These cancers in common involve sexually transmitted infection of HPV to the stratified epithelial tissue.[29] [2][3]
An estimated 561,200 new cancer cases worldwide (5.2% of all new cancers) were attributable to HPV in 2002, making HPV one of the most important infectious causes of cancer.[29] HPV-associated cancers make up over 5% of total diagnosed cancer cases worldwide, and this incidence is higher in developing countries where it is estimated to cause almost half a million cases each year.[29]
In the United States, about 27,000 cases of cancer due to HPV occur each year.[6]
| Cancer area | Average annual number of cases | HPV attributable (estimated) | HPV 16/18 attributable (estimated) |
|---|---|---|---|
| Cervix | 11,967 | 11,500 | 9,100 |
| Vulva | 3,136 | 1,600 | 1,400 |
| Vagina | 729 | 500 | 400 |
| Penis | 1,046 | 400 | 300 |
| Anus (women) | 3,089 | 2,900 | 2,700 |
| Anus (men) | 1,678 | 1,600 | 1,500 |
| Oropharynx (women) | 2,370 | 1,500 | 1,400 |
| Oropharynx (men) | 9,356 | 5,900 | 5,600 |
| Total (women) | 21,291 | 18,000 | 15,000 |
| Total (men) | 12,080 | 7,900 | 7,600 |
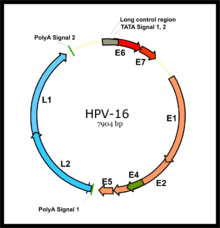
In some infected individuals, their immune systems may fail to control HPV. Lingering infection with high-risk HPV types, such as types 16, 18, 31, and 45, can favor the development of cancer.[31] Co-factors such as cigarette smoke can also enhance the risk of such HPV-related cancers.[32][33]
HPV-induced cancers arise when viral sequences are accidentally integrated into the DNA of host cells. Some of the "early genes" carried by the HPV virus, such as genes E6 and E7, act as oncogenes that promote tumor growth and malignant transformation. Furthermore, HPV can induce a tumorigenic process through integration into a host genome which is associated with alterations in DNA copy number.[34]
E6 produces a protein (also called E6) that binds to and inactivates a protein in the host cell called p53. Normally, p53 acts to prevent cell growth, and promotes cell death in the presence of DNA damage. p53 also upregulates the p21 protein, which blocks the formation of the cyclin D/Cdk4 complex, thereby preventing the phosphorylation of RB, and in turn, halting cell cycle progression by preventing the activation of E2F. In short, p53 is a tumor-suppressor protein that arrests the cell cycle and prevents cell growth and survival when DNA damage occurs. Thus, inactivation of p53 by E6 can promote unregulated cell division, cell growth, and cell survival, characteristics of cancer.
E6 also has a close relationship with the cellular protein E6-associated protein (E6-AP), which is involved in the ubiquitin ligase pathway, a system that acts to degrade proteins. E6-AP binds ubiquitin to the p53 protein, thereby flagging it for proteosomal degradation.
Studies have also shown a link between a wide range of HPV types and squamous cell carcinoma of the skin. In such cases, in vitro studies suggest that the E6 protein of the HPV virus may inhibit apoptosis induced by ultraviolet light.[35]
Cervical cancer
Nearly all cases of cervical cancer are associated with HPV infection, with two types, HPV16 and HPV18, present in 70% of cases.[2][4][36][37][38][39]
HPV type 16 is the most malignant strain, present in 41 to 54% of all cervical cancers,[36][40] and in many cases of vaginal/vulvar cancer,[41] penile cancers, anal cancers, and cancers of the head and neck.[42]
In 2012, about 528,000 new cases and 266,000 deaths from cervical cancer occurred worldwide.[8] Around 85% of these occurred in the developing world.[2]
Most HPV infections of the cervix are cleared rapidly by the immune system and do not progress to cervical cancer (see below the Clearance subsection in Virology). Because the process of transforming normal cervical cells into cancerous ones is slow, cancer occurs in people having been infected with HPV for a long time, usually over a decade or more (persistent infection).[26][43]
Genital cancers
Studies show a link between HPV infection and penile and anal cancers. Sexually transmitted HPVs are found in a large percentage of anal cancers.[29] Moreover, the risk for anal cancer is 17 to 31 times higher among gay and bisexual men than among heterosexual men[44][45] - though one survey did not find a difference between the HPV infection rate of men who had sex with men versus those who had sex only with women.[46]
Anal Pap smear screening for anal cancer might benefit some subpopulations of men or women engaging in anal sex.[47] No consensus exists, though, that such screening is beneficial, or who should get an anal Pap smear.[48][49]
Cancers of the head and neck
High-risk carcinogenic HPV types (including HPV 16 and HPV 18) are associated with an increasing number of head and neck cancers.[38]
Sexually transmitted forms of HPV account for about 25% of cancers of the mouth and upper throat (the oropharynx).[29] The latter commonly present in the tonsil area, and HPV is linked to the increase in oral cancers in nonsmokers.[50][51] Engaging in anal or oral sex with an HPV-infected partner may increase the risk of developing these types of cancers.[52] Oral infection with several types of HPV, in particular type 16, have been found to be associated with HPV-positive oropharyngeal cancer, a form of head and neck cancer.[52][53] This association is independent of tobacco and alcohol use.[53] In the United States, HPV is expected to replace tobacco as the main causal agent for oral cancer, and the number of newly diagnosed, HPV-associated head and neck cancers is expected to surpass that of cervical cancer cases by 2020.[54][55]
In recent years, the United States has experienced an increase in the number of cases of throat cancer caused by HPV type 16. Throat cancers associated with HPV have been estimated to have increased from 0.8 cases per 100,000 people in 1988 to 2.6 per 100,000 in 2004.[56] Researchers explain these recent data by an increase in oral sex. Moreover, findings indicate this type of cancer is much more prevalent in men than in women, something that needs to be further explored.[57] Currently, two immunizations, Gardasil and Cervarix, are recommended to girls to prevent HPV-related cervical cancer, but not as a precaution against HPV-related throat cancer.[58]
The mutational profile of HPV-positive and HPV-negative head and neck cancer has been reported, further demonstrating that they are fundamentally distinct diseases.[59]
Lung cancer
Some evidence links HPV to benign and malignant tumors of the upper respiratory tract. The International Agency for Research on Cancer has found that people with lung cancer were significantly more likely to have several high-risk forms of HPV antibodies compared to those who did not have lung cancer.[60] Researchers looking for HPV among 1,633 lung cancer patients and 2,729 people without the lung disease found that people with lung cancer had more types of HPV than noncancer patients did, and among lung cancer patients, the chances of having eight types of serious HPV were significantly increased.[61] In addition, expression of HPV structural proteins by immunohistochemistry and in vitro studies suggest HPV presence in bronchial cancer and its precursor lesions.[62] Another study detected HPV in the EBC, bronchial brushing and neoplastic lung tissue of cases, and found a presence of an HPV infection in 16.4% of the subjects affected by nonsmall cell lung cancer, but in none of the controls.[63] The reported average frequencies of HPV in lung cancers were 17% and 15% in Europe and the Americas, respectively, and the mean number of HPV in Asian lung cancer samples was 35.7%, with a considerable heterogeneity between certain countries and regions.[64]
Immunocompromised individuals
In very rare cases, HPV may cause epidermodysplasia verruciformis in immunocompromised individuals. The virus, unchecked by the immune system, causes the overproduction of keratin by skin cells, resulting in lesions resembling warts or cutaneous horns.[65]
Cause
Transmission
Risk factors for persistent genital HPV infections include early age of first sexual intercourse, multiple partners, smoking, and immunosuppression.[2] Genital HPV is typically spread by sustained direct skin-to-skin contact, with vaginal and anal sex being the most common method.[5] Occasionally it can spread from a mother to her baby during pregnancy. It does not spread via common items like toilet seats.[6]
Perinatal
Although genital HPV types can be transmitted from mother to child during birth, the appearance of genital HPV-related diseases in newborns is rare. However, the lack of appearance does not rule out asymptomatic latent infection, as the virus has proven to be capable of hiding for decades. Perinatal transmission of HPV types 6 and 11 can result in the development of juvenile-onset recurrent respiratory papillomatosis (JORRP). JORRP is very rare, with rates of about 2 cases per 100,000 children in the United States.[26] Although JORRP rates are substantially higher if a woman presents with genital warts at the time of giving birth, the risk of JORRP in such cases is still less than 1%.
Genital infections
Since cervical and female genital infection by specific HPV types is highly associated with cervical cancer, those types of HPV infection have received most of the attention from scientific studies.
