Robot-assisted surgery
This article contains promotional content. (December 2014) |
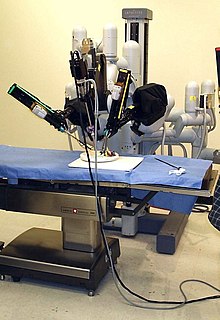
Robotic surgery, computer-assisted surgery, and robotically-assisted surgery are terms for technological developments that use robotic systems to aid in surgical procedures. Robotically-assisted surgery was developed to overcome the limitations of pre-existing minimally-invasive surgical procedures and to enhance the capabilities of surgeons performing open surgery.
In the case of robotically-assisted minimally-invasive surgery, instead of directly moving the instruments, the surgeon uses one of two methods to control the instruments; either a direct telemanipulator or through computer control. A telemanipulator is a remote manipulator that allows the surgeon to perform the normal movements associated with the surgery whilst the robotic arms carry out those movements using end-effectors and manipulators to perform the actual surgery on the patient. In computer-controlled systems the surgeon uses a computer to control the robotic arms and its end-effectors, though these systems can also still use telemanipulators for their input. One advantage of using the computerised method is that the surgeon does not have to be present, but can be anywhere in the world, leading to the possibility for remote surgery.
In the case of enhanced open surgery, autonomous instruments (in familiar configurations) replace traditional steel tools, performing certain actions (such as rib spreading) with much smoother, feedback-controlled motions than could be achieved by a human hand. The main object of such smart instruments is to reduce or eliminate the tissue trauma traditionally associated with open surgery without requiring more than a few minutes' training on the part of surgeons. This approach seeks to improve open surgeries, particularly cardio-thoracic, that have so far not benefited from minimally-invasive techniques.
Robotic surgery has been criticized for its expense, by one estimate costing $1,500 to $2000 more per patient.[1]
Comparison to traditional methods
Major advances aided by surgical robots have been remote surgery, minimally invasive surgery and unmanned surgery. Due to robotic use, the surgery is done with precision, miniaturization, smaller incisions; decreased blood loss, less pain, and quicker healing time. Articulation beyond normal manipulation and three-dimensional magnification helps resulting in improved ergonomics. Due to these techniques there is a reduced duration of hospital stays, blood loss, transfusions, and use of pain medication.[2] The existing open surgery technique has many flaws like limited access to surgical area, long recovery time, long hours of operation, blood loss, surgical scars and marks.[3]
The robot normally costs $1,390,000 and while its disposable supply cost is normally $1,500 per procedure, the cost of the procedure is higher.[4] Additional surgical training is needed to operate the system.[5] Numerous feasibility studies have been done to determine whether the purchase of such systems are worthwhile. As it stands, opinions differ dramatically. Surgeons report that, although the manufacturers of such systems provide training on this new technology, the learning phase is intensive and surgeons must operate on twelve to eighteen patients before they adapt. During the training phase, minimally invasive operations can take up to twice as long as traditional surgery, leading to operating room tie ups and surgical staffs keeping patients under anesthesia for longer periods. Patient surveys indicate they chose the procedure based on expectations of decreased morbidity, improved outcomes, reduced blood loss and less pain.[2] Higher expectations may explain higher rates of dissatisfaction and regret.[5]
Compared with other minimally invasive surgery approaches, robot-assisted surgery gives the surgeon better control over the surgical instruments and a better view of the surgical site. In addition, surgeons no longer have to stand throughout the surgery and do not tire as quickly. Naturally occurring hand tremors are filtered out by the robot's computer software. Finally, the surgical robot can continuously be used by rotating surgery teams.[6]
Critics of the system, including the American Congress of Obstetricians and Gynecologists,[7] say there is a steep learning curve for surgeons who adopt use of the system and that there's a lack of studies that indicate long-term results are superior to results following traditional laparoscopic surgery.[4] Articles in the newly created Journal of Robotic Surgery tend to report on one surgeon's experience.[4]
A Medicare study found that some procedures that have traditionally been performed with large incisions can be converted to "minimally invasive" endoscopic procedures with the use of the Da Vinci, shortening length-of-stay in the hospital and reducing recovery times. But because of the hefty cost of the robotic system it is not clear that it is cost-effective for hospitals and physicians despite any benefits to patients since there is no additional reimbursement paid by the government or insurance companies when the system is used.[4]
