Smoking cessation: Difference between revisions
Fixing reference errors |
m Journal cites, using AWB (9944) |
||
| Line 31: | Line 31: | ||
* For one-to-one or person-to-person counselling sessions, the duration of each session, the total amount of contact time, and the number of sessions all correlated with the effectiveness of smoking cessation. For example, "Higher intensity" interventions (>10 minutes) produced a quit rate of 22.1% as opposed to 10.9% for "no contact"; over 300 minutes of contact time produced a quit rate of 25.5% as opposed to 11.0% for "no minutes"; and more than 8 sessions produced a quit rate of 24.7% as opposed to 12.4% for 0–1 sessions.<ref name=USDHHS2008/>{{rp|83–86}} |
* For one-to-one or person-to-person counselling sessions, the duration of each session, the total amount of contact time, and the number of sessions all correlated with the effectiveness of smoking cessation. For example, "Higher intensity" interventions (>10 minutes) produced a quit rate of 22.1% as opposed to 10.9% for "no contact"; over 300 minutes of contact time produced a quit rate of 25.5% as opposed to 11.0% for "no minutes"; and more than 8 sessions produced a quit rate of 24.7% as opposed to 12.4% for 0–1 sessions.<ref name=USDHHS2008/>{{rp|83–86}} |
||
* Both physicians and non-physicians increased abstinence rates compared with self-help or no clinicians.<ref name=USDHHS2008/>{{rp|87–88}} For example, a Cochrane review of 31 studies found that nursing interventions increased the likelihood of quitting by 28%.<ref>{{Cite journal | author = Rice VH, Stead LF | editor1-last = Rice | editor1-first = Virginia Hill | title = Nursing interventions for smoking cessation | journal = Cochrane Database Syst Rev | issue = 1 | pages = CD001188 | year = 2008 | doi = 10.1002/14651858.CD001188.pub3 | pmid = 18253987}}</ref> |
* Both physicians and non-physicians increased abstinence rates compared with self-help or no clinicians.<ref name=USDHHS2008/>{{rp|87–88}} For example, a Cochrane review of 31 studies found that nursing interventions increased the likelihood of quitting by 28%.<ref>{{Cite journal | author = Rice VH, Stead LF | editor1-last = Rice | editor1-first = Virginia Hill | title = Nursing interventions for smoking cessation | journal = Cochrane Database Syst Rev | issue = 1 | pages = CD001188 | year = 2008 | doi = 10.1002/14651858.CD001188.pub3 | pmid = 18253987}}</ref> |
||
* Dental professionals also provide a key component in increasing tobacco abstinence rates in the community through counseling patients on the effects of tobacco on oral health in conjunction with an oral exam.<ref>Carr AB, Ebbert J. interventions for tobacco cessation in the dental setting. Cochrane Database of Systematic Reviews 2012, Issue 6. Art. No.: CD005084. DOI |
* Dental professionals also provide a key component in increasing tobacco abstinence rates in the community through counseling patients on the effects of tobacco on oral health in conjunction with an oral exam.<ref>Carr AB, Ebbert J. interventions for tobacco cessation in the dental setting. ''Cochrane Database of Systematic Reviews'' 2012, Issue 6. Art. No.: CD005084. {{DOI|10.1002/14651858.CD005084.pub3}}</ref> |
||
* According to the 2008 Guideline, based on two studies the training of clinicians in smoking cessation methods may increase abstinence rates<ref name=USDHHS2008/>{{rp|130}}; however, a Cochrane review found "a measurable effect" that such training decreased smoking in patients.<ref>{{cite journal |author=Carson KV, Verbiest ME, Crone MR, ''et al.'' |title=Training health professionals in smoking cessation |journal=Cochrane Database Syst Rev |volume=5 |issue= |pages=CD000214 |year=2012 |pmid=22592671 |doi=10.1002/14651858.CD000214.pub2 |editor1-last=Carson |editor1-first=Kristin V}}</ref> |
* According to the 2008 Guideline, based on two studies the training of clinicians in smoking cessation methods may increase abstinence rates<ref name=USDHHS2008/>{{rp|130}}; however, a Cochrane review found "a measurable effect" that such training decreased smoking in patients.<ref>{{cite journal |author=Carson KV, Verbiest ME, Crone MR, ''et al.'' |title=Training health professionals in smoking cessation |journal=Cochrane Database Syst Rev |volume=5 |issue= |pages=CD000214 |year=2012 |pmid=22592671 |doi=10.1002/14651858.CD000214.pub2 |editor1-last=Carson |editor1-first=Kristin V}}</ref> |
||
* Reducing or eliminating the costs of cessation therapies for smokers increased quit rates in three meta-analyses.<ref name=USDHHS2008/>{{rp|139–140}}<ref name=Hopkins2001/>{{rp|38–40}}<ref>{{Cite journal | author = Reda AA, Kaper J, Fikrelter H, Severens JL, van Schayck CP | editor1-last = Van Schayck | editor1-first = Constant Paul | title = Healthcare financing systems for increasing the use of tobacco dependence treatment | journal = Cochrane Database Syst Rev | issue = 2 | pages = CD004305 | year = 2009 | doi = 10.1002/14651858.CD004305.pub3 | pmid = 19370599}}</ref> |
* Reducing or eliminating the costs of cessation therapies for smokers increased quit rates in three meta-analyses.<ref name=USDHHS2008/>{{rp|139–140}}<ref name=Hopkins2001/>{{rp|38–40}}<ref>{{Cite journal | author = Reda AA, Kaper J, Fikrelter H, Severens JL, van Schayck CP | editor1-last = Van Schayck | editor1-first = Constant Paul | title = Healthcare financing systems for increasing the use of tobacco dependence treatment | journal = Cochrane Database Syst Rev | issue = 2 | pages = CD004305 | year = 2009 | doi = 10.1002/14651858.CD004305.pub3 | pmid = 19370599}}</ref> |
||
| Line 42: | Line 42: | ||
===Biochemical feedback=== |
===Biochemical feedback=== |
||
Various methods exist which allow a smoker to see the impact of their tobacco use, and the immediate effects of quitting. Using biochemical feedback methods can allow tobacco-users to be identified and assessed, and the use of monitoring throughout an effort to quit can increase motivation to quit.<ref name="Bittoun">Bittoun R. (2008). Carbon monoxide meter: The essential clinical tool- the ‘stethoscope"-of smoking cessation. Journal of Smoking Cessation, 3(2); 69-70.</ref><ref>Jamrozik K, Vessey M, Fowler G, Nicholas W, Parker G, and van Vunakis H. (1984). Controlled trial of three different anti-smoking interventions in general practice. |
Various methods exist which allow a smoker to see the impact of their tobacco use, and the immediate effects of quitting. Using biochemical feedback methods can allow tobacco-users to be identified and assessed, and the use of monitoring throughout an effort to quit can increase motivation to quit.<ref name="Bittoun">Bittoun R. (2008). Carbon monoxide meter: The essential clinical tool- the ‘stethoscope"-of smoking cessation. Journal of Smoking Cessation, 3(2); 69-70.</ref><ref>Jamrozik K, Vessey M, Fowler G, Nicholas W, Parker G, and van Vunakis H. (1984). Controlled trial of three different anti-smoking interventions in general practice. ''British Medical Journal'', 288; 1499-1503.</ref> |
||
* Breath carbon monoxide (CO) monitoring: Because [[carbon monoxide]] is a significant component of cigarette smoke, a [[breath carbon monoxide monitor]] can be used to detect recent cigarette use. Carbon monoxide concentration in breath has been shown to be directly correlated with the CO concentration in blood, known as percent [[carboxyhemoglobin]]. The value of demonstrating blood CO concentration to a smoker through a non-invasive breath sample is that it links the smoking habit with the physiological harm associated with smoking.<ref>Irving JM, Clark EC, Crombie IK, and Smith WC. (1988). Evaluation of a portable measure of expired-air carbon monoxide. Preventive Medicine, 17; 109-115.</ref> Within hours of quitting, CO concentrations show a noticeable decrease, and this can be encouraging for someone working to quit. Breath CO monitoring has been utilized in smoking cessation as a tool to provide patients with biomarker feedback, similar to the way in which other diagnostic tools such as the stethoscope, the blood pressure cuff, and the cholesterol test have been used by treatment professionals in medicine.<ref name="Bittoun" /> |
* Breath carbon monoxide (CO) monitoring: Because [[carbon monoxide]] is a significant component of cigarette smoke, a [[breath carbon monoxide monitor]] can be used to detect recent cigarette use. Carbon monoxide concentration in breath has been shown to be directly correlated with the CO concentration in blood, known as percent [[carboxyhemoglobin]]. The value of demonstrating blood CO concentration to a smoker through a non-invasive breath sample is that it links the smoking habit with the physiological harm associated with smoking.<ref>Irving JM, Clark EC, Crombie IK, and Smith WC. (1988). Evaluation of a portable measure of expired-air carbon monoxide. Preventive Medicine, 17; 109-115.</ref> Within hours of quitting, CO concentrations show a noticeable decrease, and this can be encouraging for someone working to quit. Breath CO monitoring has been utilized in smoking cessation as a tool to provide patients with biomarker feedback, similar to the way in which other diagnostic tools such as the stethoscope, the blood pressure cuff, and the cholesterol test have been used by treatment professionals in medicine.<ref name="Bittoun" /> |
||
* Cotinine: A [[metabolite]] of nicotine, cotinine is present in smokers. Like carbon monoxide, a cotinine test can serve as a reliable biomarker to determine smoking status.<ref>Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G (February 2009). "Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology". Therapeutic Drug Monitoring 31 (1): 14–30. {{doi|10.1097/FTD.0b013e3181957a3b}} |
