Samarium(II) chloride
Appearance
| |

| |
| Names | |
|---|---|
| IUPAC name
Samarium(II) chloride
| |
| Other names
Samarium dichloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.034.196 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SmCl2 | |
| Molar mass | 221.27 g/mol |
| Appearance | dark brown crystals[1] |
| Density | 3.69 g/cm3, solid |
| Melting point | 855 °C (1,571 °F; 1,128 K) |
| Boiling point | 1,310 °C (2,390 °F; 1,580 K) |
| ? | |
| Structure | |
| Orthorhombic | |
| Pbnm, No. 62[2] | |
| Related compounds | |
Other anions
|
Samarium(II) bromide Samarium(II) iodide |
Other cations
|
Samarium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Samarium(II) chloride (SmCl2) is a chemical compound, used as a radical generating agent in the ketone-mediated intraannulation reaction.
Preparation
[edit]Reduction of samarium(III) chloride with samarium metal in a vacuum at a temperature of 800 °C to 900 °C, or with hydrogen gas at 350 °C yields samarium(II) chloride:[1]
- 2 SmCl3 + Sm → 3 SmCl2
- 2 SmCl3 + H2 → 2 SmCl2 + 2 HCl
Samarium(II) chloride can also be prepared by reducing samarium(III) chloride with lithium metal/naphthalene in THF:[3]
- SmCl3 + Li → SmCl2 + LiCl
A similar reaction has been observed with sodium.[2]
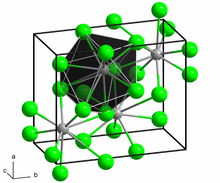
Structure
[edit]Samarium(II) chloride adopts the PbCl2 (cotunnite) structure.[2]
References
[edit]- ^ a b Brauer, Georg; Baudler, Marianne (1975). Handbuch der Präparativen Anorganischen Chemie, Band I. (3rd ed.). Stuttgart: Ferdinand Enke. ISBN 3-432-02328-6.
- ^ a b c Meyer, Gerd; Schleid, Thomas (1986-02-01). "The metallothermic reduction of several rare-earth trichlorides with lithium and sodium". Journal of the Less Common Metals. 116 (1): 187–197. doi:10.1016/0022-5088(86)90228-6.
- ^ Rossmainth, Kurt (1979-01-01). "Herstellung der klassischen Seltenerd(II)-chloride in Lösung" [Preparation of the classical rare earth(II) chlorides in solution]. Anorganische, Struktur- und Physikalische Chemie. 110 (4): 109–114. doi:10.1007/BF00903752. S2CID 91731356.