Robot-assisted pancreatectomies have been found to be associated with "longer operating time, lower estimated blood loss, a higher spleen-preservation rate, and shorter hospital stay[s]" than laparoscopic pancreatectomies; there was "no significant difference in transfusion, conversion to open surgery, overall complications, severe complications, pancreatic fistula, severe pancreatic fistula, ICU stay, total cost, and 30-day mortality between the two groups."[8]
Robotic surgery has been criticized for its expense, by one estimate costing $1,500 to $2000 more per patient.[1]
Uses
General surgery
In early 2000 the field of general surgical interventions with the daVinci device was explored by surgeons at Ohio State University. Reports were published in esophageal and pancreatic surgery for the first time in the world and further data was subsequently published by Horgan and his group at the University of Illinois and then later at the same institution by others.[9][10] In 2007, the University of Illinois at Chicago medical team, led by Prof. Pier Cristoforo Giulianotti, reported a pancreatectomy and also the Midwest's first fully robotic Whipple surgery. In April 2008, the same team of surgeons performed the world's first fully minimally invasive liver resection for living donor transplantation, removing 60% of the patient's liver, yet allowing him to leave the hospital just a couple of days after the procedure, in very good condition. Furthermore, the patient can also leave with less pain than a usual surgery due to the four puncture holes and not a scar by a surgeon.[11]
Cardiothoracic surgery
Robot-assisted MIDCAB and Endoscopic coronary artery bypass (TECAB) operations are being performed with the Da Vinci system. Mitral valve repairs and replacements have been performed. The Ohio State University, Columbus has performed CABG, mitral valve, esophagectomy, lung resection, tumor resections, among other robotic assisted procedures and serves as a training site for other surgeons. In 2002, surgeons at the Cleveland Clinic in Florida reported and published their preliminary experience with minimally invasive "hybrid" procedures. These procedures combined robotic revascularization and coronary stenting and further expanded the role of robots in coronary bypass to patients with disease in multiple vessels. Ongoing research on the outcomes of robotic assisted CABG and hybrid CABG is being done.
Cardiology and electrophysiology
The Stereotaxis Magnetic Navigation System (MNS) has been developed to increase precision and safety in ablation procedures for arrhythmias and atrial fibrillation while reducing radiation exposure for the patient and physician, and the system utilizes two magnets to remotely steerable catheters. The system allows for automated 3-D mapping of the heart and vasculature, and MNS has also been used in interventional cardiology for guiding stents and leads in PCI and CTO procedures, proven to reduce contrast usage and access tortuous anatomy unreachable by manual navigation. Dr. Andrea Natale has referred to the new Stereotaxis procedures with the magnetic irrigated catheters as "revolutionary."[12]
The Hansen Medical Sensei robotic catheter system uses a remotely operated system of pulleys to navigate a steerable sheath for catheter guidance. It allows precise and more forceful positioning of catheters used for 3-D mapping of the heart and vasculature. The system provides doctors with estimated force feedback information and feasible manipulation within the left atrium of the heart. The Sensei has been associated with mixed acute success rates compared to manual, commensurate with higher procedural complications, longer procedure times but lower fluoroscopy dosage to the patient.[13][14][15]
At present, three types of heart surgery are being performed on a routine basis using robotic surgery systems.[16] These three surgery types are:
- Atrial septal defect repair – the repair of a hole between the two upper chambers of the heart,
- Mitral valve repair – the repair of the valve that prevents blood from regurgitating back into the upper heart chambers during contractions of the heart,
- Coronary artery bypass – rerouting of blood supply by bypassing blocked arteries that provide blood to the heart.
As surgical experience and robotic technology develop, it is expected that the applications of robots in cardiovascular surgery will expand.
Colon and rectal surgery
Many studies have been undertaken in order to examine the role of robotic procedures in the field of colorectal surgery.[17][18]
Results to date indicate that robotic-assisted colorectal procedures outcomes are "no worse" than the results in the now "traditional" laparoscopic colorectal operations. Robotic-assisted colorectal surgery appears to be safe as well.[19] Most of the procedures have been performed for malignant colon and rectal lesions. However, surgeons are now moving into resections for diverticulitis and non-resective rectopexies (attaching the colon to the sacrum in order to treat rectal prolapse.)
When evaluated for several variables, robotic-assisted procedures fare equally well when compared with laparoscopic, or open abdominal operations. Study parameters have looked at intraoperative patient preparation time, length of time to perform the operation, adequacy of the removed surgical specimen with respect to clear surgical margins and number of lymph nodes removed, blood loss, operative or postoperative complications and long-term results.