* Cotinine: A [[metabolite]] of nicotine, cotinine is present in smokers. Like carbon monoxide, a cotinine test can serve as a reliable biomarker to determine smoking status.<ref>Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G (February 2009). "Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology". Therapeutic Drug Monitoring 31 (1): 14–30. .{{doi|10.1097/FTD.0b013e3181957a3b}} PMID 19125149.</ref> Cotinine levels can be tested through urine, saliva, blood, or hair samples, with one of the main concerns of cotinine testing being the invasiveness of typical sampling methods. |
||
While both measures offer high sensitivity and specificity, they differ in usage method and cost. As an example, breath CO monitoring is non-invasive, while cotinine testing relies on a bodily fluid. These two methods can be used either alone or together, for example, in a situation where abstinence verification needs additional confirmation.<ref>5. McClure JB. (2002). Are biomarkers useful treatment aids for promoting health behavior change? American Journal of Preventive Medicine, 22 (3); 200-207.</ref> |
While both measures offer high sensitivity and specificity, they differ in usage method and cost. As an example, breath CO monitoring is non-invasive, while cotinine testing relies on a bodily fluid. These two methods can be used either alone or together, for example, in a situation where abstinence verification needs additional confirmation.<ref>5. McClure JB. (2002). Are biomarkers useful treatment aids for promoting health behavior change? ''American Journal of Preventive Medicine'', 22 (3); 200-207.</ref> |
||
===Single medications=== |
===Single medications=== |
||
| Line 59: | Line 59: | ||
*# [[inhaler]]s. |
*# [[inhaler]]s. |
||
:A study found that 93 percent of over-the-counter NRT users relapse and return to smoking within six months.<ref>{{cite news|author=Millstone K|title=Nixing the patch: Smokers quit cold turkey|url=http://jscms.jrn.columbia.edu/cns/2007-02-13/millstone-coldturkeyquitters.html|date=2007-02-13 |publisher=Columbia.edu News Service|accessdate=2011-02-21}}</ref> |
:A study found that 93 percent of over-the-counter NRT users relapse and return to smoking within six months.<ref>{{cite news|author=Millstone K|title=Nixing the patch: Smokers quit cold turkey|url=http://jscms.jrn.columbia.edu/cns/2007-02-13/millstone-coldturkeyquitters.html|date=2007-02-13 |publisher=Columbia.edu News Service|accessdate=2011-02-21}}</ref> |
||
* [[Antidepressant]]: [[Bupropion]] is FDA-approved and is marketed under the brand name Zyban. Bupropion is contraindicated in [[epilepsy]], seizure disorder; anorexia/bulimia (eating disorders), patients' use of antidepressant drugs (MAO inhibitors) within 14 days, patients undergoing abrupt discontinuation of ethanol or sedatives (including benzodiazepines such as Valium).<ref>{{Cite book | author = Lacy CF et al| title = Drug information handbook | edition= 12th | year = 2004 | publisher = Lexi-Comp | location =Hudson, OH | isbn = 1-59195-083-X }}</ref><ref name=Hughes2007>{{Cite journal | author = Hughes JR, Stead LF, Lancaster T | editor1-last = Hughes | editor1-first = John R | title = Antidepressants for smoking cessation | journal = Cochrane Database Syst Rev | issue = 1 | pages = CD000031 | year = 2007 | doi = 10.1002/14651858.CD000031.pub3 | pmid = 17253443}}</ref>Evidence from a systematic review suggests that antidepressants such as Bupropion and Nortriptyline help in long-term smoking cessation and that adverse events with both drugs are rarely serious enough to cause stopping of the medication. The evidence also points out that Bupropion is less effective than Varenicline however this needs to be further validated.<ref>{{cite journal | author=John R Hughes et al. | title= Antidepressants for smoking cessation. | journal=Cochrane Database of Systematic Reviews | year=2014 | volume=1 | doi= 10.1002/14651858.CD000031.pub4}}</ref> |
* [[Antidepressant]]: [[Bupropion]] is FDA-approved and is marketed under the brand name Zyban. Bupropion is contraindicated in [[epilepsy]], seizure disorder; anorexia/bulimia (eating disorders), patients' use of antidepressant drugs (MAO inhibitors) within 14 days, patients undergoing abrupt discontinuation of ethanol or sedatives (including benzodiazepines such as Valium).<ref>{{Cite book | author = Lacy CF et al| title = Drug information handbook | edition= 12th | year = 2004 | publisher = Lexi-Comp | location =Hudson, OH | isbn = 1-59195-083-X }}</ref><ref name=Hughes2007>{{Cite journal | author = Hughes JR, Stead LF, Lancaster T | editor1-last = Hughes | editor1-first = John R | title = Antidepressants for smoking cessation | journal = Cochrane Database Syst Rev | issue = 1 | pages = CD000031 | year = 2007 | doi = 10.1002/14651858.CD000031.pub3 | pmid = 17253443}}</ref> Evidence from a systematic review suggests that antidepressants such as Bupropion and Nortriptyline help in long-term smoking cessation and that adverse events with both drugs are rarely serious enough to cause stopping of the medication. The evidence also points out that Bupropion is less effective than Varenicline however this needs to be further validated.<ref>{{cite journal | author=John R Hughes et al. | title= Antidepressants for smoking cessation. | journal=Cochrane Database of Systematic Reviews | year=2014 | volume=1 | doi= 10.1002/14651858.CD000031.pub4}}</ref> |
||
* [[Nicotinic receptor]] [[partial agonist]]s: |
* [[Nicotinic receptor]] [[partial agonist]]s: |
||
** [[Cytisine]] (Tabex) is a plant extract that has been in use since the 1960s in former Soviet-bloc countries.<ref>{{Cite journal | author = Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM | title = Cytisine for smoking cessation: a research agenda | journal = Drug Alcohol Depend | volume = 92 | issue = 1–3 | pages = 3–8 | year = 2008 | doi = 10.1016/j.drugalcdep.2007.06.017 | pmid = 17825502 }}</ref> It was the first medication approved as an aid to smoking cessation, and has very few side effects in small doses.<ref>{{cite book|author=Herrick C, Herrick C, Mitchell M|title=100 questions & answers about how to quit smoking|location=Sudbury, MA |publisher=Jones and Bartlett|year=2009|isbn=0-7637-5741-1|page=112}}</ref><ref name=WestShiffman2007>{{cite book|author=West R, Shiffman S|title=Fast facts: smoking cessation|publisher=Health Press Ltd|location=Abingdon, England|year=2007|edition=2nd|isbn=978-1-903734-98-8}}</ref>{{rp|70}} |
** [[Cytisine]] (Tabex) is a plant extract that has been in use since the 1960s in former Soviet-bloc countries.<ref>{{Cite journal | author = Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM | title = Cytisine for smoking cessation: a research agenda | journal = Drug Alcohol Depend | volume = 92 | issue = 1–3 | pages = 3–8 | year = 2008 | doi = 10.1016/j.drugalcdep.2007.06.017 | pmid = 17825502 }}</ref> It was the first medication approved as an aid to smoking cessation, and has very few side effects in small doses.<ref>{{cite book|author=Herrick C, Herrick C, Mitchell M|title=100 questions & answers about how to quit smoking|location=Sudbury, MA |publisher=Jones and Bartlett|year=2009|isbn=0-7637-5741-1|page=112}}</ref><ref name=WestShiffman2007>{{cite book|author=West R, Shiffman S|title=Fast facts: smoking cessation|publisher=Health Press Ltd|location=Abingdon, England|year=2007|edition=2nd|isbn=978-1-903734-98-8}}</ref>{{rp|70}} |
||
| Line 282: | Line 282: | ||
== Further reading == |
== Further reading == |
||
{{Commons category}} |
{{Commons category}} |
||
* {{Cite journal | author = Jason Wright | title = |
* {{Cite journal | author = Jason Wright | title = Knowing How To Quit Smoking | volume = 1 | issue = 1 | pages = 38 | year = 2013| ISBN = 1494238454 |ISBN = 978-1494238452 | url=http://www.amazon.co.uk/gp/product/1494238454}} |
||
* {{Cite journal | author = Henningfield J, Fant R, Buchhalter A, Stitzer M | title = Pharmacotherapy for nicotine dependence | journal = CA Cancer J Clin | volume = 55 | issue = 5 | pages = 281–99; quiz 322–3, 325 | year = 2005| pmid = 16166074 | doi = 10.3322/canjclin.55.5.281 }} |
* {{Cite journal | author = Henningfield J, Fant R, Buchhalter A, Stitzer M | title = Pharmacotherapy for nicotine dependence | journal = CA Cancer J Clin | volume = 55 | issue = 5 | pages = 281–99; quiz 322–3, 325 | year = 2005| pmid = 16166074 | doi = 10.3322/canjclin.55.5.281 }} |
||
* {{Cite journal | doi = 10.1111/j.1360-0443.2004.00540.x | author = Hughes JR, Keely J, Naud S | title = Shape of the relapse curve and long-term abstinence among untreated smokers | journal = Addiction | volume = 99 | issue = 1 | pages = 29–38 |year = 2004 | pmid = 14678060 }} |
* {{Cite journal | doi = 10.1111/j.1360-0443.2004.00540.x | author = Hughes JR, Keely J, Naud S | title = Shape of the relapse curve and long-term abstinence among untreated smokers | journal = Addiction | volume = 99 | issue = 1 | pages = 29–38 |year = 2004 | pmid = 14678060 }} |
||
Revision as of 12:30, 1 March 2014
Smoking cessation (colloquially quitting smoking) is the process of discontinuing tobacco smoking. Tobacco contains nicotine, which is addictive.[1]
Smoking cessation can be achieved with or without assistance from healthcare professionals or the use of medications.[2] Methods that have been found to be effective include interventions directed at or via health care providers and health care systems; medications including nicotine replacement therapy (NRT) and varenicline; individual and group counselling; and Web-based or stand-alone and computer programs. Although stopping smoking can cause short-term side effects such as reversible weight gain, smoking cessation services and activities are cost-effective because of the positive health benefits.