HPV infections in that area are transmitted primarily via sexual activity.[66]
Of the 120 known human papillomaviruses, 51 species and three subtypes infect the genital mucosa.[67] 15 are classified as high-risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82), 3 as probable high-risk (26, 53, and 66), and 12 as low-risk (6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, and CP6108).[68]
If a woman has at least one different partner per year for four years, the probability that she will leave college with an HPV infection is greater than 85%.[69] Condoms do not completely protect from the virus because the areas around the genitals including the inner thigh area are not covered, thus exposing these areas to the infected person’s skin.[69]
Hands
Studies have shown HPV transmission between hands and genitals of the same person and sexual partners. Hernandez tested the genitals and dominant hand of each person in 25 couples every other month for an average of 7 months. She found 2 couples where the man's genitals infected the woman's hand with high-risk HPV, 2 where her hand infected his genitals, 1 where her genitals infected his hand, 2 each where he infected his own hand, and she infected her own hand.[70][71] Hands were not the main source of transmission in these 25 couples, but they were significant.
Partridge reports men's fingertips became positive for high risk HPV at more than half the rate (26% per 2 years) as their genitals (48%).[72] Winer reports 14% of fingertip samples from sexually active women were positive.[73]
Non-sexual hand contact seems to have little or no role in HPV transmission. Winer found all 14 fingertip samples from virgin women negative at the start of her fingertip study.[73] In a separate report on genital HPV infection, 1% of virgin women (1 of 76) with no sexual contact tested positive for HPV, while 10% of virgin women reporting non-penetrative sexual contact were positive (7 of 72).[74]
Shared objects
Sharing of possibly contaminated objects may transmit HPV.[75][76][77] Although possible, transmission by routes other than sexual intercourse is less common for female genital HPV infection.[66] Fingers-genital contact is a possible way of transmission but unlikely to be a significant source.[73][78]
Blood
Though it has traditionally been assumed that HPV is not transmissible via blood—as it is thought to only infect cutaneous and mucosal tissues—recent studies have called this notion into question. Historically, HPV DNA has been detected in the blood of cervical cancer patients.[79] In 2005, a group reported that, in frozen blood samples of 57 sexually naive pediatric patients who had vertical or transfusion-acquired HIV infection, 8 (14.0%) of these samples also tested positive for HPV-16.[80] This seems to indicate that it may be possible for HPV to be transmitted via blood transfusion. However, as non-sexual transmission of HPV by other means is not uncommon, this could not be definitively proven. In 2009, a group tested Australian Red Cross blood samples from 180 healthy male donors for HPV, and subsequently found DNA of one or more strains of the virus in 15 (8.3%) of the samples.[81] However, it is important to note that detecting the presence of HPV DNA in blood is not the same as detecting the virus itself in blood, and whether or not the virus itself can or does reside in blood in infected individuals is still unknown. As such, it remains to be determined whether HPV can or cannot be transmitted via blood.[79] This is of concern, as blood donations are not currently screened for HPV, and at least some organizations such as the American Red Cross and other Red Cross societies do not presently appear to disallow HPV-positive individuals from donating blood.[82]
Surgery
Hospital transmission of HPV, especially to surgical staff, has been documented. Surgeons, including urologists and/or anyone in the room, is subject to HPV infection by inhalation of noxious viral particles during electrocautery or laser ablation of a condyloma (wart).[83] There has been a case report of a laser surgeon who developed extensive laryngeal papillomatosis after providing laser ablation to patients with anogenital condylomata.[83]
Virology
| Human papillomavirus infection | |
|---|---|

| |
| TEM of papillomavirus | |
| Virus classification | |
| Group: | Group I (dsDNA)
|
| Order: | Unranked
|
| Family: | |
| Genera | |
|
Alphapapillomavirus | |
HPV infection is limited to the basal cells of stratified epithelium, the only tissue in which they replicate.[84] The virus cannot bind to live tissue; instead, it infects epithelial tissues through micro-abrasions or other epithelial trauma that exposes segments of the basement membrane.[84] The infectious process is slow, taking 12–24 hours for initiation of transcription. It is believed that involved antibodies play a major neutralizing role while the virions still reside on the basement membrane and cell surfaces.[84]
HPV lesions are thought to arise from the proliferation of infected basal keratinocytes. Infection typically occurs when basal cells in the host are exposed to the infectious virus through a disturbed epithelial barrier as would occur during sexual intercourse or after minor skin abrasions. HPV infections have not been shown to be cytolytic; rather, viral particles are released as a result of degeneration of desquamating cells. HPV can survive for many months and at low temperatures without a host; therefore, an individual with plantar warts can spread the virus by walking barefoot.[24]
HPV is a small DNA virus with a genome of approximately 8000 base pairs.[85] The HPV life cycle strictly follows the differentiation program of the host keratinocyte. It is thought that the HPV virion infects epithelial tissues through micro-abrasions, whereby the virion associates with putative receptors such as alpha integrins and laminins, leading to entry of the virions into basal epithelial cells through clathrin-mediated endocytosis and/or caveolin-mediated endocytosis depending on the type of HPV. At this point, the viral genome is transported to the nucleus by unknown mechanisms and establishes itself at a copy number of 10-200 viral genomes per cell. A sophisticated transcriptional cascade then occurs as the host keratinocyte begins to divide and become increasingly differentiated in the upper layers of the epithelium.
The phylogeny of the various strains of HPV generally reflects the migration patterns of Homo sapiens and suggests that HPV may have diversified along with the human population. Studies suggest that HPV evolved along five major branches that reflect the ethnicity of human hosts, and diversified along with the human population.[86] Researchers have identified two major variants of HPV16, European (HPV16-E), and Non-European (HPV16-NE).[87]
E6/E7 proteins
The two primary oncoproteins of high risk HPV types are E6 and E7. The “E” designation indicates that these two proteins are expressed early in the HPV life cycle, while the "L" designation indicates late expression.[38] The HPV genome is composed of six early (E1, E2, E4, E5, E6, and E7) ORFs, two late (L1 and L2) ORFs, and a non-coding long control region (LCR).[88] After the host cell is infected viral early promoter is activated and a polycistronic primary RNA containing all six early ORFs is transcribed. This polycistronic RNA then undergoes active RNA splicing to generate multiple isoforms of mRNAs.[89] One of the spliced isoform RNAs, E6*I, serves as an E7 mRNA to translate E7 protein.[90] However, viral early transcription subjects to viral E2 regulation and high E2 levels repress the transcription. HPV genomes integrate into host genome by disruption of E2 ORF, preventing E2 repression on E6 and E7. Thus, viral genome integration into host DNA genome increases E6 and E7 expression to promote cellular proliferation and the chance of malignancy. The degree to which E6 and E7 are expressed is correlated with the type of cervical lesion that can ultimately develop.[85]
- Role in cancer
The E6/E7 proteins inactivate two tumor suppressor proteins, p53 (inactivated by E6) and pRb (inactivated by E7).[13] The viral oncogenes E6 and E7[91] are thought to modify the cell cycle so as to retain the differentiating host keratinocyte in a state that is favourable to the amplification of viral genome replication and consequent late gene expression. E6 in association with host E6-associated protein, which has ubiquitin ligase activity, acts to ubiquitinate p53, leading to its proteosomal degradation. E7 (in oncogenic HPVs) acts as the primary transforming protein. E7 competes for retinoblastoma protein (pRb) binding, freeing the transcription factor E2F to transactivate its targets, thus pushing the cell cycle forward. All HPV can induce transient proliferation, but only strains 16 and 18 can immortalize cell lines in vitro. It has also been shown that HPV 16 and 18 cannot immortalize primary rat cells alone; there needs to be activation of the ras oncogene. In the upper layers of the host epithelium, the late genes L1 and L2 are transcribed/translated and serve as structural proteins that encapsidate the amplified viral genomes. Once the genome is encapsidated, the capsid appears to undergo a redox-dependent assembly/maturation event, which is tied to a natural redox gradient that spans both suprabasal and cornified epithelial tissue layers. This assembly/maturation event stabilizes virions, and increases their specific infectivity.[92] Virions can then be sloughed off in the dead squames of the host epithelium and the viral lifecycle continues.[93] A 2010 study has found that E6 and E7 are involved in beta-catenin nuclear accumulation and activation of Wnt signaling in HPV-induced cancers.[94]
Latency period
Once an HPV virion invades a cell, an active infection occurs, and the virus can be transmitted. Several months to years may elapse before squamous intraepithelial lesions (SIL) develop and can be clinically detected. The time from active infection to clinically detectable disease may make it difficult for epidemiologists to establish which partner was the source of infection.[95]
Clearance
Most HPV infections are cleared up by most people without medical action or consequences. The table provides data for high-risk types (i.e. the types found in cancers).
| Months after initial positive test | 8 months | 12 months | 18 months |
|---|---|---|---|
| % of men tested negative[96] | 70% | 80% | 100% |
Clearing an infection does not always create immunity if there is a new or continuing source of infection. Hernandez' 2005-6 study of 25 couples reports "A number of instances indicated apparent reinfection [from partner] after viral clearance."[70]
Diagnosis
There are multiple types of HPV, sometimes called "low-risk" and "high-risk" types. Low-risk types cause warts and high-risk types can cause lesions or cancer.[97]
Health guidelines recommend HPV testing in patients with specific indications including certain abnormal Pap test results.[needs update]
Cervical testing
According to the National Cancer Institute, “The most common test[which?] detects DNA from several high-risk HPV types, but it cannot identify the type(s) that are present. Another test[which?] is specific for DNA from HPV types 16 and 18, the two types that cause most HPV-associated cancers. A third test[which?] can detect DNA from several high-risk HPV types and can indicate whether HPV-16 or HPV-18 is present. A fourth test[which?] detects RNA from the most common high-risk HPV types. These tests can detect HPV infections before cell abnormalities are evident.