More difficult to evaluate are issues related to the view of the operative field, the types of procedures that should be performed using robotic assistance and the potential added cost for a robotic operation.
Many surgeons feel that the optics of the 3-dimensional, two camera stereo optic robotic system are superior to the optical system used in laparoscopic procedures. The pelvic nerves are clearly visualized during robotic-assisted procedures. Less clear however is whether or not these supposedly improved optics and visualization improve patient outcomes with respect to postoperative impotence or incontinence, and whether long-term patient survival is improved by using the 3-dimensional optic system. Additionally, there is often a need for a wider, or "larger" view of the operative field than is routinely provided during robotic operations.,[20] The close-up view of the area under dissection may hamper visualization of the "bigger view", especially with respect to ureteral protection.
Questions remain unanswered, even after many years of experience with robotic-assisted colorectal operations. Ongoing studies may help clarify many of the issues of confusion associated with this novel surgical approach.
Gastrointestinal surgery
Multiple types of procedures have been performed with either the 'Zeus' or da Vinci robot systems, including bariatric surgery and gastrectomy[21] for cancer. Surgeons at various universities initially published case series demonstrating different techniques and the feasibility of GI surgery using the robotic devices.[10] Specific procedures have been more fully evaluated, specifically esophageal fundoplication for the treatment of gastroesophageal reflux[22] and Heller myotomy for the treatment of achalasia.[23][24]
Other gastrointestinal procedures including colon resection, pancreatectomy, esophagectomy and robotic approaches to pelvic disease have also been reported.
Gynecology
Robotic surgery in gynecology is of uncertain benefit with it being unclear if it affects rates of complications. Gynecologic procedures may take longer with robot-assisted surgery but may be associated with a shorter hospital stay following hysterectomy.[25] In the United States, robotic-assisted hysterectomy for benign conditions has been shown to be more expensive than conventional laparoscopic hysterectomy, with no difference in overall rates of complications.[26]
This includes the use of the da Vinci surgical system in benign gynecology and gynecologic oncology. Robotic surgery can be used to treat fibroids, abnormal periods, endometriosis, ovarian tumors, uterine prolapse, and female cancers. Using the robotic system, gynecologists can perform hysterectomies, myomectomies, and lymph node biopsies.
Neurosurgery
Several systems for stereotactic intervention are currently on the market. The NeuroMate was the first neurosurgical robot, commercially available in 1997.[27] Originally developed in Grenoble by Alim-Louis_Benabid's team, it is now owned by Renishaw. With installations in the United States, Europe and Japan, the system has been used in 8000 stereotactic brain surgeries by 2009. IMRIS Inc.'s SYMBIS(TM) Surgical System[28] will be the version of NeuroArm, the world's first MRI-compatible surgical robot, developed for world-wide commercialization. Medtech's Rosa is being used by several institutions, including the Cleveland Clinic in the U.S, and in Canada at Sherbrooke University and the Montreal Neurological Institute and Hospital in Montreal (MNI/H). Between June 2011 and September 2012, over 150 neurosurgical procedures at the MNI/H have been completed robotized stereotaxy, including in the placement of depth electrodes in the treatment of epilepsy, selective resections, and stereotaxic biopsies.
Orthopedics
The ROBODOC system was released in 1992 by Integrated Surgical Systems, Inc. which merged into CUREXO Technology Corporation.[29] Also, The Acrobot Company Ltd. developed the "Acrobot Sculptor", a robot that constrained a bone cutting tool to a pre-defined volume. The "Acrobot Sculptor" was sold to Stanmore Implants in August 2010. Stanmore received FDA clearance in February 2013 for US surgeries but sold the Sculptor to Mako Surgical in June 2013 to resolve a patent infringement lawsuit.[30] Another example is the CASPAR robot produced by U.R.S.-Ortho GmbH & Co. KG, which is used for total hip replacement, total knee replacement and anterior cruciate ligament reconstruction.[31] MAKO Surgical Corp (founded 2004) produces the RIO (Robotic Arm Interactive Orthopedic System) which combines robotics, navigation, and haptics for both partial knee and total hip replacement surgery.[32] Blue Belt Technologies received FDA clearance in November 2012 for the Navio™ Surgical System. The Navio System is a navigated, robotics-assisted surgical system that uses a CT free approach to assist in partial knee replacement surgery.[33]
Pediatrics
Surgical robotics has been used in many types of pediatric surgical procedures including: tracheoesophageal fistula repair, cholecystectomy, nissen fundoplication, morgagni's hernia repair, kasai portoenterostomy, congenital diaphragmatic hernia repair, and others. On 17 January 2002, surgeons at Children's Hospital of Michigan in Detroit performed the nation's first advanced computer-assisted robot-enhanced surgical procedure at a children's hospital.