- In a growing number of countries, there are more ex-smokers than smokers.[2]
- Early "failure" is a normal part of trying to stop, and more than one attempt at stopping smoking prior to longer-term success is common.[2]
- NRT, other prescribed pharmaceuticals, and professional counselling or support also help many smokers.[2]
- However, up to three-quarters of ex-smokers report having quit without assistance ("cold turkey" or cut down then quit), and cessation without professional support or medication may be the most common method used by ex-smokers.[3]
Tobacco contains nicotine. Smoking cigarettes can lead to nicotine addiction.[4]: 2300–2301 The addiction begins when nicotine acts on nicotinic acetylcholine receptors to release neurotransmitters such as dopamine, glutamate, and gamma-aminobutyric acid.[4]: 2296 Cessation of smoking leads to symptoms of nicotine withdrawal such as anxiety and irritability.[4]: 2298 Professional smoking cessation support methods generally endeavour to address both nicotine addiction and nicotine withdrawal symptoms.
Studies have shown that it takes between 6 to 12 weeks post quitting before the amount of nicotinic receptors in the brain return to the level of a non smoker.[5]
Methods
Major reviews of the scientific literature on smoking cessation include:
- Systematic reviews of the Cochrane Tobacco Addiction Group of the Cochrane Collaboration.[6] As of 2012, this independent, international, not-for-profit organization has published over 60 systematic reviews "on interventions to prevent and treat tobacco addiction"[6] which will be referred to as "Cochrane reviews."
- Clinical Practice Guideline: Treating Tobacco Use and Dependence: 2008 Update of the United States Department of Health and Human Services, which will be referred to as the "2008 Guideline."[7] The Guideline was originally published in 1996[8] and revised in 2000.[9] For the 2008 Guideline, experts screened over 8700 research articles published between 1975 and 2007.[7]: 13–14 More than 300 studies were used in meta-analyses of relevant treatments; an additional 600 reports were not included in meta-analyses, but helped formulate the recommendations.[7]: 22 Limitations of the 2008 Guideline include its not evaluating studies of "cold turkey" methods ("unaided quit attempts") and its focus on studies that followed up subjects only to about 6 months after the "quit date" (even though almost one-third of former smokers who relapse before one year will do so 7–12 months after the "quit date").[7]: 19, 23 [10][11]
Unassisted methods
As it is common for ex-smokers to have made a number of attempts (often using different approaches on each occasion) to stop smoking before achieving long-term abstinence, identifying which approach or technique is eventually most successful is difficult; it has been estimated, for example, that "only about 4% to 7% of people are able to quit smoking on any given attempt without medicines or other help.".[12] However, in analysing a 1986 U.S. survey, Fiore et al. (1990) found that 95% of former smokers who had been abstinent for 1–10 years had made an unassisted last quit attempt.[13] The most frequent unassisted methods were "cold turkey" and "gradually decreased number" of cigarettes.[13] A 1995 meta-analysis estimated that the quit rate from unaided methods was 7.3% after an average of 10 months of follow-up.[14]
Cold turkey
"Cold turkey" is a colloquial term indicating abrupt withdrawal from an addictive drug, and in this context indicates sudden and complete cessation of all nicotine use. In three studies, it was the quitting method cited by 76%,[15] 85%,[13] or 88%[16] of long-term successful quitters. In a large British study of ex-smokers in the 1980s, before the advent of pharmacotherapy, 53% of the ex-smokers said that it was "not at all difficult" to stop, 27% said it was "fairly difficult", and the remaining 20% found it very difficult.[2] Cold turkey methods have been advanced by J. Wayne McFarland and Elman J. Folkenburg;[17][18] Joel Spitzer and John R. Polito;[19] Allen Carr,[20] and Jason Wright.[21]
Healthcare provider and systems
Interventions delivered via healthcare providers and healthcare systems have been shown to improve smoking cessation among people who visit those providers.
- A clinic screening system (e.g., computer prompts) to identify whether or not a person smokes doubled abstinence rates, from 3.1% to 6.4%.[7]: 78–79 Similarly, the Task Force on Community Preventive Services determined that provider reminders alone or with provider education are effective in promoting smoking cessation.[22]: 33–38
- A 2008 Guideline meta-analysis estimated that physician advice to quit smoking led to a quit rate of 10.2%, as opposed to a quit rate of 7.9% among patients who did not receive physician advice to quit smoking.[7]: 82–83 A Cochrane review found that even brief advice from physicians had "a small effect on cessation rates."[23] However, one study from Ireland involving vignettes found that physicians' probability of giving smoking cessation advice declines with the patient's age,[24] and another study from the U.S. found that only 81% of smokers age 50 or greater received advice on quitting from their physicians in the preceding year.[25]
- For one-to-one or person-to-person counselling sessions, the duration of each session, the total amount of contact time, and the number of sessions all correlated with the effectiveness of smoking cessation. For example, "Higher intensity" interventions (>10 minutes) produced a quit rate of 22.1% as opposed to 10.9% for "no contact"; over 300 minutes of contact time produced a quit rate of 25.5% as opposed to 11.0% for "no minutes"; and more than 8 sessions produced a quit rate of 24.7% as opposed to 12.4% for 0–1 sessions.[7]: 83–86
- Both physicians and non-physicians increased abstinence rates compared with self-help or no clinicians.[7]: 87–88 For example, a Cochrane review of 31 studies found that nursing interventions increased the likelihood of quitting by 28%.[26]
- Dental professionals also provide a key component in increasing tobacco abstinence rates in the community through counseling patients on the effects of tobacco on oral health in conjunction with an oral exam.[27]
- According to the 2008 Guideline, based on two studies the training of clinicians in smoking cessation methods may increase abstinence rates[7]: 130 ; however, a Cochrane review found "a measurable effect" that such training decreased smoking in patients.[28]
- Reducing or eliminating the costs of cessation therapies for smokers increased quit rates in three meta-analyses.[7]: 139–140 [22]: 38–40 [29]
- In one systematic review and meta-analysis, multi-component interventions increased quit rates in primary care settings.[30] "Multi-component" interventions were defined as those that combined two or more of the following strategies known as the "5 A's"[7]: 38–43 :
- Ask — Systematically identify all tobacco users at every visit
- Advise — Strongly urge all tobacco users to quit

Breath CO monitor displaying carbon monoxide concentration of an exhaled breath sample (in ppm) with its corresponding percent concentration of carboxyhemoglobin. - Assess — Determine willingness to make a quit attempt
- Assist — Aid the patient in quitting (provide counselling-style support and medication)
- Arrange — Ensure follow-up contact
Biochemical feedback
Various methods exist which allow a smoker to see the impact of their tobacco use, and the immediate effects of quitting. Using biochemical feedback methods can allow tobacco-users to be identified and assessed, and the use of monitoring throughout an effort to quit can increase motivation to quit.[31][32]
- Breath carbon monoxide (CO) monitoring: Because carbon monoxide is a significant component of cigarette smoke, a breath carbon monoxide monitor can be used to detect recent cigarette use. Carbon monoxide concentration in breath has been shown to be directly correlated with the CO concentration in blood, known as percent carboxyhemoglobin. The value of demonstrating blood CO concentration to a smoker through a non-invasive breath sample is that it links the smoking habit with the physiological harm associated with smoking.[33] Within hours of quitting, CO concentrations show a noticeable decrease, and this can be encouraging for someone working to quit. Breath CO monitoring has been utilized in smoking cessation as a tool to provide patients with biomarker feedback, similar to the way in which other diagnostic tools such as the stethoscope, the blood pressure cuff, and the cholesterol test have been used by treatment professionals in medicine.[31]
- Cotinine: A metabolite of nicotine, cotinine is present in smokers. Like carbon monoxide, a cotinine test can serve as a reliable biomarker to determine smoking status.[34] Cotinine levels can be tested through urine, saliva, blood, or hair samples, with one of the main concerns of cotinine testing being the invasiveness of typical sampling methods.
While both measures offer high sensitivity and specificity, they differ in usage method and cost. As an example, breath CO monitoring is non-invasive, while cotinine testing relies on a bodily fluid. These two methods can be used either alone or together, for example, in a situation where abstinence verification needs additional confirmation.[35]
Single medications
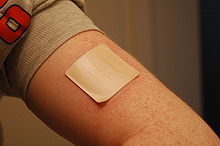
The American Cancer Society estimates that "between about 25% and 33% of smokers who use medicines can stay smoke-free for over 6 months."[12] Single medications include:
- Nicotine replacement therapy (NRT): Five medications approved by the U.S. Food and Drug Administration (FDA) deliver nicotine in a form that does not involve the risks of smoking. NRTs are meant to be used for a short period of time and should be tapered down to a low dose before stopping. The five NRT medications, which in a Cochrane review increased the chances of stopping smoking by 50 to 70% compared to placebo or to no treatment,[36] are:
- transdermal nicotine patches deliver doses of the addictive chemical nicotine, thus reducing the unpleasant effects of nicotine withdrawal. These patches can give smaller and smaller doses of nicotine, slowly reducing dependence upon nicotine and thus tobacco. A Cochrane review found further increased chance of success in a combination of the nicotine patch and a faster acting form.[36] Also, this method becomes most effective when combined with other medication and psychological support.[37]
- gum
- lozenges
- sprays
- inhalers.