“Theoretically, the HPV DNA and RNA tests could be used to identify HPV infections in cells taken from any part of the body. However, the tests are approved by the FDA for only two indications: for follow-up testing of women who seem to have abnormal Pap test results and for cervical cancer screening in combination with a Pap test among women over age 30.” [98]
In April 2011, the Food and Drug Administration approved the cobas HPV Test, manufactured by Roche.[99] This cervical cancer screening test “specifically identifies types HPV 16 and HPV 18 while concurrently detecting the rest of the high risk types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68).”[99]
The cobas HPV Test was evaluated in the ATHENA trial, which studied more than 47,000 U.S. women 21 years old and older undergoing routine cervical cancer screening.[100] Results from the ATHENA trial demonstrated that 1 in 10 women, age 30 and older, who tested positive for HPV 16 and/or 18, actually had cervical pre-cancer even though they showed normal results with the Pap test.[100]
In March 2003, the U.S. Food and Drug Administration (FDA) approved the Hybrid Capture 2 test manufactured by Qiagen/Digene,[101] which is a "hybrid-capture" test[102][103] as an adjunct to Pap testing. The test may be performed during a routine Pap smear. It detects the DNA of 13 "high-risk" HPV types that most commonly affect the cervix, it does not determine the specific HPV types. Hybrid Capture 2 is the most widely studied commercially available HPV assay and the majority of the evidence for HPV primary testing in population-based screening programs is based on the Hybrid Capture 2 assay.[104]
The recent outcomes in the identification of molecular pathways involved in cervical cancer provide helpful information about novel bio- or oncogenic markers that allow monitoring of these essential molecular events in cytological smears, histological, or cytological specimens. These bio- or onco- markers are likely to improve the detection of lesions that have a high risk of progression in both primary screening and triage settings. E6 and E7 mRNA detection PreTect HPV-Proofer, (HPV OncoTect) or p16 cell-cycle protein levels are examples of these new molecular markers. According to published results, these markers, which are highly sensitive and specific, allow to identify cells going through malignant transformation.[105][106]
In October 2011 the US Food and Drug Administration approved the Aptima HPV Assay test for RNA created when and if any HPV strains start creating cancers (see virology).[107][108][109]
The vulva/vagina has been sampled with Dacron swabs and shows more HPV than the cervix. Among women who were HPV positive in either place, 90% were positive in the vulvovaginal region, 46% in the cervix.[74]
Mouth testing
Studies have found heightened HPV in mouth cell samples from people with squamous cell carcinoma of the mouth. Studies have not found significant HPV in mouth cells after sampling with toothbrushes (5 of 2,619 samples)[74] and cytobrushes (no oral transmission found).[70]
Testing men
Research studies have tested for and found HPV, including high-risk types (i.e. the types found in cancers), on fingers, mouth, saliva, anus, urethra, urine, semen, blood, scrotum and penis. However, most research tests have used Dacron swabs and custom analysis not available to the general public.[110][needs update]
A Brazilian study used the readily available Qiagen/Digene test mentioned above (off label) to test men's penis, scrotum and anus.[111] Each of the 50 men had been a partner for at least 6 months of a woman who was positive for high-risk HPV. They found high-risk HPV on 60% of these men, primarily the penis. "The specimens were obtained using a vigorous motion of the conical brush included in the Digene kit after spraying the anogenital region with saline solution."[111][needs update]
A slightly different method also used cytobrushes (but custom lab analysis) and found 37% of 582 Mexican army recruits positive for high risk HPV.[112][needs update] They were told not to wash genitals for 12 hours before sampling. (Other studies are silent on washing, a particular gap in studies of hands). They included the urethra as well as scrotum and penis, but the urethra added less than 1% to the HPV rate. Studies like this led Giuliano to recommend sampling the glans, shaft and crease between them and scrotum, since sampling the urethra or anus added very little to diagnosis.[72] Dunne recommends glans, shaft, their crease, and foreskin.[110]
A small study of cytobrushes on 10 US men where the brush was wet, rather than the skin, found 2 of 10 men were positive for HPV (type not reported).[113][needs update] Their lab analysis was not the same as either study above. This small study found 4 of 10 men positive for HPV when the skin was rubbed with 600 grit emery paper, then swabbed with a wet Dacron swab. Since emery paper and brush were analyzed together at the lab, it is not known if the emery paper collected viruses or loosened them for the swab to collect.
Studies have found collection by men from their own skin (with emery paper and Dacron swabs) as effective as by a clinician, sometimes more so, since patients were more willing to scrape vigorously.[114][needs update][115][116][needs update]
Other studies have used similar cytobrushes to sample fingertips and under the fingernails, though without wetting the area or brush.[73][78][117][needs update]
Other studies analyzed urine, semen, and blood and found varying amounts of HPV,[110] but there is no publicly available test for them.
There is not a wide range of tests even though HPV is common. Clinicians depend on the vaccine among young people and high clearance rates (see Clearance subsection in Virology) to create a low risk of disease and mortality, and treat the cancers when they appear. Others believe that reducing HPV infection in more men and women, even when it has no symptoms, is important (herd immunity) to prevent more cancers rather than just treating them.[118][119][needs update] Where tests are used, negative test results show safety from transmission, and positive test results show where shielding (condoms, gloves) is needed to prevent transmission until the infection clears.[120]
Other testing
Although it is possible to test for HPV DNA in other kinds of infections,[121] there are no FDA-approved tests for general screening in the United States[44] or tests approved by the Canadian government,[122] since the testing is inconclusive and considered medically unnecessary.[123]
Genital warts are the only visible sign of low-risk genital HPV and can be identified with a visual check. These visible growths, however, are the result of non-carcinogenic HPV types. Five percent acetic acid (vinegar) is used to identify both warts and squamous intraepithelial neoplasia (SIL) lesions with limited success[citation needed] by causing abnormal tissue to appear white, but most doctors have found this technique helpful only in moist areas, such as the female genital tract.[citation needed] At this time, HPV test for males are used only in research.[citation needed]
Research into testing for HPV by antibody presence has been done. The approach is looking for an immune response in blood, which would contain antibodies for HPV if the patient is HPV positive.[124][125][126][127] The reliability of such tests hasn't been proven, as there hasn't been a FDA approved product as of March 2014; testing by blood would be a less invasive test for screening purposes.
Prevention
The HPV vaccines can prevent the most common types of infection.[5] To be effective they must be used before an infection occurs and are therefore recommended between the ages of nine and thirteen. Cervical cancer screening, such as with the Papanicolaou test (pap) or looking at the cervix after using acetic acid, can detect early cancer or abnormal cells that may develop into cancer. This allows for early treatment which results in better outcomes.[2] Screening has reduced both the number and deaths from cervical cancer in the developed world.[7] Warts can be removed by freezing.[1]
Methods of reducing the chances of infection include sexual abstinence, condoms, vaccination and microbicides.
Vaccines
Three vaccines are available to prevent infection by some HPV types: Gardasil, Cervarix, and Gardasil 9. Both protect against initial infection with HPV types 16 and 18, which cause most of the HPV-associated cancer cases. Gardasil also protects against HPV types 6 and 11, which cause 90% of genital warts. Gardasil is a recombinant quadrivalent vaccine, whereas Cervarix is bivalent, and is prepared from virus-like particles (VLP) of the L1 capsid protein. Gardasil 9 is nonavalent, it has the potential to prevent about 90% of cervical, vulvar, vaginal, and anal cancers. It can protect for HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. The latter five additional types cause up to 20% of cervical cancers, which were not previously covered beforehand.[128]
The vaccines provide little benefit to women having already been infected with HPV types 16 and 18.[129] For this reason, the vaccine is recommended primarily for those women not yet having been exposed to HPV during sex. The World Health Organization position paper on HPV vaccination clearly outlines appropriate, cost-effective strategies for using HPV vaccine in public sector programs.[130][needs update]
Both vaccines are delivered in three shots over six months. In most countries, they are funded only for female use, but are approved for male use in many countries, and funded for teenage boys in Australia. The vaccine does not have any therapeutic effect on existing HPV infections or cervical lesions.[131] In 2010, 49% of teenage girls in the US got the HPV vaccine.
Following studies suggesting that the vaccine is more effective in younger girls[132] than in older teenagers, the United Kingdom, Switzerland, Mexico, the Netherlands and Quebec began offering the vaccine in a two-dose schedule for girls aged under 15 in 2014.