The Center for Robotic Surgery at Children's Hospital Boston provides a high level of expertise in pediatric robotic surgery. Specially-trained surgeons use a high-tech robot to perform complex and delicate operations through very small surgical openings. The results are less pain, faster recoveries, shorter hospital stays, smaller scars, and happier patients and families.
In 2001, Children's Hospital Boston was the first pediatric hospital to acquire a surgical robot. Today, surgeons use the technology for many procedures and perform more pediatric robotic operations than any other hospital in the world. Children's Hospital physicians have developed a number of new applications to expand the use of the robot, and train surgeons from around the world on its use.[34]
Radiosurgery
The CyberKnife Robotic Radiosurgery System uses image guidance and computer controlled robotics to treat tumors throughout the body by delivering multiple beams of high-energy radiation to the tumor from virtually any direction. The system uses a German KUKA KR 240. Mounted on the robot is a compact X-band linac that produces 6MV X-ray radiation. Mounting the radiation source on the robot allows very fast repositioning of the source, which enables the system to deliver radiation from many different directions without the need to move both the patient and source as required by current gantry configurations.
Transplant surgery
Transplant surgery (organ transplantation) has been considered as highly technically demanding and virtually unobtainable by means of conventional laparoscopy. For many years, transplant patients were unable to benefit from the advantages of minimally invasive surgery. The development of robotic technology and its associated high resolution capabilities, three dimensional visual system, wrist type motion and fine instruments, gave opportunity for highly complex procedures to be completed in a minimally invasive fashion. Subsequently, the first fully robotic kidney transplantations were performed in the late 2000s. After the procedure was proven to be feasible and safe, the main emerging challenge was to determine which patients would benefit most from this robotic technique. As a result, recognition of the increasing prevalence of obesity amongst patients with kidney failure on hemodialysis posed a significant problem. Due to the abundantly higher risk of complications after traditional open kidney transplantation, obese patients were frequently denied access to transplantation, which is the premium treatment for end stage kidney disease. The use of the robotic-assisted approach has allowed kidneys to be transplanted with minimal incisions, which has virtually alleviated wound complications and significantly shortened the recovery period. The University of Illinois Medical Center reported the largest series of 104 robotic-assisted kidney transplants for obese recipients (mean body mass index > 42). Amongst this group of patients, no wound infections were observed and the function of transplanted kidneys was excellent. In this way, robotic kidney transplantation could be considered as the biggest advance in surgical technique for this procedure since its creation more than half a century ago.[35][36][37]
Urology
Robotic surgery in the field of urology has become very popular, especially in the United States.[38] It has been most extensively applied for excision of prostate cancer because of difficult anatomical access. It is also utilized for kidney cancer surgeries and to lesser extent surgeries of the bladder.
As of 2014, there is little evidence of increased benefits compared to standard surgery to justify the increased costs.[39] Some have found tentative evidence of more complete removal of cancer and less side effects from surgery for prostatectomy.[40]
In 2000, the first robot-assisted laparoscopic radical prostatectomy was performed.[5]
Vascular surgery
In September 2010, the first robotic operations with Hansen Medical's Magellan Robotic System at the femoral vasculature were performed at the University Medical Centre Ljubljana (UMC Ljubljana), Slovenia. The research was led by Borut Geršak, the head of the Department of Cardiovascular Surgery at the centre. Geršak explained that the robot used was the first true robot in the history of robotic surgery, meaning the user interface was not resembling surgical instruments and the robot was not simply imitating the movement of human hands but was guided by pressing buttons, just like one would play a video game. The robot was imported to Slovenia from the United States.[41][42]
Miniature robotics
As scientists seek to improve the versatility and utility of robotics in surgery, some are attempting to miniaturize the robots. For example, the University of Nebraska Medical Center has led a multi-campus effort to provide collaborative research on mini-robotics among surgeons, engineers and computer scientists.[43]
History
The first robot to assist in surgery was the Arthrobot, which was developed and used for the first time in Vancouver in 1983.[44] Intimately involved were biomedical engineer, Dr. James McEwen, Geof Auchinleck, a UBC engineering physics grad, and Dr. Brian Day as well as a team of engineering students. The robot was used in an orthopaedic surgical procedure on 12 March 1984, at the UBC Hospital in Vancouver. Over 60 arthroscopic surgical procedures were performed in the first 12 months, and a 1985 National Geographic video on industrial robots, The Robotics Revolution, featured the device. Other related robotic devices developed at the same time included a surgical scrub nurse robot, which handed operative instruments on voice command, and a medical laboratory robotic arm. A YouTube video entitled Arthrobot illustrates some of these in operation.