- A study found that 93 percent of over-the-counter NRT users relapse and return to smoking within six months.[38]
- Antidepressant: Bupropion is FDA-approved and is marketed under the brand name Zyban. Bupropion is contraindicated in epilepsy, seizure disorder; anorexia/bulimia (eating disorders), patients' use of antidepressant drugs (MAO inhibitors) within 14 days, patients undergoing abrupt discontinuation of ethanol or sedatives (including benzodiazepines such as Valium).[39][40] Evidence from a systematic review suggests that antidepressants such as Bupropion and Nortriptyline help in long-term smoking cessation and that adverse events with both drugs are rarely serious enough to cause stopping of the medication. The evidence also points out that Bupropion is less effective than Varenicline however this needs to be further validated.[41]
- Nicotinic receptor partial agonists:
- Cytisine (Tabex) is a plant extract that has been in use since the 1960s in former Soviet-bloc countries.[42] It was the first medication approved as an aid to smoking cessation, and has very few side effects in small doses.[43][44]: 70
- Varenicline tartrate is a prescription drug marketed by Pfizer as Chantix in the U.S. (under FDA approval) and as Champix outside the U.S.[45] Synthesized as an improvement upon cytisine,[46] varenicline decreases the urge to smoke and reduces withdrawal symptoms.[47] Two systematic reviews and meta-analyses supported by unrestricted funding from Pfizer, one in 2006[48] and one in 2009,[49] found varenicline more effective than NRT or bupropion. A table in the 2008 Guideline indicates that 2 mg/day of varenicline leads to the highest abstinence rate (33.2%) of any single therapy, while 1 mg/day leads to an abstinence rate of 25.4%.[7]: 109 A 2011 Cochrane review of 15 studies (13 of which had been sponsored by Pfizer) found that varenicline was significantly superior to bupropion at one year but that varenicline and nicotine patches produced the same level of abstinence at 24 weeks.[50] A 2011 review of double-blind studies found that varenicline has increased risk of serious adverse cardiovascular events compared with placebo.[51] Varenicline may cause neuropsychiatric side effects; for example, in 2008 the UK. Medicines and Healthcare products Regulatory Agency issued a warning about possible suicidal thoughts and suicidal behavior associated with varenicline.[52]
- Moclobemide has been tested in heavy dependent smokers against placebo based on the theory that tobacco smoking could be a form of self medicating of major depression,[53] and moclobemide could therefore help increase abstinence rates due to moclobemide mimicking the MAO-A inhibiting effects of tobacco smoke. Moclobemide was administered for 3 months and then stopped; at 6 months follow-up it was found those who had taken moclobemide for 3 months had a much higher successful quit rate than those in the placebo group. However, at 12 month follow-up the difference between the placebo group and the moclobemide group was no longer significant.[54]
Two other medications have been used in trials for smoking cessation, although they are not approved by the FDA for this purpose. They may be used under careful physician supervision if the first line medications are contraindicated for the patient.
- Clonidine may reduce withdrawal symptoms and "approximately doubles abstinence rates when compared to a placebo," but its side effects include dry mouth and sedation, and abruptly stopping the drug can cause high blood pressure and other side effects.[7]: 55, 116–117 [55]
- Nortriptyline, another antidepressant, has similar success rates to bupropion but has side effects including dry mouth and sedation.[7]: 56, 117–118 [40]
Combinations of medications
The 2008 US Guideline specifies that three combinations of medications are effective[7]: 118–120 :
- Long-term nicotine patch and ad libitum NRT gum or spray
- Nicotine patch and nicotine inhaler
- Nicotine patch and bupropion (the only combination that the US FDA has approved for smoking cessation)
Cut down to quit
Gradual reduction involves slowly reducing one's daily intake of nicotine. This can theoretically be accomplished through repeated changes to cigarettes with lower levels of nicotine, by gradually reducing the number of cigarettes smoked each day, or by smoking only a fraction of a cigarette on each occasion. A 2009 systematic review by researchers at the University of Birmingham found that gradual nicotine replacement therapy could be effective in smoking cessation.[56][57] A 2010 Cochrane review found that abrupt cessation and gradual reduction with pre-quit NRT produced similar quit rates whether or not pharmacotherapy or psychological support was used. [58][59] According to a more recent 2012 Cochrane systematic review analysis of 10 studies and 3670 patients, overall relative risk reduction between smokers who attempted to quit with abrupt cessation or with gradual reduction techniques was 0.06. This analysis demonstrated that there was no significant difference in quit rates between smokers who quit by gradual reduction or abrupt cessation as measured by abstinence from smoking of at least six months from the quit day, suggesting that patients who want to quit can choose between these two methods.[60]
Community interventions
A Cochrane review found evidence that community interventions using "multiple channels to provide reinforcement, support and norms for not smoking" had an effect on smoking cessation outcomes among adults.[61] Specific methods used in the community to encourage smoking cessation among adults include:
- Policies making workplaces[15] and public places smoke-free. It is estimated that "comprehensive clean indoor laws" can increase smoking cessation rates by 12%–38%.[62] In 2008, the New York State of Alcoholism and Substantance Abuse Services banned smoking by patients, staff and volunteers at 1,300 addiction treatment centers.[63]
- Voluntary rules making homes smoke-free, which are thought to promote smoking cessation.[15][64]
- Initiatives to educate the public regarding the health effects of second-hand smoke.
- Increasing the price of tobacco products, for example by taxation. The US Task Force on Community Preventive Services found "strong scientific evidence" that this is effective in increasing tobacco use cessation.[22]: 28–30 It is estimated that an increase in price of 10% will increase smoking cessation rates by 3–5%.[62]
- Mass media campaigns. The US Task Force on Community Preventive Services declared that "strong scientific evidence" existed for these when "combined with other interventions"[22]: 30–32 , but a Cochrane review concluded that it was "difficult to establish their independent role and value".[65]
Competitions and incentives
One 2008 Cochrane review concluded that "incentives and competitions have not been shown to enhance long-term cessation rates."[66] However, a trial published in 2009 found that financial incentives for smoking cessation led to significantly higher rates of smoking cessation 15–18 months after enrollment.[67] Furthermore, a different 2008 Cochrane review found that one type of competition, "Quit and Win," did increase quit rates among participants.[68]
Psychosocial approaches
- Great American Smokeout is an annual event that invites smokers to quit for one day, hoping they will be able to extend this forever.
- The World Health Organization's World No Tobacco Day is held on May 31 each year.
- Smoking-cessation support is often offered over the internet, over the telephone quitlines[69][70] (e.g., the US toll-free number 1-800-QUIT-NOW), or in person. Three meta-analyses have concluded that telephone cessation support is effective when compared with minimal or no counselling or self-help, and that telephone cessation support with medication is more effective than medication alone.[7]: 91–92 [22]: 40–42 [71]
- Group or individual psychological support can help people who want to quit. This form of counselling can be effective alone; combining it with medication is more effective, and the number of sessions of support with medication correlates with effectiveness.[7]: 89–90, 101–103 [72][73] The counselling styles that have been effective in smoking cessation activities include motivational interviewing,[74][75][76] cognitive behavioural therapy[77] and Acceptance and Commitment Therapy.[78][78]
- The Freedom From Smoking group clinic includes eight sessions and features a step-by-step plan for quitting smoking. Each session is designed to help smokers gain control over their behavior. The clinic format encourages participants to work on the process and problems of quitting both individually and as part of a group[79]
- Multiple formats of psychosocial interventions increase quit rates: 10.8% for no intervention, 15.1% for one format, 18.5% for 2 formats, and 23.2% for three or four formats.[7]: 91
- The Transtheoretical Model including "stages of change" has been used in tailoring smoking cessation methods to individuals.[80][81][82][83] However, a 2010 Cochrane review concluded that "stage-based self-help interventions (expert systems and/or tailored materials) and individual counselling were neither more nor less effective than their non-stage-based equivalents."[84]
Self-help
A 2005 Cochrane review found that self-help materials may produce only a small increase in quit rates.[85] In the 2008 Guideline, "the effect of self-help was weak," and the number of types of self-help did not produce higher abstinence rates.[7]: 89–91 Nevertheless, self-help modalities for smoking cessation include:
- In-person self-help groups such as Nicotine Anonymous[86][87] or electronic self-help groups such as Stomp It Out.[88]
- Newsgroups: The Usenet group alt.support.stop-smoking has been used by people quitting smoking as a place to go to for support from others.[89] It is accessible through Google Groups.[90] and is now also available as an online forum, at http://www.as3-web.org/discuss .
- Interactive web-based and stand-alone computer programs and online communities which assist participants in quitting, such as EX and QuitNet. For example, "quit meters" keep track of statistics such as how long a person has remained abstinent.[91] In the 2008 US Guideline, there was no meta-analysis of computerised interventions, but they were described as "highly promising."[7]: 93–94 A meta-analysis published in 2009,[92] a Cochrane review published in 2010,[93] and a 2011 systematic review[94] found the evidence base for such interventions weak.
- Mobile phone-based interventions: A 2009 Cochrane review stated that "more evidence is needed" to determine the effectiveness of such interventions.[95] As of 2009, a randomised trial of mobile phone-based smoking cessation support was underway in the UK.[96]
- Self-help books such as Allen Carr's Easy Way to Stop Smoking.[20]
- Spirituality: In one survey of adult smokers, 88% reported a history of spiritual practice or belief, and of those more than three-quarters were of the opinion that using spiritual resources may help them quit smoking.[97]
Substitutes for cigarettes
- Electronic cigarette: Shaped like a cigarette to emulate the tactile experience of smoking, electronic cigarettes contain a rechargeable battery and a heating element which vaporises liquid nicotine and other flavorings from an insertable cartridge. Proponents of electronic cigarettes often market them as a smoking cessation device. Many claim that electronic cigarettes deliver the experience of smoking without the adverse health effects usually associated with tobacco smoke, or at least greatly reduce those risks.[98] However, in September 2008, the World Health Organization issued a release proclaiming that it does not consider the electronic cigarette to be a legitimate smoking cessation aid, stating that "no rigorous, peer-reviewed studies have been conducted showing that the electronic cigarette is a safe and effective nicotine replacement therapy."[99]
- The US National Institute of Health recommends chewing cinnamon sticks when trying to quit the use of tobacco. In addition to raw cinnamon sticks, cinnamon-flavoured toothpicks are used to help curb the urge for tobacco.[100][101]
- Plastic cigarette substitute: In one 2006 study, giving people a free "Better Quit" hollow tube resembling a cigarette did not improve quit rates.[102]
Alternative approaches
- Acupuncture: Acupuncture has been explored as an adjunct treatment method for smoking cessation.[103] A Cochrane review concluded that acupuncture "do[es] not appear to help smokers who are trying to quit",[104] a meta-analysis from the 2008 Guideline showed no difference between acupuncture and placebo,[7]: 99–100 and the 2008 Guideline found no scientific studies supporting laser therapy based on acupuncture principles but without the needles.[7]: 99
- Aromatherapy: A 2006 book reviewing the scientific literature on aromatherapy[105] identified only one study on smoking cessation and aromatherapy; the study found that "inhalation of vapor from black pepper extract reduces smoking withdrawal symptoms".[106]
- Hypnosis: Hypnosis often involves the hypnotherapist suggesting to the patient the unpleasant outcomes of smoking.[107] Clinical trials studying hypnosis and hypnotherapy as a method for smoking cessation have been inconclusive[7]: 100 ;[108][109][110] however, a randomized trial published in 2008 found that hypnosis and nicotine patches "compares favorably" with standard behavioral counseling and nicotine patches in 12-month quit rates.[111]
- Herbs: Many herbs have been studied as a method for smoking cessation, including lobelia and St John's wort.[112][113] The results are inconclusive, but St. Johns Wort shows few adverse events. Lobelia has been used to treat respiratory diseases like asthma and bronchitis, and has been used for smoking cessation because of chemical similarities to tobacco; lobelia is now listed in the FDA's Poisonous Plant Database.[114][115] Lobelia can still be found in many products sold for smoking cessation and should be used with caution.