It remains a recommendation that women continue cervical screening, such as Pap smear testing, even after receiving the vaccine. Cervical cancer screening recommendations have not changed for females who receive HPV vaccine.[131][needs update]
Both men and women are carriers of HPV.[133] The Gardasil vaccine also protects men against anal cancers and warts and genital warts.[134]
Duration of both vaccines' efficacy has been observed since they were first developed, and is expected to be longlasting.[135]
In December 2014, the FDA approved a nine-valent Gardasil-based vaccine, Gardasil 9, to protect against infection with the four strains of HPV covered by the first generation of Gardasil as well as five other strains responsible for 20% of cervical cancers (HPV-31, HPV-33, HPV-45, HPV-52, and HPV-58).[136]
Condoms
The Centers for Disease Control and Prevention says that male "condom use may reduce the risk for genital human papillomavirus (HPV) infection" but provides a lesser degree of protection compared with other sexual transmitted diseases "because HPV also may be transmitted by exposure to areas (e.g., infected skin or mucosal surfaces) that are not covered or protected by the condom."[137]
Female condoms provide somewhat greater protection than male condoms, as the female condom allows for less skin contact.[138]
Studies have suggested that regular condom use can effectively limit the ongoing persistence and spread of HPV to additional genital sites in individuals already infected.[needs update]
Disinfection
The virus is relatively hardy and immune to many common disinfectants. Exposure to 90% ethanol for at least 1 minute, 2% glutaraldehyde, 30% Savlon, and/or 1% sodium hypochlorite can disinfect the pathogen.[139]
The virus is resistant to drying and heat, but killed at 100 °C (212 °F) and by ultraviolet radiation.[139]
Treatment
There is currently no specific treatment for HPV infection.[140][141][142] However, the viral infection, more often than not, clears to undetectable levels by itself.[143] According to the Centers for Disease Control and Prevention, the body's immune system clears HPV naturally within two years for 90% of cases (see Clearance subsection in Virology for more detail).[140] However, experts do not agree on whether the virus is completely eliminated or reduced to undetectable levels, and it is difficult to know when it is contagious.[144]
Follow up care is usually recommended and practiced by many health clinics.[145] Follow-up is sometimes not successful because a portion of those treated do not return to be evaluated. In addition to the normal methods of phone calls and mail, text messaging and email can improve the number of people who return for care.[146]
Epidemiology
Globally HPV is estimated to infect about 12% of women at any given time.[147] HPV infection is the most frequently sexually transmitted disease in the world.[148]
United States
| Age (years) | Prevalence (%) |
|---|---|
| 14 to 19 | 24.5% |
| 20 to 24 | 44.8% |
| 25 to 29 | 27.4% |
| 30 to 39 | 27.5% |
| 40 to 49 | 25.2% |
| 50 to 59 | 19.6% |
| 14 to 59 | 26.8% |
HPV is estimated to be the most common sexually transmitted infection in the United States.[149] Most sexually active men and women will probably acquire genital HPV infection at some point in their lives.[36] The American Social Health Association estimates that about 75–80% of sexually active Americans will be infected with HPV at some point in their lifetime.[150][151] By the age of 50 more than 80% of American women will have contracted at least one strain of genital HPV.[149][152] It was estimated that, in the year 2000, there were approximately 6.2 million new HPV infections among Americans aged 15–44; of these, an estimated 74% occurred to people between ages of 15 and 24.[153] Of the STDs studied, genital HPV was the most commonly acquired.[153] In the United States, it is estimated that 10% of the population has an active HPV infection, 4% has an infection that has caused cytological abnormalities, and an additional 1% has an infection causing genital warts.[154]
Estimates of HPV prevalence vary from 14% to more than 90%.[155] One reason for the difference is that some studies report women who currently have a detectable infection, while other studies report women who have ever had a detectable infection.[156][157] Another cause of discrepancy is the difference in strains that were tested for.
One study found that, during 2003–2004, at any given time, 26.8% of women aged 14 to 59 were infected with at least one type of HPV. This was higher than previous estimates; 15.2% were infected with one or more of the high-risk types that can cause cancer.[149][158]
The prevalence for high-risk and low-risk types is roughly similar over time.[149]
Human papillomavirus is not included among the diseases that are typically reportable to the CDC as of 2011.[159][160]
History
In 1972, the association of the human papillomaviruses with skin cancer in epidermodysplasia verruciformis was proposed by Stefania Jabłońska in Poland. In 1978, Jabłońska and Gerard Orth at the Pasteur Institute discovered HPV-5 in skin cancer.[161][page needed] In 1976 Harald zur Hausen published the hypothesis that human papilloma virus plays an important role in the cause of cervical cancer. In 1983 and 1984 zur Hausen and his collaborators identified HPV16 and HPV18 in cervical cancer.[162]
The HeLa cell line contains extra DNA in its genome that originated from HPV type 18.[163]
Research
- Ludwig-McGill HPV Cohort, large longitudinal study of the natural history of human papillomavirus infection and cervical cancer risk
References
- ^ a b c d e f Milner, Danny A. (2015). Diagnostic Pathology: Infectious Diseases. Elsevier Health Sciences. p. 40. ISBN 9780323400374.
- ^ a b c d e f g h i j k l m "Human papillomavirus (HPV) and cervical cancer". WHO. June 2016. Retrieved 10 August 2016.
- ^ a b c Ljubojevic, Suzana; Skerlev, Mihael (2014). "HPV-associated diseases". Clinics in Dermatology. 32 (2): 227–234. doi:10.1016/j.clindermatol.2013.08.007. ISSN 0738-081X. PMID 24559558.
- ^ a b c "The Link Between HPV and Cancer". CDC. 30 September 2015. Retrieved 11 August 2016.
- ^ a b c d e f g h "What is HPV?". CDC. 28 December 2015. Retrieved 10 August 2016.
- ^ a b c d "Human Papillomavirus (HPV) Questions and Answers". CDC. 28 December 2015. Retrieved 11 August 2016.
- ^ a b Sawaya, GF; Kulasingam, S; Denberg, TD; Qaseem, A; Clinical Guidelines Committee of American College of, Physicians (16 June 2015). "Cervical Cancer Screening in Average-Risk Women: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians". Annals of Internal Medicine. 162 (12): 851–9. doi:10.7326/M14-2426. PMID 25928075.
- ^ a b c d World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.12. ISBN 9283204298.
- ^ a b Tyring, Stephen; Moore, Angela Yen; Lupi, Omar (2016). Mucocutaneous Manifestations of Viral Diseases: An Illustrated Guide to Diagnosis and Management (2 ed.). CRC Press. p. 207. ISBN 9781420073133.
- ^ EHPV.
- ^ Ghittoni, R; Accardi, R; Chiocca, S; Tommasino, M (2015), "Role of human papillomaviruses in carcinogenesis", ecancer, 9 (526), doi:10.3332/ecancer.2015.526, PMC 4431404, PMID 25987895
- ^ Bzhalava, D; Guan, P; Franceschi, S; Dillner, J; Clifford, G (2013), "A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types", Virology, 445 (1–2): 224–31, doi:10.1016/j.virol.2013.07.015, PMID 23928291.
- ^ a b Chaturvedi, Anil; Maura L. Gillison (4 March 2010). "Human Papillomavirus and Head and Neck Cancer". In Andrew F. Olshan (ed.). Epidemiology, Pathogenesis, and Prevention of Head and Neck Cancer (1st ed.). New York: Springer. ISBN 978-1-4419-1471-2.
- ^ Muñoz, N; Bosch, F. X.; De Sanjosé, S; Herrero, R; Castellsagué, X; Shah, K. V.; Snijders, P. J.; Meijer, C. J.; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group (2003), "Epidemiologic classification of human papillomavirus types associated with cervical cancer", The New England Journal of Medicine, 348 (6): 518–27, doi:10.1056/NEJMoa021641, PMID 12571259
- ^ a b c Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Mitchell, Richard (2007). "Chapter 19 The Female Genital System and Breast". Robbins Basic Pathology (8 ed.). Philadelphia: Saunders. ISBN 1-4160-2973-7.
- ^ Palefsky, Joel M.; Holly, Elizabeth A.; Ralston, Mary L.; Jay, Naomi (February 1988). "Prevalence and Risk Factors for Human Papillomavirus Infection of the Anal Canal in Human Immunodeficiency Virus (HIV)–Positive and HIV-Negative Homosexual Men" (PDF). Departments of Laboratory Medicine, Stomatology, and Epidemiology Biostatistics, University of California, San Francisco. The Journal of Infectious Diseases Oxford University Press. Retrieved 2 March 2014.
- ^ a b Muñoz N, Castellsagué X, de González AB, Gissmann L; Castellsagué; De González (2006). "Chapter 1: HPV in the etiology of human cancer". Vaccine. 24 (3): S1–S10. doi:10.1016/j.vaccine.2006.05.115. PMID 16949995.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG (2000). "The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses". J. Virol. 74 (24): 11636–41. doi:10.1128/JVI.74.24.11636-11641.2000. PMC 112445. PMID 11090162.
- ^ Mayo Clinic.com, Common warts, http://www.mayoclinic.com/print/common-warts/DS00370/DSECTION=all&METHOD=print
- ^ De Koning MN, Quint KD, Bruggink SC, Gussekloo J, Bouwes Bavinck JN, Feltkamp MC, Quint WG, Eekhof JA (2014). "High prevalence of cutaneous warts in elementary school children and ubiquitous presence of wart-associated HPV on clinically normal skin". The British Journal of Dermatology. 172: 196–201. doi:10.1111/bjd.13216. PMID 24976535.