In 1985 a robot, the Unimation Puma 200, was used to place a needle for a brain biopsy using CT guidance.[45] In 1992, the PROBOT, developed at Imperial College London, was used to perform prostatic surgery by Dr. Senthil Nathan at Guy's and St Thomas' Hospital, London. This was the first pure robotic surgery in the world. Also the Robot Puma 560, a robot developed in 1985 by Kwoh et al. Puma 560 was used to perform neurosurgical biopsies with greater precision. Just like with any other technological innovation, this system led to the development of new and improved surgical robot called PROBOT. The PROBOT was specifically designed for transurethral resection of the prostate. Meanwhile, when PROBOT was being developed, ROBODOC, a robotic system designed to assist hip replacement surgeries was the first surgical robot that was approved by the FDA.[46] The ROBODOC from Integrated Surgical Systems (working closely with IBM) was introduced in 1992 to mill out precise fittings in the femur for hip replacement.[47] The purpose of the ROBODOC was to replace the previous method of carving out a femur for an implant, the use of a mallet and broach/rasp.
Further development of robotic systems was carried out by SRI International and Intuitive Surgical with the introduction of the da Vinci Surgical System and Computer Motion with the AESOP and the ZEUS robotic surgical system.[48] The first robotic surgery took place at The Ohio State University Medical Center in Columbus, Ohio under the direction of Robert E. Michler.[49] Examples of using ZEUS include a fallopian tube reconnection in July 1998,[50] a beating heart coronary artery bypass graft in October 1999,[51] and the Lindbergh Operation, which was a cholecystectomy performed remotely in September 2001.[52]
The original telesurgery robotic system that the da Vinci was based on was developed at SRI International in Menlo Park with grant support from DARPA and NASA.[53] Although the telesurgical robot was originally intended to facilitate remotely performed surgery in battlefield and other remote environments, it turned out to be more useful for minimally invasive on-site surgery. The patents for the early prototype were sold to Intuitive Surgical in Mountain View, California. The da Vinci senses the surgeon's hand movements and translates them electronically into scaled-down micro-movements to manipulate the tiny proprietary instruments. It also detects and filters out any tremors in the surgeon's hand movements, so that they are not duplicated robotically. The camera used in the system provides a true stereoscopic picture transmitted to a surgeon's console. Examples of using the da Vinci system include the first robotically assisted heart bypass (performed in Germany) in May 1998, and the first performed in the United States in September 1999;[citation needed] and the first all-robotic-assisted kidney transplant, performed in January 2009.[54] The da Vinci Si was released in April 2009, and initially sold for $1.75 million.[55]
In May 2006 the first artificial intelligence doctor-conducted unassisted robotic surgery on a 34-year-old male to correct heart arythmia. The results were rated as better than an above-average human surgeon. The machine had a database of 10,000 similar operations, and so, in the words of its designers, was "more than qualified to operate on any patient".[56][57] In August 2007, Dr. Sijo Parekattil of the Robotics Institute and Center for Urology (Winter Haven Hospital and University of Florida) performed the first robotic assisted microsurgery procedure denervation of the spermatic cord for chronic testicular pain.[58] In February 2008, Dr. Mohan S. Gundeti of the University of Chicago Comer Children's Hospital performed the first robotic pediatric neurogenic bladder reconstruction.[59]
On 12 May 2008, the first image-guided MR-compatible robotic neurosurgical procedure was performed at University of Calgary by Dr. Garnette Sutherland using the NeuroArm.[60] In June 2008, the German Aerospace Centre (DLR) presented a robotic system for minimally invasive surgery, the MiroSurge.[61] In September 2010, the Eindhoven University of Technology announced the development of the Sofie surgical system, the first surgical robot to employ force feedback.[62] In September 2010, the first robotic operation at the femoral vasculature was performed at the University Medical Centre Ljubljana by a team led by Borut Geršak.[41][42]
See also
- Bone segment navigation
- Computer-assisted surgery
- Computer-integrated surgery
- Minimally invasive surgery
- Patient registration
- Stereolithography (medicine)
- Surgical Segment Navigator
- Telemedicine
References
- ^ a b http://www.nytimes.com/2010/02/14/health/14robot.html?_r=0.