- Smokeless tobacco: There is little smoking in Sweden, which is reflected in the very low cancer rates for Swedish men. Use of snus (a form of steam-pasteurised, rather than heat-pasteurised, air-cured smokeless tobacco) is an observed cessation method for Swedish men and even recommended by some Swedish doctors.[116]
- There are many other measures used in an effort to quit smoking which lack evidence including: a substance put on the cigarette called NicoBloc.[117] Due to the lack of evidence they are typically not recommended.[118]
Special populations
Children and adolescents
Methods used with children and adolescents include:
- Motivational enhancement[119]
- Psychological support[119]
- Youth anti-tobacco activities, such as sport involvement
- School-based curricula, such as life-skills training
- School-based nurse counseling sessions[120]
- Access reduction to tobacco
- Anti-tobacco media
- Family communication
A Cochrane review, mainly of studies combining motivational enhancement and psychological support, concluded that "complex approaches" for smoking cessation among young people show promise.[119] The 2008 US Guideline recommends counselling-style support for adolescent smokers on the basis of a meta-analysis of seven studies.[7]: 159–161 Neither the Cochrane review nor the 2008 Guideline recommends medications for adolescents who smoke.
Pregnant women
Smoking during pregnancy can cause adverse health effects in both the woman and the fetus. The 2008 US Guideline determined that "person-to-person psychosocial interventions" (typically including "intensive counseling") increased abstinence rates in pregnant women who smoke to 13.3%, compared with 7.6% in usual care.[7]: 165–167 Mothers who smoke during pregnancy have a greater tendency towards premature births. Their babies are often underdeveloped, have smaller organs, and weigh much less compared with the normal baby. In addition, these babies have worse immune systems, making them more susceptible to many diseases in early childhood, such as middle ear inflammations and asthmatic bronchitis which can bring about a lot of agony and suffering. As well, there is a high chance that they will become smokers themselves when grown up.
It is a widely spread myth that a female smoker can cause harm to her fetus by quitting immediately upon discovering that she is with child. Though this idea does seem to follow logic, it is not based on any medical study or fact.[121]
Workers
A 2008 Cochrane review of smoking cessation activities in work-places concluded that "interventions directed towards individual smokers increase the likelihood of quitting smoking."[122] A 2010 systematic review determined that worksite incentives and competitions needed to be combined with additional interventions to produce significant increases in smoking cessation rates.[123]
Hospitalised smokers

Smokers who are hospitalised may be particularly motivated to quit.[7]: 149–150 A 2007 Cochrane review found that interventions beginning during a hospital stay and continuing for one month or more after discharge were effective in producing abstinence.[124] But the use of Swedish snus should be considered for use by patients who require not only the nicotine but the other alkaloids present in tobacco that users enjoy.
Comparison of success rates
Comparison of success rates across interventions can be difficult because of different definitions of "success" across studies.[12] Robert West and Saul Shiffman, authorities in this field recognised by government health departments in a number of countries [44]: 73, 76, 80 , have concluded that, used together, "behavioural support" and "medication" can quadruple the chances that a quit attempt will be successful. In 2010 the US National Tobacco Cessation Collaborative (NTCC) created "What Works to Quit: A Guide to Quit Smoking Methods" which compares the efficacy and cost of 17 smoking cessation methods.[125] The guide, based on the 2008 Guideline, reports that smokers using a combination method of pharmacological and psychosocial approaches have the most success compared to those who use pharmaceutical or psychosocial approaches in isolation.[125]
A 2008 systematic review in the European Journal of Cancer Prevention found that group behavioural therapy was the most effective intervention strategy for smoking cessation, followed by bupropion, intensive physician advice, nicotine replacement therapy, individual counselling, telephone counselling, nursing interventions, and tailored self-help interventions; the study did not discuss varenicline.[62]

Factors affecting success
Quitting can be harder for individuals with dark pigmented skin compared to individuals with pale skin since nicotine has an affinity for melanin-containing tissues. Studies suggest this can cause the phenomenon of increased nicotine dependence and lower smoking cessation rate in darker pigmented individuals.[127]
There is an important social component to smoking. A 2008 study of a densely interconnected network of over 12,000 individuals found that smoking cessation by any given individual reduced the chances of others around them lighting up by the following amounts: a spouse by 67%, a sibling by 25%, a friend by 36%, and a coworker by 34%.[128] Nevertheless, a Cochrane review determined that interventions to increase social support for a smoker's cessation attempt did not increase long-term quit rates.[129]
Smokers who are trying to quit are faced with social influences that may persuade them to conform and continue smoking. Cravings are easier to detain when ones environment does not provoke the habit. If a person who stopped smoking has close relationships with active smokers they are often put into situations that make the urge to conform more tempting. However, in a small group with at least one other not smoking, the likelihood of conformity decreases. The social influence to smoke cigarettes has been proven to rely on simple variables. One researched variable depends on whether the influence is from a friend or non-friend.[130] the research shows that individuals are 77% more likely to conform to non-friends, while close friendships decrease conformity. Therefore, if an acquaintance offers a cigarette as a polite gesture, the person who has stopped smoking will be more likely to break his commitment than if a friend had offered.
Smokers with major depressive disorder may be less successful at quitting smoking than non-depressed smokers.[7]: 81 [131]
Relapse (resuming smoking after quitting) has been related to psychological issues such as low self-efficacy[132] or non-optimal coping responses;[133] however, psychological approaches to prevent relapse have not been proven to be successful.[134] In contrast, varenicline may help some relapsed smokers.[134]
Side effects
| Craving for tobacco | 3 to 8 weeks[135] |
| Dizziness | Few days[135] |
| Insomnia | 1 to 2 weeks[135] |
| Headaches | 1 to 2 weeks[135] |
| Chest discomfort | 1 to 2 weeks[135] |
| Constipation | 1 to 2 weeks[135] |
| Irritability | 2 to 4 weeks[135] |
| Fatigue | 2 to 4 weeks[135] |
| Cough or nasal drip | Few weeks[135] |
| Lack of concentration | Few weeks[135] |
| Hunger | Up to several weeks[135] |
Symptoms
In a 2007 review of the effects of abstinence from tobacco, Hughes concluded that "anger, anxiety, depression, difficulty concentrating, impatience, insomnia, and restlessness are valid withdrawal symptoms that peak within the first week and last 2–4 weeks."[40] In contrast, "constipation, cough, dizziness, increased dreaming, and mouth ulcers" may or may not be symptoms of withdrawal, while drowsiness, fatigue, and certain physical symptoms ("dry mouth, flu symptoms, headaches, heart racing, skin rash, sweating, tremor") were not symptoms of withdrawal.[40]
Weight gain
Giving up smoking is associated with an average weight gain of 4–5 kilograms (8.8–11.0 lb) after 12 months, most of which occurs within the first three months of quitting.[136]
The possible causes of the weight gain include:
- Smoking over-expresses the gene AZGP1 which stimulates lipolysis, so smoking cessation may decrease lipolysis.[137]
- Smoking suppresses appetite, which may be caused by nicotine's effect on central autonomic neurons (e.g., via regulation of melanin concentrating hormone neurons in the hypothalamus).[138]
- Heavy smokers are reported to burn 200 calories per day more than non-smokers eating the same diet.[139] Possible reasons for this phenomenon include nicotine's ability to increase energy metabolism or nicotine's effect on peripheral neurons.[138]
The 2008 Guideline suggests that sustained-release bupropion, nicotine gum, and nicotine lozenge be used "to delay weight gain after quitting."[7]: 173–176 However, a 2012 Cochrane review concluded that "The data are not sufficient to make strong clinical recommendations for effective programmes" for preventing weight gain.[140]
Depression
Like other physically addictive drugs, nicotine withdrawal causes down-regulation of the production of dopamine and other stimulatory neurotransmitters as the brain attempts to compensate for artificial stimulation. Therefore, when people stop smoking, depressive symptoms or actual depression may result.[131][141] This side effect of smoking cessation may be particularly common in women, as depression is more common among women than among men.[142]
Anxiety
A recent study by The British Journal of Psychiatry has found that smokers who successfully quit feel less anxious afterwards with the effect being greater among those who had mood and anxiety disorders than those that smoked for pleasure.[143]
Health benefits
Many of tobacco's detrimental health effects can be reduced or largely removed through smoking cessation. The health benefits over time of stopping smoking include:[144]
- Within 20 minutes after quitting, blood pressure and heart rate decrease
- Within 12 hours, carbon monoxide levels in the blood decrease to normal
- Within 48 hours, nerve endings and sense of smell and taste both start recovering
- Within 3 months, circulation and lung function improve
- Within 9 months, there are decreases in cough and shortness of breath
- Within 1 year, the risk of coronary heart disease is cut in half