- ^ Lountzis NI, Rahman O (2008). "Images in clinical medicine. Digital verrucae". N. Engl. J. Med. 359 (2): 177. doi:10.1056/NEJMicm071912. PMID 18614785.
- ^ MedlinePlus, Warts, https://www.nlm.nih.gov/medlineplus/warts.html#cat42 (general reference with links). Also, see
- ^ Greer CE, Wheeler CM, Ladner MB, Beutner K, Coyne MY, Liang H, Langenberg A, Yen TS, Ralston R (1995). "Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts". J. Clin. Microbiol. 33 (8): 2058–63. PMC 228335. PMID 7559948.
- ^ a b Human Papillomavirus at eMedicine
- ^ "Photos of larynx Papillomas — Voice Medicine, New York". Voicemedicine.com. Retrieved 29 August 2010.
- ^ a b c d Sinal SH, Woods CR (2005). "Human papillomavirus infections of the genital and respiratory tracts in young children". Seminars in Pediatric Infectious Diseases. 16 (4): 306–16. doi:10.1053/j.spid.2005.06.010. PMID 16210110.
- ^ Wu R, Sun S, Steinberg BM (2003). "Requirement of STAT3 activation for differentiation of mucosal stratified squamous epithelium". Mol. Med. 9 (3–4): 77–84. doi:10.2119/2003-00001.Wu. PMC 1430729. PMID 12865943.
- ^ Moore CE, Wiatrak BJ, McClatchey KD, Koopmann CF, Thomas GR, Bradford CR, Carey TE (1999). "High-risk human papillomavirus types and squamous cell carcinoma in patients with respiratory papillomas". Otolaryngol. Head Neck Surg. 120 (5): 698–705. doi:10.1053/hn.1999.v120.a91773. PMID 10229596.
- ^ a b c d e f Parkin DM (2006). "The global health burden of infection-associated cancers in the year 2002". Int. J. Cancer. 118 (12): 3030–44. doi:10.1002/ijc.21731. PMID 16404738.
- ^ http://www.medscape.org/viewarticle/768633_slide(subscription required)[clarification needed]
- ^ Schiffman M, Castle PE (2005). "The promise of global cervical-cancer prevention". N. Engl. J. Med. 353 (20): 2101–4. doi:10.1056/NEJMp058171. PMID 16291978.
- ^ Alam S, Conway MJ, Chen HS, Meyers C (2007). "Cigarette Smoke Carcinogen Benzo[a]pyrene Enhances Human Papillomavirus Synthesis". J Virol. 82 (2): 1053–8. doi:10.1128/JVI.01813-07. PMC 2224590. PMID 17989183.
- ^ Lu B, Hagensee ME, Lee JH, Wu Y, Stockwell HG, Nielson CM, Abrahamsen M, Papenfuss M, Harris RB, Giuliano AR (February 2010). "Epidemiologic factors associated with seropositivity to human papillomavirus type 16 and 18 virus-like particles and risk of subsequent infection in men". Cancer Epidemiol. Biomarkers Prev. 19 (2): 511–6. doi:10.1158/1055-9965.EPI-09-0790. PMID 20086109.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Parfenov, Michael. "Characterization of HPV and host genome interactions in primary head and neck cancers". Proceedings of the National Academy of Sciences. 111: 15544–15549. doi:10.1073/pnas.1416074111.
- ^ Karagas MR, Waterboer T, Li Z, Nelson HH, Michael KM, Bavinck JN, Perry AE, Spencer SK, Daling J, Green AC, Pawlita M (2010). "Genus β human papillomaviruses and incidence of basal cell and squamous cell carcinomas of skin: population based case-control study". BMJ. 341: 2986. doi:10.1136/bmj.c2986. PMC 2900549. PMID 20616098.
- ^ a b c Baseman JG, Koutsky LA (2005). "The epidemiology of human papillomavirus infections". J. Clin. Virol. 32 (Suppl 1): S16–24. doi:10.1016/j.jcv.2004.12.008. PMID 15753008.
Overall, these DNA-based studies, combined with measurements of type-specific antibodies against HPV capsid antigens, have shown that most (>50%) sexually active women have been infected by one or more genital HPV types at some point in time [S17].
- ^ Cohen J (2005). "Public health. High hopes and dilemmas for a cervical cancer vaccine". Science. 308 (5722): 618–21. doi:10.1126/science.308.5722.618. PMID 15860602.
- ^ a b c Ault KA (2006). "Epidemiology and Natural History of Human Papillomavirus Infections in the Female Genital tract". Infectious Diseases in Obstetrics and Gynecology. 2006: 1–5. doi:10.1155/IDOG/2006/40470. PMC 1581465. PMID 16967912.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kreimer, Aimee R.; Clifford, Gary M.; Boyle, Peter; Franceschi, Silvia (1 February 2005). "Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review". Cancer Epidemiology, Biomarkers & Prevention. 14 (2): 467–475. doi:10.1158/1055-9965.EPI-04-0551. ISSN 1055-9965. PMID 15734974.
- ^ Noel J, Lespagnard L, Fayt I, Verhest A, Dargent J (2001). "Evidence of human papilloma virus infection but lack of Epstein-Barr virus in lymphoepithelioma-like carcinoma of uterine cervix: report of two cases and review of the literature". Hum. Pathol. 32 (1): 135–8. doi:10.1053/hupa.2001.20901. PMID 11172309.
- ^ "Vulvar Intraepithelial Neoplasia: Varied signs, varied symptoms: what you need to know". www.advanceweb.com. Retrieved 5 August 2009.
- ^ Bolt J, Vo QN, Kim WJ, McWhorter AJ, Thomson J, Hagensee ME, Friedlander P, Brown KD, Gilbert J (2005). "The ATM/p53 pathway is commonly targeted for inactivation in squamous cell carcinoma of the head and neck (SCCHN) by multiple molecular mechanisms". Oral Oncol. 41 (10): 1013–20. doi:10.1016/j.oraloncology.2005.06.003. PMID 16139561.
- ^ Greenblatt, R. J. (2005). "Human papillomaviruses: Diseases, diagnosis, and a possible vaccine". Clinical Microbiology Newsletter. 27 (18): 139–145. doi:10.1016/j.clinmicnews.2005.09.001.
- ^ a b "HPV and Men — CDC Fact Sheet". Centers for Disease Control and Prevention (CDC). 3 April 2008. Retrieved 13 November 2009.
- ^ Frisch M, Smith E, Grulich A, Johansen C (2003). "Cancer in a population-based cohort of men and women in registered homosexual partnerships". Am. J. Epidemiol. 157 (11): 966–72. doi:10.1093/aje/kwg067. PMID 12777359.
However, the risk for invasive anal squamous carcinoma, which is believed to be caused by certain types of sexually transmitted human papillomaviruses, a notable one being type 16, was significantly 31-fold elevated at a crude incidence of 25.6 per 100,000 person-years
- ^ Thomas W. Gaither; et al. (2015). "The Influence of Sexual Orientation and Sexual Role on Male Grooming-Related Injuries and Infections". J Sex Med. 12 (3): 631–640. doi:10.1111/jsm.12780.
- ^ Chin-Hong PV, Vittinghoff E, Cranston RD, Browne L, Buchbinder S, Colfax G, Da Costa M, Darragh T, Benet DJ, Judson F, Koblin B, Mayer KH, Palefsky JM (2005). "Age-related prevalence of anal cancer precursors in homosexual men: the EXPLORE study". J. Natl. Cancer Inst. 97 (12): 896–905. doi:10.1093/jnci/dji163. PMID 15956651.
- ^ "AIDSmeds Web Exclusives : Pap Smears for Anal Cancer? — by David Evans". AIDSmeds.com. Retrieved 29 August 2010.
- ^ Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Palefsky JM (June 2000). "Cost-effectiveness of screening for anal squamous intraepithelial lesions and anal cancer in human immunodeficiency virus-negative homosexual and bisexual men". Am. J. Med. 108 (8): 634–41. doi:10.1016/S0002-9343(00)00349-1. PMID 10856411.
- ^ Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D (2000). "Evidence for a causal association between human papillomavirus and a subset of head and neck cancers". J. Natl. Cancer Inst. 92 (9): 709–20. doi:10.1093/jnci/92.9.709. PMID 10793107.
- ^ Gillison ML (2006). "Human papillomavirus and prognosis of oropharyngeal squamous cell carcinoma: implications for clinical research in head and neck cancers". J. Clin. Oncol. 24 (36): 5623–5. doi:10.1200/JCO.2006.07.1829. PMID 17179099.
- ^ a b D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, Westra WH, Gillison ML (2007). "Case-control study of human papillomavirus and oropharyngeal cancer". N. Engl. J. Med. 356 (19): 1944–56. doi:10.1056/NEJMoa065497. PMID 17494927.
- ^ a b Ridge JA, Glisson BS, Lango MN, et al. "Head and Neck Tumors" in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management: A Multidisciplinary Approach. 11 ed. 2008.
- ^ "Oral Cancer on the rise among non-smokers under 50" (PDF). California Dental Hygienists’ Association. Retrieved 10 January 2011.