- ^ a b Estey, EP (2009). "Robotic prostatectomy: The new standard of care or a marketing success?". Canadian Urological Association Journal. 3 (6): 488–90. PMC 2792423. PMID 20019980.
- ^ O'Toole, M. D.; Bouazza-Marouf, K.; Kerr, D.; Gooroochurn, M.; Vloeberghs, M. (2009). "A methodology for design and appraisal of surgical robotic systems". Robotica. 28 (2): 297–310. doi:10.1017/S0263574709990658.

- ^ a b c d Kolata, Gina (13 February 2010). "Results Unproven, Robotic Surgery Wins Converts". The New York Times. Retrieved 11 March 2010.
- ^ a b c Finkelstein J; Eckersberger E; Sadri H; Taneja SS; Lepor H; Djavan B (2010). "Open Versus Laparoscopic Versus Robot-Assisted Laparoscopic Prostatectomy: The European and US Experience". Reviews in Urology. 12 (1): 35–43. PMC 2859140. PMID 20428292.
- ^ Gerhardus, D (July–August 2003). "Robot-assisted surgery: the future is here". Journal of Healthcare Management. 48 (4): 242–251. PMID 12908224.
- ^ Breeden, James T., MD, President of ACOG, [1] Statement on Robotic Surgery, 14 March 2013
- ^ Zhou, JY; Xin, C; Mou, YP; Xu, XW; Zhang, MZ; Zhou, YC; Lu, C; Chen, RG (2016). "Robotic versus Laparoscopic Distal Pancreatectomy: A Meta-Analysis of Short-Term Outcomes". PloS one. 11 (3): e0151189. PMID 26974961.
- ^ Melvin, W. S.; Needleman, B. J.; Krause, K. R.; Ellison, E. C. (February 2003). "Robotic Resection of Pancreatic Neuroendocrine Tumor". Journal of Laparoendoscopic & Advanced Surgical Techniques. 13 (1): 33–36. doi:10.1089/109264203321235449.

- ^ a b Talamini, M. A.; Chapman, S.; Horgan, S.; Melvin, W. S. (October 2003). "A prospective analysis of 211 robotic-assisted surgical procedures". Surgical Endoscopy. 17 (10): 1521–1524. doi:10.1007/s00464-002-8853-3. PMID 12915974.

- ^ Ahmed, K.; Khan, M. S.; Vats, A.; Nagpal, K.; Priest, O.; Patel, V.; Vecht, J. A.; Ashrafian, H.; et al. (October 2009). "Current status of robotic assisted pelvic surgery and future developments". International Journal of Surgery. 7 (5): 431–440. doi:10.1016/j.ijsu.2009.08.008. PMID 19735746.

- ^ TCAI Press Release, 3 March 2009
- ^ Natale et al., Lessons Learned and Techniques Altered Following Early Experience of the Hansen Robotic System During Catheter Ablation of Atrial Fibrillation, Poster Session II, HRS 2008
- ^ Barnebei et al., Lahey Clinic, presented at HRS 2009: PO04-35 – Robotic versus Manual Catheter Ablation for Atrial Fibrillation
- ^ R. Liew, L. Richmond, V. Baker, F. Goromonzi, G. Thomas, M. Finlay, M. Dhinoja, M. Earley, S. Sporton, R. Schilling, National Heart Centre – Singapore – Singapore, Barts and the London NHS Trust – London – United Kingdom European Heart Journal ( 2009 ) 30 ( Abstract Supplement ), 910
- ^ Kypson, Alan P; Chitwood Jr., W. Randolph (2004). "Robotic Applications in Cardiac Surgery". International Journal of Advanced Robotic Systems. 1 (2): 87–92. arXiv:cs/0412055. Bibcode:2004cs.......12055K.
- ^ D'Annibale, A et al. Diseases of the Colon and Rectum. December 2004. Volume 47, issue 12, pp. 2162–2168
- ^ Spinoglio, G. Diseases of the Colon and Rectum. November 2008. Volume 51, issue 11, pp. 1627–1632
- ^ Delaney, C. et al. Diseases of the colon and rectum. December 2003. Volume 46, pp. 1633–1639
- ^ Braumann, C. et al. Diseases of the Colon and Rectum. December 2005. Volume 48, Number 9, pp1820-1827.