- Within 5 years, the risk of stroke falls to the same as a non-smoker, and the risks of many cancers (mouth, throat, esophagus, bladder, cervix) decrease significantly
- Within 10 years, the risk of dying from lung cancer is cut in half,[145] and the risks of larynx and pancreas cancers decrease
- Within 15 years, the risk of coronary heart disease drops to the level of a non-smoker; lowered risk for developing COPD (chronic obstructive pulmonary disease)
The British doctors study showed that those who stopped smoking before they reached 30 years of age lived almost as long as those who never smoked.[146] Stopping in one's sixties can still add three years of healthy life.[146] A randomized trial from the U.S. and Canada showed that a smoking cessation program lasting 10 weeks decreased mortality from all causes over 14 years later.[147]
Another published study, "Smoking Cessation Reduces Postoperative Complications: A Systematic Review and Meta-analysis," examined six randomized trials and 15 observational studies to look at the effects of preoperative smoking cessation on postoperative complications. The findings were: 1) taken together, the studies demonstrated decreased likelihood of postoperative complications in patients who ceased smoking prior to surgery; 2) overall, each week of cessation prior to surgery increased the magnitude of the effect by 19%. A significant positive effect was noted in trials where smoking cessation occurred at least four weeks prior to surgery; 3) For the six randomized trials, they demonstrated on average a relative risk reduction of 41% for postoperative complications.[148]
Cost-effectiveness

Cost-effectiveness analyses of smoking cessation activities have shown that they increase quality-adjusted life years (QALYs) at costs comparable with other types of interventions to treat and prevent disease.[7]: 134–137 Studies of the cost-effectiveness of smoking cessation include:
- In a 1997 U.S. analysis, the estimated cost per QALY varied by the type of cessation approach, ranging from group intensive counselling without nicotine replacement at $1108 per QALY to minimal counselling with nicotine gum at $4542 per QALY.[149]
- A study from Erasmus University Rotterdam limited to people with chronic obstructive pulmonary disease found that the cost-effectiveness of minimal counselling, intensive counselling, and drug therapy were €16,900, €8,200, and €2,400 per QALY gained respectively.[150]
- Among National Health Service smoking cessation clients in Glasgow, pharmacy one-to-one counselling cost £2,600 per QALY gained and group support cost £4,800 per QALY gained.[151]
Statistical trends
The frequency of smoking cessation among smokers varies across countries. Smoking cessation increased in Spain between 1965 and 2000,[152] in Scotland between 1998 and 2007,[153] and in Italy after 2000.[154] In contrast, in the U.S. the cessation rate was "stable (or varied little)" between 1998 and 2008,[155] and in China smoking cessation rates declined between 1998 and 2003.[156]
Nevertheless, in a growing number of countries there are now more ex-smokers than smokers.[2] For example, in the U.S. as of 2010, there were 47 million ex-smokers and 46 million smokers.[157]
See also
- American Legacy Foundation
- Carbon monoxide breath monitor
- Coerced abstinence
- Health promotion
- Massachusetts Tobacco Cessation and Prevention Program
- National Tobacco Cessation Collaborative
- Nicotine Anonymous
- Nicotine replacement therapy
- Smoking cessation programs in Canada
- Tobacco and health
- Tobacco cessation clinic
- Tobacco Control
- Trifolium pratense
- U.S. government and smoking cessation
- Youth Tobacco Cessation Collaborative
Bibliography
- ^ "Guide to quitting smoking". American Cancer Society. 2011-01-31. Retrieved 2011-02-15.
- ^ a b c d e f Chapman S, MacKenzie R (2010-02-09). "The global research neglect of unassisted smoking cessation: causes and consequences". PLoS Medicine. 7 (2). Public Library of Science: e1000216. doi:10.1371/journal.pmed.1000216. PMC 2817714. PMID 20161722.
{{cite journal}}: CS1 maint: unflagged free DOI (link) Cite error: The named reference "Chapman-MacKenzie" was defined multiple times with different content (see the help page). - ^ Cite error: The named reference
Chapman-MacKenzi ewas invoked but never defined (see the help page). - ^ a b c Benowitz NL; Benowitz, Neal L. (2010). "Nicotine addiction". N Engl J Med. 362 (24): 2295–303. doi:10.1056/NEJMra0809890. PMC 2928221. PMID 20554984.
- ^ "Abstinent Smokers' Nicotinic Receptors Take More Than a Month to Normalize".
- ^ a b Cochrane Tobacco Addiction Group (2010-03-02). "Welcome". Retrieved 2011-02-17.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac Fiore MC, Jaén CR, Baker TB; et al. (2008). Clinical practice guideline: treating tobacco use and dependence: 2008 update (PDF). Rockville, MD: U.S. Department of Health and Human Services, Public Health Service. Retrieved 2011-02-16.
{{cite book}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Fiore MC, Bailey WC, Cohen SJ; et al. (1996). Smoking cessation. Clinical practice guideline no. 18. AHCPR publication no. 96-0692. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research.
{{cite book}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Fiore MC, Bailey WC, Cohen SJ; et al. (2000). Clinical practice guideline: treating tobacco use and dependence (PDF). Rockville, MD: U.S. Department of Health and Human Services, Public Health Service. Retrieved 2011-02-16.
{{cite book}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "Conflict over officials' stop-smoking advice". msnbc.com. Associated Press. 2008-05-07. Retrieved 2011-02-19.
- ^ Ferguson J, Bauld L, Chesterman J, Judge K (2005). "The English smoking treatment services: one-year outcomes" (PDF). Addiction. 100 (Suppl 2): 59–69. doi:10.1111/j.1360-0443.2005.01028.x. PMID 15755262.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c "Guide to quitting smoking. A word about quitting success rates". American Cancer Society. 2011-01-31. Retrieved 2011-02-17.
- ^ a b c Fiore MC, Novotny TE, Pierce JP, Giovino GA, Hatziandreu EJ, Newcomb PA, Surawicz TS, Davis RM (1990). "Methods used to quit smoking in the United States. Do cessation programs help?". JAMA. 263 (20): 2760–5. doi:10.1001/jama.263.20.2760. PMID 2271019.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Baillie AJ, Mattick RP, Hall W (1995). "Quitting smoking: estimation by meta-analysis of the rate of unaided smoking cessation". Aust J Public Health. 19 (2): 129–31. doi:10.1111/j.1753-6405.1995.tb00361.x. PMID 7786936.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Lee CW, Kahende J (2007). "Factors associated with successful smoking cessation in the United States, 2000". Am J Public Health. 97 (8): 1503–9. doi:10.2105/AJPH.2005.083527. PMC 1931453. PMID 17600268.
- ^ Doran CM, Valenti L, Robinson M, Britt H, Mattick RP (2006). "Smoking status of Australian general practice patients and their attempts to quit". Addict Behav. 31 (5): 758–66. doi:10.1016/j.addbeh.2005.05.054. PMID 16137834.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "New book details history of LLU bringing 'Health to the People'". Loma Linda University. 2008-03-31. Retrieved 2011-02-15.
- ^ McFarland JW, Folkenberg EJ (1964). How to stop smoking in five days (PDF). Englewood Cliffs, NJ: Prentice-Hall.
- ^ "WhyQuit". WhyQuit. Retrieved 2011-02-20.
- ^ a b Carr A (2004). The easy way to stop smoking. New York: Sterling. ISBN 1-4027-7163-0.
- ^ Jason Wright (2013). Knowing How To Quit Smoking. Lincoln, England: CreateSpace.com. ISBN 1-4942-3845-4.
- ^ a b c d e Hopkins DP, Briss PA, Ricard CJ, Husten CG, Carande-Kulis VG, Fielding JE, Alao MO, McKenna JW, Sharp DJ, Harris JR, Woollery TA, Harris KW; Task Force on Community Preventive Services (2001). "Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke" (PDF). Am J Prev Med. 20 (2 Suppl): 16–66. doi:10.1016/S0749-3797(00)00297-X. PMID 11173215.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stead LF, Bergson G, Lancaster T (2008). Stead, Lindsay F (ed.). "Physician advice for smoking cessation". Cochrane Database Syst Rev (2): CD000165. doi:10.1002/14651858.CD000165.pub3. PMID 18425860.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Maguire CP, Ryan J, Kelly A, O'Neill D, Coakley D, Walsh JB (2000). "Do patient age and medical condition influence medical advice to stop smoking?". Age Ageing. 29 (3): 264–6. doi:10.1093/ageing/29.3.264. PMID 10855911.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ossip-Klein DJ, McIntosh S, Utman C, Burton K, Spada J, Guido J (2000). "Smokers ages 50+: who gets physician advice to quit?" (PDF). Prev Med. 31 (4): 364–9. doi:10.1006/pmed.2000.0721. PMID 11006061.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rice VH, Stead LF (2008). Rice, Virginia Hill (ed.). "Nursing interventions for smoking cessation". Cochrane Database Syst Rev (1): CD001188. doi:10.1002/14651858.CD001188.pub3. PMID 18253987.
- ^ Carr AB, Ebbert J. interventions for tobacco cessation in the dental setting. Cochrane Database of Systematic Reviews 2012, Issue 6. Art. No.: CD005084. doi:10.1002/14651858.CD005084.pub3
- ^ Carson KV, Verbiest ME, Crone MR; et al. (2012). Carson, Kristin V (ed.). "Training health professionals in smoking cessation". Cochrane Database Syst Rev. 5: CD000214. doi:10.1002/14651858.CD000214.pub2. PMID 22592671.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Reda AA, Kaper J, Fikrelter H, Severens JL, van Schayck CP (2009). Van Schayck, Constant Paul (ed.). "Healthcare financing systems for increasing the use of tobacco dependence treatment". Cochrane Database Syst Rev (2): CD004305. doi:10.1002/14651858.CD004305.pub3. PMID 19370599.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Papadakis S, McDonald P, Mullen KA, Reid R, Skulsky K, Pipe A (2010). "Strategies to increase the delivery of smoking cessation treatments in primary care settings: a systematic review and meta-analysis". Prev Med. 51 (3–4): 199–213. doi:10.1016/j.ypmed.2010.06.007. PMID 20600264.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Bittoun R. (2008). Carbon monoxide meter: The essential clinical tool- the ‘stethoscope"-of smoking cessation. Journal of Smoking Cessation, 3(2); 69-70.