- ^ Chaturvedi, Anil K.; Engels, Eric A.; Pfeiffer, Ruth M.; Hernandez, Brenda Y.; Xiao, Weihong; Kim, Esther; Jiang, Bo; Goodman, Marc T.; Sibug-Saber, Maria (10 November 2011). "Human papillomavirus and rising oropharyngeal cancer incidence in the United States". Journal of Clinical Oncology. 29 (32): 4294–4301. doi:10.1200/JCO.2011.36.4596. ISSN 1527-7755. PMC 3221528. PMID 21969503.
- ^ Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML (October 2011). "Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States". Journal of Clinical Oncology. 29 (32): 4294–301. doi:10.1200/JCO.2011.36.4596. PMC 3221528. PMID 21969503.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Ernster JA, Sciotto CG, O'Brien MM, Finch JL, Robinson LJ, Willson T, Mathews M (December 2007). "Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus". The Laryngoscope. 117 (12): 2115–28. doi:10.1097/MLG.0b013e31813e5fbb. PMID 17891052.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Kidon MI, Shechter E, Toubi E (January 2011). "[Vaccination against human papilloma virus and cervical cancer]". Harefuah (in Hebrew). 150 (1): 33–6, 68. PMID 21449154.
- ^ Lechner M, Frampton GM, Fenton T, Feber A, Palmer G, Jay A, Pillay N, Forster M, Cronin MT, Lipson D, Miller VA, Brennan TA, Henderson S, Vaz F, O'Flynn P, Kalavrezos N, Yelensky R, Beck S, Stephens PJ, Boshoff C; Boshoff, G. (2013). "Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV- tumors". Genome Medicine. 5 (5): 49. doi:10.1186/gm453. PMID 23718828.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ "Lung Cancer Risk Rises in the Presence of HPV Antibodies". Lung Cancer Risk Rises in the Presence of HPV Antibodies.
- ^ "Lung Cancer Patients More Likely to Have High-Risk Human Papillomavirus". NPIN.
- ^ Syrjänen K, Syrjänen S, Kellokoski J, Kärjä J, Mäntyjärvi R (1989). "Human papillomavirus (HPV) type 6 and 16 DNA sequences in bronchial squamous cell carcinomas demonstrated by in situ DNA hybridization". Lung. 167 (1): 33–42. doi:10.1007/BF02714928. PMID 2537916.
- ^ Carpagnano GE, Koutelou A, Natalicchio MI, Martinelli D, Ruggieri C, Di Taranto A, Antonetti R, Carpagnano F, Foschino-Barbaro MP (11 October 2011). "HPV in exhaled breath condensate of lung cancer patients". British Journal of Cancer. 105 (8): 1183–90. doi:10.1038/bjc.2011.354. PMC 3208494. PMID 21952627.
- ^ Klein F, Amin Kotb WF, Petersen I (July 2009). "Incidence of human papillomavirus in lung cancer". Lung Cancer. 65 (1): 13–8. doi:10.1016/j.lungcan.2008.10.003. PMID 19019488.
- ^ Moore, Matthew (12 November 2007). "Tree man 'who grew roots' may be cured". The Daily Telegraph. London.
- ^ a b Burchell AN, Winer RL, de Sanjosé S, Franco EL (August 2006). "Chapter 6: Epidemiology and transmission dynamics of genital HPV infection". Vaccine. 24 Suppl 3: S3/52–61. doi:10.1016/j.vaccine.2006.05.031. ISSN 0264-410X. PMID 16950018.
- ^ Schmitt M, Depuydt C, Benoy I, Bogers J, Antoine J, Arbyn M, Pawlita M; on behalf of the VALGENT study group (201) Prevalence and viral load of 51 genital human papillomavirus types and 3 subtypes. Int J Cancer doi:10.1002/ijc.27891
- ^ Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ (2003). "Epidemiologic classification of human papillomavirus types associated with cervical cancer". N. Engl. J. Med. 348 (6): 518–27. doi:10.1056/NEJMoa021641. PMID 12571259.
- ^ a b Egendorf, Laura. Sexually Transmitted Diseases (At Issue Series). New York: Greenhaven Press, 2007.
- ^ a b c Hernandez BY, Wilkens LR, Zhu X, Thompson P, McDuffie K, Shvetsov YB, Kamemoto LE, Killeen J, Ning L, Goodman MT (2008). "Transmission of human papillomavirus in heterosexual couples". Emerging Infectious Diseases. 14 (6): 888–894. doi:10.3201/eid1406.070616. PMC 2600292. PMID 18507898.
- ^ Appendix Table. HPV transmission events in male-female couples by anatomic site
- ^ a b Giuliano AR, Nielson CM, Flores R, Dunne EF, Abrahamsen M, Papenfuss MR, Markowitz LE, Smith D, Harris RB (2007). "The Optimal Anatomic Sites for Sampling Heterosexual Men for Human Papillomavirus (HPV) Detection: The HPV Detection in Men Study". The Journal of Infectious Diseases. 196 (8): 1146–1152. doi:10.1086/521629. PMID 17955432.
- ^ a b c d Winer RL, Hughes JP, Feng Q, Xi LF, Cherne S, O'Reilly S, Kiviat NB, Koutsky LA (2010). "DETECTION OF GENITAL HPV TYPES IN FINGERTIP SAMPLES FROM NEWLY SEXUALLY ACTIVE FEMALE UNIVERSITY STUDENTS". Cancer Epidemiology Biomarkers & Prevention. 19 (7): 1682–1685. doi:10.1158/1055-9965.EPI-10-0226. PMC 2901391. PMID 20570905.
- ^ a b c Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA (2003). "Genital human papillomavirus infection: Incidence and risk factors in a cohort of female university students". American Journal of Epidemiology. 157 (3): 218–226. doi:10.1093/aje/kwf180. PMID 12543621.
- ^ Tay SK (July 1995). "Genital oncogenic human papillomavirus infection: a short review on the mode of transmission" (Free full text). Annals of the Academy of Medicine, Singapore. 24 (4): 598–601. ISSN 0304-4602. PMID 8849195.
- ^ Pao CC, Tsai PL, Chang YL, Hsieh TT, Jin JY (March 1993). "Possible non-sexual transmission of genital human papillomavirus infections in young women". European Journal of Clinical Microbiology & Infectious Diseases. 12 (3): 221–222. doi:10.1007/BF01967118. ISSN 0934-9723. PMID 8389707.
- ^ Tay SK, Ho TH, Lim-Tan SK (August 1990). "Is genital human papillomavirus infection always sexually transmitted?" (Free full text). The Australian & New Zealand Journal of Obstetrics & Gynaecology. 30 (3): 240–242. doi:10.1111/j.1479-828X.1990.tb03223.x. ISSN 0004-8666. PMID 2256864.
- ^ a b Sonnex C, Strauss S, Gray JJ (October 1999). "Detection of human papillomavirus DNA on the fingers of patients with genital warts". Sexually Transmitted Infections. 75 (5): 317–319. doi:10.1136/sti.75.5.317. ISSN 1368-4973. PMC 1758241. PMID 10616355.
- ^ a b Hans Krueger; Gavin Stuart; Richard Gallagher; Dan Williams, Jon Kerner (12 April 2010). HPV and Other Infectious Agents in Cancer:Opportunities for Prevention and Public Health: Opportunities for Prevention and Public Health. Oxford University Press. p. 34. ISBN 978-0-19-973291-3. Retrieved 24 December 2012.
- ^ Bodaghi S, Wood LV, Roby G, Ryder C, Steinberg SM, Zheng ZM (November 2005). "Could human papillomaviruses be spread through blood?". J. Clin. Microbiol. 43 (11): 5428–34. doi:10.1128/JCM.43.11.5428-5434.2005. PMC 1287818. PMID 16272465.
- ^ Chen AC, Keleher A, Kedda MA, Spurdle AB, McMillan NA, Antonsson A (October 2009). "Human papillomavirus DNA detected in peripheral blood samples from healthy Australian male blood donors". J. Med. Virol. 81 (10): 1792–6. doi:10.1002/jmv.21592. PMID 19697401.
- ^ "Eligibility Criteria by Topic - American Red Cross".
- ^ a b "Human Papillomavirus: Confronting the Epidemic—A Urologist's Perspective". nih.gov.
- ^ a b c Schiller JT, Day PM, Kines RC (2010). "Current understanding of the mechanism of HPV infection". Gynecologic Oncology. 118 (1 Suppl): S12. doi:10.1016/j.ygyno.2010.04.004. PMC 3493113. PMID 20494219.
- ^ a b Scheurer ME, Tortolero-Luna G, Adler-Storthz K (2005). "Human papillomavirus infection: biology, epidemiology, and prevention". International Journal of Gynecological Cancer. 15 (5): 727–746. doi:10.1111/j.1525-1438.2005.00246.x. PMID 16174218.