- ^ Myung-Han, Hyun; Chung-Ho Lee; Hyun-Jung Kim; YiXin Tong; Sung-Soo Park (November 2013). "Systematic review and meta-analysis of robotic surgery compared with conventional laparoscopic and open resection for gastric carcinoma". British Journal of Surgery. 100 (12): 1566–78. doi:10.1002/bjs.9242. PMID 24264778.
{{cite journal}}: Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help) - ^ Melvin, W. Scott; Needleman, Bradley J.; Krause, Kevin R.; Schneider, Carol; Ellison, E. Christopher (2002). "Computer-Enhanced vs. Standard Laparoscopic Antireflux Surgery". Journal of Gastrointestinal Surgery. 6 (1): 11–15, discussion 15–6. doi:10.1016/S1091-255X(01)00032-4. PMID 11986012.

- ^ Melvin, W. S.; Dundon, J. M.; Talamini, M.; Horgan, S. (October 2005). "Computer-enhanced robotic telesurgery minimizes esophageal perforation during Heller myotomy". Surgery. 138 (4): 553–558, discussion 558–9. doi:10.1016/j.surg.2005.07.025. PMID 16269282.

- ^ Shaligram A; Unnirevi J; Simorov A; Kothari VM; Oleynikov D (April 2012). "How does the robot affect outcomes? A retrospective review of open, laparoscopic, and robotic Heller myotomy for achalasia". Surgical Endoscopy. 26 (4): 1047–50. doi:10.1007/s00464-011-1994-5. PMID 22038167.
- ^ Liu, H; Lawrie, TA; Lu, D; Song, H; Wang, L; Shi, G (10 December 2014). "Robot-assisted surgery in gynaecology". The Cochrane database of systematic reviews. 12: CD011422. doi:10.1002/14651858.CD011422. PMID 25493418.
- ^ "Committee Opinion: Robotic Surgery in Gynecolgy". Obstetrics and Gynecolgy. 125: 760–767. March 2015. doi:10.1097/01.AOG.0000461761.47981.07.
{{cite journal}}:|access-date=requires|url=(help) - ^ "Robot-Assisted Surgery: Neurosurgery". Biomed.brown.edu. Retrieved 25 June 2013.
- ^ "SYMBIS Homepage on IMRIS Website".
- ^ ROBODOC history. Robodoc.com. Retrieved 29 November 2011.
- ^ Acrobot Sculptor[dead link]
- ^ Siebert, W.; Mai, Sabine; Kober, Rudolf; Heeckt, Peter F. (30 December 2004). "Chapter 12 – Total knee replacement: robotic assistive technique". In DiGioia, Anthony M.; Jaramaz, Branislav; Picard, Frederic; Nolte, Lutz-Peter (eds.). Computer and robotic assisted hip and knee surgery. Oxford University Press. pp. 127–156. ISBN 0-19-850943-X.
- ^ "Home | MAKO Surgical Corp". Makosurgical.com. 3 June 2013. Retrieved 25 June 2013.
- ^ "Home | Blue Belt Technologies, Inc". Bluebelttech.com. 24 June 2014. Retrieved 24 June 2014.
- ^ "Robotic Surgery". Children's Hospital Center. Archived from the original on 16 April 2014. Retrieved 29 November 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Giulianotti PC, Gorodner V, Sbrana F, Tzvetanov I, Jeon H, Bianco F, Kinzer K, Oberholzer J, Benedetti E (2010). "Robotic Trans-Abdominal Kidney Transplantation in a Morbidly Obese Patient". AJT. 10: 1–5.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Oberholzer J, Giulianotti P, Danielson KK, Spaggiari M, Bejarano-Pineda L, Bianco F, Tzvetanov I, Ayloo S, Jeon H, Garcia-Roca R, Thielke J, Tang I, Akkina S, Becker B, Kinzer K, Patel A, Benedetti E (2013). "Minimally invasive robotic kidney transplantation for obese patients previously denied access to transplantation". American Journal of Transplantation. 13 (3): 721–8. doi:10.1111/ajt.12078. PMID 23437881.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tzvetanov I, Giulianotti PC, Bejarano-Pineda L, Jeon H, Garcia-Roca R, Bianco F, Oberholzer J, Benedetti E (December 2013). "Robotic-assisted kidney transplantation". Surg Clin North Am. 93 (6): 1309–23. doi:10.1016/j.suc.2013.08.003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lee, DI (April 2009). "Robotic prostatectomy: what we have learned and where we are going". Yonsei Med J. 50 (2): 177–81. doi:10.3349/ymj.2009.50.2.177. PMC 2678689. PMID 19430547.