- ^ Jamrozik K, Vessey M, Fowler G, Nicholas W, Parker G, and van Vunakis H. (1984). Controlled trial of three different anti-smoking interventions in general practice. British Medical Journal, 288; 1499-1503.
- ^ Irving JM, Clark EC, Crombie IK, and Smith WC. (1988). Evaluation of a portable measure of expired-air carbon monoxide. Preventive Medicine, 17; 109-115.
- ^ Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G (February 2009). "Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology". Therapeutic Drug Monitoring 31 (1): 14–30. .doi:10.1097/FTD.0b013e3181957a3b PMID 19125149.
- ^ 5. McClure JB. (2002). Are biomarkers useful treatment aids for promoting health behavior change? American Journal of Preventive Medicine, 22 (3); 200-207.
- ^ a b Stead LF, Perera R, Bullen C, Mant D, Lancaster T (2008). Stead, Lindsay F (ed.). "Nicotine replacement therapy for smoking cessation". Cochrane Database Syst Rev (1): CD000146. doi:10.1002/14651858.CD000146.pub3. PMID 18253970.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Noble HB (1999-03-02). "New from the smoking wars: success". New York Times. Retrieved 2011-02-19.
- ^ Millstone K (2007-02-13). "Nixing the patch: Smokers quit cold turkey". Columbia.edu News Service. Retrieved 2011-02-21.
- ^ Lacy CF; et al. (2004). Drug information handbook (12th ed.). Hudson, OH: Lexi-Comp. ISBN 1-59195-083-X.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ a b c d Hughes JR, Stead LF, Lancaster T (2007). Hughes, John R (ed.). "Antidepressants for smoking cessation". Cochrane Database Syst Rev (1): CD000031. doi:10.1002/14651858.CD000031.pub3. PMID 17253443.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "Hughes2007" was defined multiple times with different content (see the help page). - ^ John R Hughes; et al. (2014). "Antidepressants for smoking cessation". Cochrane Database of Systematic Reviews. 1. doi:10.1002/14651858.CD000031.pub4.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM (2008). "Cytisine for smoking cessation: a research agenda". Drug Alcohol Depend. 92 (1–3): 3–8. doi:10.1016/j.drugalcdep.2007.06.017. PMID 17825502.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Herrick C, Herrick C, Mitchell M (2009). 100 questions & answers about how to quit smoking. Sudbury, MA: Jones and Bartlett. p. 112. ISBN 0-7637-5741-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c West R, Shiffman S (2007). Fast facts: smoking cessation (2nd ed.). Abingdon, England: Health Press Ltd. ISBN 978-1-903734-98-8.
- ^ Pfizer (2009). "Doing things differently. Annual review 2008" (PDF). Retrieved 2011-02-22.
- ^ Yarnell A (2005-05-31). "Design of an antismoking pill. The makers of varenicline got their inspiration from two natural products". Chemical & Engineering News. Retrieved 2011-02-22.
- ^ Pfizer Canada Inc (2010-05-28). "Product monograph. PrChampix (varenicline tartrate tablets)". Retrieved 2011-02-22.
- ^ Wu P, Wilson K, Dimoulas P, Mills EJ (2006). "Effectiveness of smoking cessation therapies: a". BMC Public Health. 6: 300. doi:10.1186/1471-2458-6-300. PMC 1764891. PMID 17156479.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Mills EJ, Wu P, Spurden D, Ebbert JO, Wilson K (2009). "Efficacy of pharmacotherapies for short-term smoking abstinence: a systematic review and meta-analysis". Harm Reduct J. 6 (1): 25. doi:10.1186/1477-7517-6-25. PMC 2760513. PMID 19761618.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Cahill K, Stead LF, Lancaster T (2011). Cahill, Kate (ed.). "Nicotine receptor partial agonists for smoking cessation". Cochrane Database Syst Rev. 2 (2): CD006103. doi:10.1002/14651858.CD006103.pub5. PMID 21328282.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Singh S, Loke Y, Spangler J, and Furberg C (July 4, 2011). "Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis". Canadian Medical Association Journal. 183 (12): 1359–66. doi:10.1503/cmaj.110218. PMC 3168618. PMID 21727225.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Medicines and Healthcare products Regulatory Agency (July 2008). "Drug safety advice. Varenicline: suicidal thoughts and behaviour" (PDF). Drug Safety Update. 1 (12): 2–3. Retrieved 2011-02-21.
- ^ Berlin, I.; Saïd, S.; Spreux-Varoquaux, O.; Launay, JM.; Olivares, R.; Millet, V.; Lecrubier, Y.; Puech, AJ. (Oct 1995). "A reversible monoamine oxidase A inhibitor (moclobemide) facilitates smoking cessation and abstinence in heavy, dependent smokers". Clin Pharmacol Ther. 58 (4): 444–52. doi:10.1016/0009-9236(95)90058-6. PMID 7586937.
- ^ Hughes, JR.; Stead, LF.; Lancaster, T. (2007). Hughes, John R (ed.). "Antidepressants for smoking cessation". Cochrane Database Syst Rev (1): CD000031. doi:10.1002/14651858.CD000031.pub3. PMID 17253443.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - ^ Gourlay SG, Stead LF, Benowitz NL (2004). Stead, Lindsay F (ed.). "Clonidine for smoking cessation". Cochrane Database Syst Rev (3): CD000058. doi:10.1002/14651858.CD000058.pub2. PMID 15266422.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Phend C (2009-04-03). "Gradual cutback with nicotine replacement boosts quit rates". MedPage Today. Retrieved 2011-02-20.
- ^ Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P (2009). "Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis". BMJ. 338: b1024. doi:10.1136/bmj.b1024. PMC 2664870. PMID 19342408.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Joseph J (March 30, 2010). "Cut down to quit approach no better". Pharmacy News. Reed Business Information.
- ^ Lindson N, Aveyard P, Hughes JR (2010). Lindson, Nicola (ed.). "Reduction versus abrupt cessation in smokers who want to quit". Cochrane Database Syst Rev. 3 (3): CD008033. doi:10.1002/14651858.CD008033.pub2. PMID 20238361.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lindson-Hawley N, Aveyard P, Hughes JR (2012). Lindson-Hawley, Nicola (ed.). "Reduction versus abrupt cessation in smokers who want to quit". Cochrane Database of Systematic Reviews. 11 (11): CD008033. doi:10.1002/14651858.CD008033.pub3. PMID 23152252.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Secker-Walker RH, Gnich W, Platt S, Lancaster T (2002). Stead, Lindsay F (ed.). "Community interventions for reducing smoking among adults". Cochrane Database Syst Rev (3): CD001745. doi:10.1002/14651858.CD001745. PMID 12137631.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Lemmens V, Oenema A, Knut IK, Brug J (2008). "Effectiveness of smoking cessation interventions among adults: a systematic review of reviews" (PDF). Eur J Cancer Prev. 17 (6): 535–44. doi:10.1097/CEJ.0b013e3282f75e48. PMID 18941375.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "State-Mandated Tobacco Ban, Integration of Cessation Services, and Other Policies Reduce Smoking Among Patients and Staff at Substance Abuse Treatment Centers". Agency for Healthcare Research and Quality. 2013-02-27. Retrieved 2013-05-13.
- ^ Centers for Disease Control and Prevention (CDC) (May 2007). "State-specific prevalence of smoke-free home rules--United States, 1992-2003". MMWR Morb. Mortal. Wkly. Rep. 56 (20): 501–4. PMID 17522588.
- ^ Bala M, Strzeszynski L, Cahill K (2008). Bala, Malgorzata (ed.). "Mass media interventions for smoking cessation in adults". Cochrane Database Syst Rev (1): CD004704. doi:10.1002/14651858.CD004704.pub2. PMID 18254058.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cahill K, Perera R (2008). Cahill, Kate (ed.). "Competitions and incentives for smoking cessation". Cochrane Database Syst Rev (3): CD004307. doi:10.1002/14651858.CD004307.pub3. PMID 18646105.
- ^ Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, Galvin R, Zhu J, Wan F, DeGuzman J, Corbett E, Weiner J, Audrain-McGovern J (2009). "A randomized, controlled trial of financial incentives for smoking cessation". N Engl J Med. 360 (7): 699–709. doi:10.1056/NEJMsa0806819. PMID 19213683.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cahill K, Perera R (2008). Cahill, Kate (ed.). "Quit and Win contests for smoking cessation". Cochrane Database Syst Rev (4): CD004986. doi:10.1002/14651858.CD004986.pub3. PMID 18843674.
- ^ Zhu SH, Anderson CM, Tedeschi GJ, Rosbrook B, Johnson CE, Byrd M, Gutiérrez-Terrell E (2002). "Evidence of real-world effectiveness of a telephone quitline for smokers". N Engl J Med. 347 (14): 1087–93. doi:10.1056/NEJMsa020660. PMID 12362011.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Helgason AR, Tomson T, Lund KE, Galanti R, Ahnve S, Gilljam H (2004). "Factors related to abstinence in a telephone helpline for smoking cessation". Eur J Public Health. 14 (3): 306–10. doi:10.1093/eurpub/14.3.306. PMID 15369039.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stead LF, Perera R, Lancaster T (2006). Stead, Lindsay F (ed.). "Telephone counselling for smoking cessation". Cochrane Database Syst Rev. 3 (3): CD002850. doi:10.1002/14651858.CD002850.pub2. PMID 16855992.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stead LF, Lancaster T (2005). Stead, Lindsay F (ed.). "Group behaviour therapy programmes for smoking cessation". Cochrane Database Syst Rev (2): CD001007. doi:10.1002/14651858.CD001007.pub2. PMID 15846610.
- ^ Lancaster T, Stead LF (2005). Lancaster, Tim (ed.). "Individual behavioural counselling for smoking cessation". Cochrane Database Syst Rev (2): CD001292. doi:10.1002/14651858.CD001292.pub2. PMID 15846616.