- ^ Chen Z, Schiffman M, Herrero R, Desalle R, Anastos K, Segondy M, Sahasrabuddhe VV, Gravitt PE, Hsing AW, Burk RD (2011). "Evolution and Taxonomic Classification of Human Papillomavirus 16 (HPV16)-Related Variant Genomes". PLOS ONE. 6 (5): 1–16. doi:10.1371/journal.pone.0020183. PMC 3103539. PMID 21673791.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Zuna RE, Tuller E, Wentzensen N, Mathews C, Allen RA, Shanesmith R, Dunn ST, Gold MA, Wang SS, Walker J, Schiffman M (2011). "HPV16 Variant Lineage, Clinical Stage, And Survival in Women With Invasive Cervical Cancer". Infectious Agents & Cancer. 6: 19–27. doi:10.1186/1750-9378-6-19. PMID 22035468.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Ganguly N, Parihar SP (2009). "Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis". Journal of Biosciences. 34 (1): 113–123. doi:10.1007/s12038-009-0013-7. PMID 19430123.
- ^ Zheng ZM, Baker CC (2006). "Papillomavirus genome structure, expression, and post-transcriptional regulation". Frontiers in Bioscience. 11: 2286–2302. doi:10.2741/1971. PMC 1472295. PMID 16720315.
- ^ Tang S, Tao M, McCoy JP, Zheng ZM (2006). "The E7 Oncoprotein is Translated from Spliced E6*I Transcripts in High-Risk Human Papillomavirus Type 16- or Type 18-Positive Cervical Cancer Cell Lines via Translation Reinitiation". Journal of Virology. 80 (9): 4249–4263. doi:10.1128/JVI.80.9.4249-4263.2006. PMC 1472016. PMID 16611884.
- ^ Münger K, Howley PM (2002). "Human papillomavirus immortalization and transformation functions". Virus Research. 89 (2): 213–228. doi:10.1016/S0168-1702(02)00190-9. PMID 12445661.
- ^
Conway MJ, Alam S, Ryndock EJ, Cruz L, Christensen ND, Roden RB, Meyers C (October 2009). "Tissue-spanning redox gradient-dependent assembly of native human papillomavirus type 16 virions". Journal of Virology. 83 (20): 10515–26. doi:10.1128/JVI.00731-09. PMC 2753102. PMID 19656879.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Bryan JT, Brown DR (March 2001). "Transmission of human papillomavirus type 11 infection by desquamated cornified cells". Virology. 281 (1): 35–42. doi:10.1006/viro.2000.0777. PMID 11222093.
- ^ Rampias T, Boutati E, Pectasides E, Sasaki C, Kountourakis P, Weinberger P, Psyrri A (2010). "Activation of Wnt signaling pathway by human papillomavirus E6 and E7 oncogenes in HPV16-positive oropharyngeal squamous carcinoma cells". Molecular Cancer Research. 8 (3): 433–443. doi:10.1158/1541-7786.MCR-09-0345. PMID 20215420.
- ^ Watson RA (2005). "Human Papillomavirus: Confronting the Epidemic—A Urologist's Perspective". Reviews in Urology. 7 (3): 135–44. PMC 1477576. PMID 16985824.
- ^ Giuliano AR, Lu B, Nielson CM, Flores R, Papenfuss MR, Lee JH, Abrahamsen M, Harris RB (2008). "Age‐Specific Prevalence, Incidence, and Duration of Human Papillomavirus Infections in a Cohort of 290 US Men". The Journal of Infectious Diseases. 198 (6): 827–835. doi:10.1086/591095. PMID 18657037.
- ^ Schiffman M, Castle PE; Castle (August 2003). "Human papillomavirus: epidemiology and public health". Arch. Pathol. Lab. Med. 127 (8): 930–4. doi:10.1043/1543-2165(2003)127<930:HPEAPH>2.0.CO;2. ISSN 1543-2165. PMID 12873163.
{{cite journal}}: Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help) - ^ "National Cancer Institute Fact Sheet: HPV and Cancer". Cancer.gov. Retrieved 23 October 2013.
- ^ a b "FDA Approval of cobas HPV Test – P1000020". U.S Food and Drug Administration. Retrieved 23 October 2013.
- ^ a b Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL (2011). "Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results". Am. J. Clin. Pathol. 136 (4): 578–586. doi:10.1309/ajcptus5exas6dkz. PMID 21917680.
- ^ Digene HPV HC2 DNA Test Qiagen HPV test
- ^ "Qiagen to Buy Digene, Maker of Tests for Cancer-Causing Virus". The New York Times. 4 June 2007.
- ^ "So Close Together for So Long, and Now One". The Washington Post. 20 August 2007.
- ^ "An Error Occurred Setting Your User Cookie". thelancet.com.
- ^
Wentzensen N, von Knebel Doeberitz M (2007). "Biomarkers in cervical cancer screening". Dis. Markers. 23 (4): 315–30. doi:10.1155/2007/678793. PMID 17627065.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Molden T, Kraus I, Skomedal H, Nordstrøm T, Karlsen F (June 2007). "PreTect HPV-Proofer: real-time detection and typing of E6/E7 mRNA from carcinogenic human papillomaviruses". J. Virol. Methods. 142 (1–2): 204–12. doi:10.1016/j.jviromet.2007.01.036. PMID 17379322.
- ^ FDA approval of APTIMA HPV Assay - P100042
- ^ Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C (2009). "Clinical performance of the APTIMA® HPV Assay for the detection of high-risk HPV and high-grade cervical lesions". Journal of Clinical Virology. 45: S55–S61. doi:10.1016/S1386-6532(09)70009-5. PMID 19651370.
- ^ "Press". gen-probe.com.
- ^ a b c Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR (2006). "Prevalence of HPV Infection among Men: A Systematic Review of the Literature". The Journal of Infectious Diseases. 194 (8): 1044–1057. doi:10.1086/507432. PMID 16991079.
- ^ a b Nicolau SM, Camargo CG, Stávale JN, Castelo A, Dôres GB, Lörincz A, de Lima GR (2005). "Human papillomavirus DNA detection in male sexual partners of women with genital human papillomavirus infection". Urology. 65 (2): 251–255. doi:10.1016/j.urology.2004.09.031. PMID 15708032.
- ^ Aguilar LV, Lazcano-Ponce E, Vaccarella S, Cruz A, Hernández P, Smith JS, Muñoz N, Kornegay JR, Hernández-Avila M, Franceschi S (2006). "Human papillomavirus in men: Comparison of different genital sites". Sexually Transmitted Infections. 82 (1): 31–33. doi:10.1136/sti.2005.015131. PMC 2563819. PMID 16461598.
- ^ Weaver BA, Feng Q, Holmes KK, Kiviat N, Lee SK, Meyer C, Stern M, Koutsky LA (2004). "Evaluation of Genital Sites and Sampling Techniques for Detection of Human Papillomavirus DNA in Men". The Journal of Infectious Diseases. 189 (4): 677–685. doi:10.1086/381395. PMID 14767822.
- ^ Hernandez BY, McDuffie K, Goodman MT, Wilkens LR, Thompson P, Zhu X, Wong W, Ning L (2006). "Comparison of Physician- and Self-Collected Genital Specimens for Detection of Human Papillomavirus in Men". Journal of Clinical Microbiology. 44 (2): 513–517. doi:10.1128/JCM.44.2.513-517.2006. PMC 1392697. PMID 16455906.
- ^ Ogilvie GS, Taylor DL, Achen M, Cook D, Krajden M (2008). "Self-collection of genital human papillomavirus specimens in heterosexual men". Sexually Transmitted Infections. 85 (3): 221–225. doi:10.1136/sti.2008.033068. PMID 19066196.
- ^ Women had similar success in self-sampling, using tampons, swabs, cytobrush and lavage. Petignat P, Faltin DL, Bruchim I, Tramèr MR, Franco EL, Coutlée F (2007). "Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis". Gynecologic Oncology. 105 (2): 530–535. doi:10.1016/j.ygyno.2007.01.023. PMID 17335880.
- ^ Partridge JM, Hughes JP, Feng Q, Winer RL, Weaver BA, Xi LF, Stern ME, Lee SK, O'Reilly SF, Hawes SE, Kiviat NB, Koutsky LA (2007). "Genital Human Papillomavirus Infection in Men: Incidence and Risk Factors in a Cohort of University Students". The Journal of Infectious Diseases. 196 (8): 1128–1136. doi:10.1086/521192. PMID 17955430.
- ^ Burchell AN, Richardson H, Mahmud SM, Trottier H, Tellier PP, Hanley J, Coutlée F, Franco EL (2006). "Modeling the Sexual Transmissibility of Human Papillomavirus Infection using Stochastic Computer Simulation and Empirical Data from a Cohort Study of Young Women in Montreal, Canada". American Journal of Epidemiology. 163 (6): 534–543. doi:10.1093/aje/kwj077. PMID 16421235.
- ^ Kim JJ (2007). "Vaccine Policy Analyses Can Benefit from Natural History Studies of Human Papillomavirus in Men". The Journal of Infectious Diseases. 196 (8): 1117–1119. doi:10.1086/521199. PMID 17955427.
- ^ "FAQs for Men". thehpvtest.com.
- ^ Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR (2006). "Prevalence of HPV infection among men: A systematic review of the literature". J. Infect. Dis. 194 (8): 1044–57. doi:10.1086/507432. PMID 16991079.