- ^ Williams, SB; Prado, K; Hu, JC (November 2014). "Economics of robotic surgery: does it make sense and for whom?". The Urologic clinics of North America. 41 (4): 591–6. doi:10.1016/j.ucl.2014.07.013. PMID 25306170.
- ^ Ramsay, C; Pickard, R; Robertson, C; Close, A; Vale, L; Armstrong, N; Barocas, DA; Eden, CG; Fraser, C; Gurung, T; Jenkinson, D; Jia, X; Lam, TB; Mowatt, G; Neal, DE; Robinson, MC; Royle, J; Rushton, SP; Sharma, P; Shirley, MD; Soomro, N (2012). "Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer". Health technology assessment (Winchester, England). 16 (41): 1–313. doi:10.3310/hta16410. PMID 23127367.
- ^ a b "V UKC Ljubljana prvič na svetu uporabili žilnega robota za posege na femoralnem žilju" (in Slovenian). 8 November 2010. Retrieved 1 April 2011.
{{cite news}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - ^ a b "UKC Ljubljana kljub finančnim omejitvam uspešen v razvoju medicine" (in Slovenian). 30 March 2011.
{{cite news}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - ^ Graham-Rowe, Duncan (26 January 2006). "Robot set loose to film your insides". New Scientist (2536). Retrieved 29 November 2011.

- ^ "Medical Post 23:1985" (PDF).
- ^ Kwoh, Y. S.; Hou, J.; Jonckheere, E. A.; Hayall, S. (February 1988). "A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery". IEEE Transactions on Biomedical Engineering. 35 (2): 153–161. doi:10.1109/10.1354.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ Lanfranco, Anthony R.; Castellanos, Andres E.; Desai, Jaydev P.; Meyers, William C. "Robotic Surgery". Annals of Surgery. 239 (1): 14–21. doi:10.1097/01.sla.0000103020.19595.7d. PMC 1356187. PMID 14685095.
- ^ "ROBODOC: Surgical Robot Success Story" (PDF). Retrieved 25 June 2013.
- ^ Meadows, Michelle. "Computer-Assisted Surgery: An Update". FDA Consumer magazine. Food and Drug Administration. Archived from the original on 1 March 2009.
- ^ McConnell, PI; Schneeberger, EW; Michler, RE (2003). "History and development of robotic cardiac surgery". Problems in General Surgery. 20 (2): 20–30. doi:10.1097/01.sgs.0000081182.03671.6e.
- ^ Leslie Versweyveld (29 September 1999). "ZEUS robot system reverses sterilization to enable birth of baby boy". Virtual Medical Worlds Monthly.
- ^ "Robotics: the Future of Minimally Invasive Heart Surgery". Biomed.brown.edu. 6 October 1999. Retrieved 29 November 2011.
- ^ "Linbergh Operation – IRCAD/EITS Laparoscopic Center". Retrieved 19 January 2011.
- ^ "Telerobotic Surgery". SRI International. Retrieved 30 September 2013.
- ^ "New Robot Technology Eases Kidney Transplants". CBS News. 22 June 2009. Retrieved 8 July 2009.
- ^ "da Vinci Si Surgical System". Intuitive Surgical. Retrieved 30 September 2013.
- ^ "Autonomous Robotic Surgeon performs surgery on first live human". Engadget. 19 May 2006.
- ^ "Robot surgeon carries out 9-hour operation by itself". Phys.Org.
- ^ Parekattil, Sijo. "Robotic Infertility". Retrieved 11 October 2012.
- ^ "Surgeons perform world's first pediatric robotic bladder reconstruction". Esciencenews.com. 20 November 2008. Retrieved 29 November 2011.
- ^ "neuroArm : revolutionary procedure a world first". ucalgary.ca. 16 May 2008. Retrieved 14 November 2012.
- ^ Hagn, U.; Nickl, M.; Jörg, S.; Tobergte, A.; Kübler, B.; Passig, G.; Gröger, M.; Fröhlich, F.; Seibold, U.; Konietschke, R.; Le-Tien, L.; Albu-Schäffer, A.; Grebenstein, M.; Ortmaier, T.; Hirzinger, G. (2008). "DLR MiroSurge – towards versatility in surgical robotics". Jahrestagung der Deutschen Gesellschaft für Computer und Roboterassistierte Chirurgie; Proceedings of CURAC. 7: 143–146.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ "Beter opereren met nieuwe Nederlandse operatierobot Sofie" (in Dutch). TU/e. 27 September 2010. Retrieved 10 October 2010.

