Vanadium(III) chloride
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Vanadium(III) chloride
Vanadium trichloride | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.028.859 | ||
PubChem CID
|
|||
| RTECS number |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| VCl3 | |||
| Molar mass | 157.30 g/mol | ||
| Appearance | violet crystals paramagnetic | ||
| Density | 3.0 g/cm3 (20 °C) | ||
| Melting point | > 300 °C (572 °F; 573 K) (decomposes) | ||
| soluble | |||
| +3030.0·10−6 cm3/mol | |||
| Structure | |||
| Trigonal, hR24 | |||
| R-3, No. 148 | |||
| Hazards | |||
| GHS labelling:[1] | |||
 
| |||
| Danger | |||
| H302, H314 | |||
| P280, P305, P310, P338, P351 | |||
| Flash point | Non-flammable | ||
| Safety data sheet (SDS) | Vanadium(III) Chloride | ||
| Related compounds | |||
Other anions
|
vanadium trifluoride, vanadium tribromide | ||
Other cations
|
titanium trichloride, chromium(III) chloride, niobium trichloride, tantalum trichloride | ||
Related compounds
|
vanadium dichloride, vanadium tetrachloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Vanadium trichloride is the inorganic compound with the formula VCl3. This purple salt is a common precursor to other vanadium(III) complexes.[2]
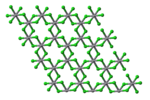
Structure
VCl3 has the common BiI3 structure, a motif that features hexagonally closest-packed chloride framework with vanadium ions occupying the octahedral holes. VBr3 and VI3 adopt the same structure, but VF3 features a structure more closely related to ReO3. VCl3 is paramagnetic and has two unpaired electrons.
Preparation and reactions
VCl3 is prepared by heating VCl4 at 160–170 °C under a flowing stream of inert gas, which sweeps out the Cl2. The bright red liquid converts to a purple solid.
Heating of VCl3 decomposes with volatilization of VCl4, leaving VCl2.[3] Upon heating under H2 at 675 °C (but less than 700 °C), VCl3 reduces to greenish VCl2.
- 2 VCl3 + H2 → 2 VCl2 + 2 HCl
Comproportionation of vanadium trichloride and vanadium(V) oxides gives vanadium oxydichloride:[4]
- V2O5 + VOCl3 + 3 VCl3 → 6 VOCl2
Vanadium trichloride catalyses the pinacol coupling reaction of benzaldehyde (PhCHO) to 1,2-diphenyl-1,2-ethanediol by various reducing metals such as zinc:[5]
- Zn + 2 H2O + 2 PhCHO → (PhCH(OH))2 + Zn(OH)2
Complexes
VCl3 forms colorful adducts and derivatives with a broad scale of ligands. VCl3 dissolves in water to give the hexahydrate, but the formula is deceptive. The salt is described by the formula [VCl2(H2O)4]Cl.2H2O. In other words, two of the water molecules are not bound to the vanadium, whose structure resembles the corresponding Fe(III) derivative. Removal of the two bound chloride ligands from [VCl2(H2O)4]+ in aqueous solution gives the green ion [V(H2O)6]3+.[6]

With tetrahydrofuran, VCl3 forms the red/pink complex VCl3(THF)3.[8] Vanadium(III) chloride reacts with acetonitrile to give the green adduct VCl3(MeCN)3. When treated with KCN, VCl3 converts to [V(CN)7]4− (early metals commonly adopt coordination numbers greater than 6 with compact ligands). Complementarily, larger metals can form complexes with rather bulky ligands. This aspect is illustrated by the isolation of VCl3(NMe3)2, containing two bulky NMe3 ligands.
Organometallic derivatives
Vanadium(III) chloride as its thf complex is a precursor toV(mesityl)3.[9]
- VCl3(THF)3 + 3 LiC6H2-2,4,6-Me3 → V(C6H2-2,4,6-Me3)3(THF) + 3 LiCl
References
- ^ "Vanadium(III) Chloride SDS". American Elements. Retrieved 2018-08-17.
- ^ Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Young, Ralph C.; Smith, Maynard E.; Moeller, Therald; Gordon, Paul G.; McCullough, Fred (2007). "Vanadium(III) Chloride". Inorganic Syntheses. pp. 128–130. doi:10.1002/9780470132357.ch43. ISBN 9780470132357.
- ^ G. Brauer (1963). "Vanadium Oxydichloride". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. NY: Academic Press. p. 1263.
- ^ Xu; Hirao, Toshikazu (2005). "Vanadium-Catalyzed Pinacol Coupling Reaction in Water". The Journal of Organic Chemistry. 70 (21): 8594–8596. doi:10.1021/jo051213f. PMID 16209617.
- ^ Donovan, William F.; Smith, Peter W. (1975). "Crystal and Molecular Structures of Aquahalogenovanadium(III) Complexes. Part I. X-Ray Crystal Structure of trans-Tetrakisaquadibromo-Vanadium(III) Bromide Dihydrate and the Isomorphous Chloro- Compound". Journal of the Chemical Society, Dalton Transactions (10): 894. doi:10.1039/DT9750000894.
- ^ Albert Cotton, F.; Duraj, Stan A.; Powell, Gregory L.; Roth, Wieslaw J. (1986). "Comparative Structural Studies of the First Row Early Transition Metal(III) Chloride Tetrahydrofuran Solvates". Inorganica Chimica Acta. 113: 81. doi:10.1016/S0020-1693(00)86863-2.
- ^ Manzer, L. E. (1982). Tetrahydrofuran Complexes of Selected Early Transition Metals. Inorganic Syntheses. Vol. 21. pp. 135–140. doi:10.1002/9780470132524.ch31.
- ^ Vivanco, Marilin; Ruiz, Javier; Floriani, Carlo; Chiesi-Villa, Angiola; Rizzoli, Corrado (1993). "Chemistry of the vanadium-carbon .sigma. Bond. 2. Oxovanadium(IV) and oxovanadium(V) containing metal-to-carbon .sigma. Bonds". Organometallics. 12 (5): 1802–1810. doi:10.1021/om00029a042.