- ^ "Human Papillomavirus (HPV) and Men: Questions and Answers". 2007. Retrieved 10 September 2008.
Currently, in Canada there is an HPV DNA test approved for women but not for men.
- ^ "What Men Need to Know About HPV". 2006. Retrieved 4 April 2007.
There is currently no FDA-approved test to detect HPV in men. That is because an effective, reliable way to collect a sample of male genital skin cells, which would allow detection of HPV, has yet to be developed.
- ^ Haedicke, Juliane; Thomas Iftner (2013). "Detection of Immunoglobulin G against E7 of Human Papillomavirus in Non-Small-Cell Lung Cancer". Journal of Oncology. Retrieved 18 March 2014.
- ^ Rocha-Zavaleta L, Ambrosio JP, Mora-Garcia Mde L, Cruz-Talonia F, Hernandez-Montes J, Weiss-Steider B, Ortiz-Navarrete V, Monroy-Garcia A (2004). "Detection of antibodies against a human papillomavirus (HPV) type 16 peptide that differentiate high-risk from low-risk HPV-associated low-grade squamous intraepithelial lesions" (PDF). Journal of General Virology. 85 (9): 2643–2650. doi:10.1099/vir.0.80077-0. PMID 15302958. Retrieved 18 March 2014.
- ^ Bolhassani, Azam; Farnaz Zahedifard, Yasaman Taslimi, Mohammad Taghikhani, Bijan Nahavandian, Sima Rafati (2009). "Antibody detection against HPV16 E7 & GP96 fragments as biomarkers in cervical cancer patients" (PDF). Indian J. Med. (130): 533–541. Retrieved 18 March 2014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fitzgerald, Kelly (18 June 2013). "Blood Test May Detect Sexually Transmitted Throat Cancer". Medical News Today. Retrieved 18 March 2014.
- ^ http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm426485.htm
- ^ "Human Papillomavirus Epidemiology and Prevention of Vaccine-Preventable Diseases". CDC.gov. Retrieved 30 January 2014.
- ^ "Human papillomavirus vaccines. WHO position paper" (PDF). Wkly. Epidemiol. Rec. 84 (15): 118–31. April 2009. PMID 19360985.
- ^ a b Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER (March 2007). "Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) (Cervical Cancer Screening Among Vaccinated Females)". MMWR Recomm Rep. 56 (RR–2): 1–24 [17]. PMID 17380109.
- ^ Simon R. M. Dobson; MD; et al. (1 May 2013). "Immunogenicity of 2 Doses of HPV Vaccine in Younger Adolescents vs 3 Doses in Young Women A Randomized Clinical Trial". JAMA. 309 (17): 1793–1802. doi:10.1001/jama.2013.1625. Retrieved 2 June 2015.
{{cite journal}}: Explicit use of et al. in:|author2=(help) - ^ "HPV Virus: Information About Human Papillomavirus". WebMD.
- ^ http://www.merck.com/product/usa/pi_circulars/g/gardasil/gardasil_pi.pdf
- ^ Yvonne Deleré; et al. (September 2014). "The Efficacy and Duration of Vaccine Protection Against Human Papillomavirus: A Systematic Review and Meta-analysis". Dtsch Arztebl Int. 111: 35–36. doi:10.3238/arztebl.2014.0584. PMC 4174682. PMID 25249360.
- ^ "FDA approves Gardasil 9 for prevention of certain cancers caused by five additional types of HPV" (press release). 10 December 2014. Retrieved 28 February 2015.
- ^ "CDC — Condom Effectiveness — Male Latex Condoms and Sexually Transmitted Diseases". Centers for Disease Control and Prevention (CDC). 22 October 2009. Retrieved 23 October 2009.
- ^ "Information About What is Human Papillomavirus (HPV)?". City of Toronto Public Health Agency. September 2010. Retrieved 20 July 2011.
- ^ a b "Human papillomavirus - Public Health Agency of Canada". phac-aspc.gc.ca.
- ^ a b "Genital HPV Infection Fact Sheet". Centers for Disease Control and Prevention (CDC). 10 April 2008. Retrieved 13 November 2009.
- ^ "HPV Vaccine Information For Young Women". Centers for Disease Control and Prevention (CDC). 26 June 2008. Retrieved 13 November 2009.
- ^ American Cancer Society. "What Are the Risk Factors for Cervical Cancer?". Archived from the original on 19 February 2008. Retrieved 21 February 2008.
- ^ "Cure for HPV". Webmd.com. Retrieved 29 August 2010.
- ^ Gilbert LK, Alexander L, Grosshans JF, Jolley L (2003). "Answering frequently asked questions about HPV". Sex. Transm. Dis. 30 (3): 193–4. doi:10.1097/00007435-200303000-00002. PMID 12616133.
- ^ "Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis". Centers for Disease Control and Prevention. Retrieved 23 October 2015.
- ^ Desai, Monica; Woodhall, Sarah C; Nardone, Anthony; Burns, Fiona; Mercey, Danielle; Gilson, Richard (2015). "Active recall to increase HIV and STI testing: a systematic review". Sexually Transmitted Infections: sextrans-2014-051930. doi:10.1136/sextrans-2014-051930. ISSN 1368-4973.
- ^ "Human papillomavirus vaccines: WHO position paper, October 2014" (PDF). Weekly Epidemiological Record. 43 (89): 465–492. 24 October 2014. PMID 25346960.
- ^ Gavillon N, Vervaet H, Derniaux E, Terrosi P, Graesslin O, Quereux C (2010). "Papillomavirus humain (HPV) : comment ai-je attrapé ça ?". Gynécologie Obstétrique & Fertilité. 38 (3): 199–204. doi:10.1016/j.gyobfe.2010.01.003. PMID 20189438.
- ^ a b c d e Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, Markowitz LE (February 2007). "Prevalence of HPV infection among females in the United States". JAMA. 297 (8): 813–9. doi:10.1001/jama.297.8.813. PMID 17327523.
- ^ "American Social Health Association — HPV Resource Center". Retrieved 17 August 2007.
- ^ "American Social Health Association — National HPV and Cervical Cancer Prevention Resource Center". Retrieved 1 July 2008.
- ^ "HPV vaccine report", STD, HIV, Planned Parenthood,
In fact, the lifetime risk for contracting HPV is at least 50 percent for all sexually active women and men, and, it is estimated that, by the age of 50, at least 80 percent of women will have acquired sexually transmitted HPV (CDC, 2004; CDC, 2006).
- ^ a b Weinstock H, Berman S, Cates W (January–February 2004). "Sexually Transmitted Diseases Among American Youth: Incidence and Prevalence Estimates, 2000". Perspectives on Sexual and Reproductive Health. 36 (1). Gutt Macher: 6–10. doi:10.1363/3600604. PMID 14982671.
- ^ Koutsky, LA (1997). "Epidemiology of human papilomavirus infection". The American Journal of Medicine. 102: 3–8. doi:10.1016/s0002-9343(97)00177-0.
- ^ Revzina NV, Diclemente RJ (2005). "Prevalence and incidence of human papillomavirus infection in women in the USA: a systematic review". International Journal of STD & AIDS. 16 (8): 528–37. doi:10.1258/0956462054679214. PMID 16105186.
The prevalence of HPV reported in the assessed studies ranged from 14% to more than 90%.
- ^ McCullough, Marie (28 February 2007). "Cancer-virus strains rarer than first estimated". The Philadelphia Inquirer. Archived from the original on 10 March 2007. Retrieved 2 March 2007.
- ^ Brown, David (28 February 2007) [The Washington Post, "More American Women Have HPV Than Previously Thought"]. "Study finds more women than expected have HPV". San Francisco Chronicle. Retrieved 2 March 2007..
- ^ Tanner, Lindsey (11 March 2008). "Study Finds 1 in 4 US Teens Has a STD". Newsvine. Associated Press. Retrieved 17 March 2008.
- ^ "MMWR: Summary of Notifiable Diseases". Morbidity and Mortality Weekly Report. CDC. Retrieved 18 August 2014.
- ^ Reportable diseases, from MedlinePlus, a service of the U.S. National Library of Medicine, from the National Institutes of Health. Update: 5/19/2013 by Jatin M. Vyas. Also reviewed by David Zieve.
- ^ Human papillomaviruses. World Health Organization, International Agency for Research on Cancer. 2007. ISBN 978-92-832-1290-4.
- ^ "HPV — the Shy Virus" (radio program). Sound print. 6 December 2008. Retrieved 6 December 2008.
- ^ Picken RN, Yang HL (1987). "The integration of HPV-18 into HeLa cells has involved duplication of part of the viral genome as well as human DNA flanking sequences". Nucleic Acids Research. 15 (23): 10068. doi:10.1093/nar/15.23.10068. PMC 306572. PMID 2827110.
External links
- Template:DMOZ
- Information Centre on HPV and Cancer, ICO.
- HPV Fact sheets, Centers for Disease Control and Prevention.
- "Human Papillomavirus (HPV) Vaccines", National Cancer Institute Fact Sheet, US National Institutes of Health, October 22, 2009.