- ^ Lai DT, Cahill K, Qin Y, Tang JL (2010). Lai, Douglas TC (ed.). "Motivational interviewing for smoking cessation". Cochrane Database Syst Rev (1): CD006936. doi:10.1002/14651858.CD006936.pub2. PMID 20091612.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hettema JE, Hendricks PS (2010). "Motivational interviewing for smoking cessation: a meta-analytic review". J Consult Clin Psychol. 78 (6): 868–84. doi:10.1037/a0021498. PMID 21114344.
- ^ Heckman CJ, Egleston BL, Hofmann MT (2010). "Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis". Tob Control. 19 (5): 410–6. doi:10.1136/tc.2009.033175. PMC 2947553. PMID 20675688.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Perkins KA, Conklin CA, Levine MD (2008). Cognitive-behavioral therapy for smoking cessation: a practical guidebook to the most effective treatment. New York: Routledge. ISBN 978-0-415-95463-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Ruiz, F. J. (2010). "A review of Acceptance and Commitment Therapy (ACT) empirical evidence: Correlational, experimental psychopathology, component and outcome studies". International Journal of Psychology and Psychological Therapy. 10 (1): 125–62.
- ^ http://www.lung.org/stop-smoking/how-to-quit/freedom-from-smoking/[full citation needed]
- ^ Prochaska JO, Velicer WF, DiClemente CC, Fava J (1988). "Measuring processes of change: applications to the cessation of smoking". J Consult Clin Psychol. 56 (4): 520–8. doi:10.1037/0022-006X.56.4.520. PMID 3198809.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS (1991). "The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change" (PDF). J Consult Clin Psychol. 59 (2): 295–304. doi:10.1037/0022-006X.59.2.295. PMID 2030191. Retrieved 2011-02-15.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Velicer WF, Prochaska JO, Rossi JS, Snow MG (1992). "Assessing outcome in smoking cessation studies". Psychol Bull. 111 (1): 23–41. doi:10.1037/0033-2909.111.1.23. PMID 1539088.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Prochaska JO, DiClemente CC, Velicer WF, Rossi JS (1993). "Standardized, individualized, interactive, and personalized self-help programs for smoking cessation" (PDF). Health Psychol. 12 (5): 399–405. doi:10.1037/0278-6133.12.5.399. PMID 8223364. Retrieved 2011-02-15.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cahill K, Lancaster T, Green N (2010). Cahill, Kate (ed.). "Stage-based interventions for smoking cessation". Cochrane Database Syst Rev (11): CD004492. doi:10.1002/14651858.CD004492.pub4. PMID 21069681.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lancaster T, Stead LF (2005). Lancaster, Tim (ed.). "Self-help interventions for smoking cessation". Cochrane Database Syst Rev (3): CD001118. doi:10.1002/14651858.CD001118.pub2. PMID 16034855.
- ^ "Nicotine Anonymous (official website)". Dallas, TX: Nicotine Anonymous World Services. Retrieved 2011-02-21.
- ^ Glasser I (2010). "Nicotine Anonymous may benefit nicotine-dependent individuals". Am J Public Health. 100 (2): 196, author reply 196–7. doi:10.2105/AJPH.2009.181545. PMC 2804638. PMID 20019295.
- ^ "Stomp It Out". San Francisco, CA: Experience Project. Retrieved 2011-02-21.
- ^ Uhler D (1995-11-15). "Breaking the habit - these tips can keep your good intentions from going up in smoke". San Antonio Express-News.
- ^ Google Groups. "alt.support.stop-smoking". Retrieved 2011-02-21.
{{cite web}}:|author=has generic name (help) - ^ Hendrick B (2009-05-26). "Computer is an ally in quit-smoking fight. Study shows web- and computer-based programs help smokers quit". WebMD Health News. Retrieved 2011-02-21.
- ^ Myung SK, McDonnell DD, Kazinets G, Seo HG, Moskowitz JM (2009). "Effects of Web- and computer-based smoking cessation programs: meta-analysis of randomized controlled trials". Arch Intern Med. 169 (10): 929–37. doi:10.1001/archinternmed.2009.109. PMID 19468084.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Civljak M, Sheikh A, Stead LF, Car J (2010). Car, Josip (ed.). "Internet-based interventions for smoking cessation". Cochrane Database Syst Rev (9): CD007078. doi:10.1002/14651858.CD007078.pub3. PMID 20824856.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hutton HE, Wilson LM, Apelberg BJ, Avila Tang E, Odelola O, Bass EB, Chander G (Feb 2011). "A systematic review of randomized controlled trials: web-based interventions for smoking cessation among adolescents, college students, and adults". Nicotine Tob Res. 13 (4): 227–38. doi:10.1093/ntr/ntq252. PMID 21350042.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Whittaker R, Borland R, Bullen C, Lin RB, McRobbie H, Rodgers A (2009). Whittaker, Robyn (ed.). "Mobile phone-based interventions for smoking cessation". Cochrane Database Syst Rev (4): CD006611. doi:10.1002/14651858.CD006611.pub2. PMID 19821377.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Free C, Whittaker R, Knight R, Abramsky T, Rodgers A, Roberts IG (2009). "Txt2stop: a pilot randomised controlled trial of mobile phone-based smoking cessation support". Tob Control. 18 (2): 88–91. doi:10.1136/tc.2008.026146. PMID 19318534.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gonzales D, Redtomahawk D, Pizacani B, Bjornson WG, Spradley J, Allen E, Lees P (2007). "Support for spirituality in smoking cessation: results of pilot survey". Nicotine Tob Res. 9 (2): 299–303. doi:10.1080/14622200601078582. PMID 17365761.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "E-Cigarette – An Excellent Subsitute to Tobacco Cigarettes". AbsolutelyeCigs.com. 25 January 2012. Retrieved 9 May 2012.
- ^ "Marketers of electronic cigarettes should halt unproved therapy claims". World Health Organization. 2008-09-19. Retrieved 2011-02-21.
- ^ "Smoking - tips on how to quit". Nlm.nih.gov. 2012-07-27. Retrieved 2012-08-05.
- ^ "Cinnamon Curbs the Urge and More". Candycrate.com. Retrieved 2012-08-05.
- ^ Bauer JE, Carlin-Menter SM, Celestino PB, Hyland A, Cummings KM (2006). "Giving away free nicotine medications and a cigarette substitute (Better Quit) to promote calls to a quitline". J Public Health Manag Pract. 12 (1): 60–7. doi:10.1097/00124784-200601000-00012. PMID 16340517.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ He, Dong; Berg, John E.; Høstmark, Arne T. (March 1997). "Effects of acupuncture on smoking cessation or reduction for motivated smokers". Preventive Medicine. 26 (2): 208–214. doi:10.1006/pmed.1996.0125. PMID 9085389.
- ^ White AR, Rampes H, Liu JP, Stead LF, Campbell J (2011). White, Adrian R (ed.). "Acupuncture and related interventions for smoking cessation". Cochrane Database Syst Rev (1): CD000009. doi:10.1002/14651858.CD000009.pub3. PMID 21249644.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lis-Balchin M (2006). Aromatherapy science: a guide for healthcare professionals. London: Pharmaceutical Press. p. 101. ISBN 0-85369-578-4.
- ^ Rose JE, Behm FM (1994). "Inhalation of vapor from black pepper extract reduces smoking withdrawal symptoms". Drug Alcohol Depend. 34 (3): 225–9. doi:10.1016/0376-8716(94)90160-0. PMID 8033760.
- ^ "Hypnosis for Quitting Smoking". WebMD. Retrieved 19 May 2012.
- ^ Barnes J, Dong CY, McRobbie H, Walker N, Mehta M, Stead LF (2010). Barnes, Jo (ed.). "Hypnotherapy for smoking cessation". Cochrane Database Syst Rev (10): CD001008. doi:10.1002/14651858.CD001008.pub2. PMID 20927723.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Johnson, D.L.; Karkut, R.T. (October 1994). "Performance by gender in a stop-smoking program combining hypnosis and aversion". Psychological reports. 75 (2): 851–7. doi:10.2466/pr0.1994.75.2.851. PMID 7862796.
- ^ Law, Malcolm; Tang, Jin Ling (1995). "An analysis of the effectiveness of interventions intended to help people stop smoking". Arch Intern Med. 155 (18): 1933–1941. doi:10.1001/archinte.1995.00430180025004. PMID 7575046.
- ^ Carmody TP, Duncan C, Simon JA, Solkowitz S, Huggins J, Lee S, Delucchi K (2008). "Hypnosis for smoking cessation: a randomized trial". Nicotine Tob Res. 10 (5): 811–8. doi:10.1080/14622200802023833. PMID 18569754.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Mayo Clinic. "St. John's Wort for Tobacco Cessation".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Mayo, Clinic. "St. John's Wort for Tobacco Cessation". US National Institute of Health. Retrieved 29 November 2006.
- ^ FDA. "FDA Poisonous Plant Database".
{{cite journal}}: Cite journal requires|journal=(help) - ^ FDA. "FDA Poisonous Plant Database". U.S. Food and Drug Administration. Retrieved 1 January 2008.
- ^ SCENIHR. Health Effects of Smokeless Tobacco Products (PDF) (Report). p. 103.
- ^ http://colinmendelsohn.com.au/files/9513/7031/1653/Mendelsohn_C._NIcotine_dependence._Australian_Doctor_How_to_Treat._June_2013.pdf[full citation needed]
- ^ McRobbie, H (Jun 20, 2008). "New Zealand smoking cessation guidelines". The New Zealand medical journal. 121 (1276): 57–70. PMID 18574510.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Grimshaw GM, Stanton A (2006). Grimshaw, Gill (ed.). "Tobacco cessation interventions for young people". Cochrane Database Syst Rev (4): CD003289. doi:10.1002/14651858.CD003289.pub4. PMID 17054164.
- ^ "Intensive Counseling of Students by School Nurses Does Not Have Larger Impact on Long-Term Smoking Rates Than Briefer Sessions". Agency for Healthcare Research and Quality. 2013-05-15. Retrieved 2013-07-10.
- ^ Philip, Owen. "Pregnancy and Smoking". Net Doctor. Retrieved 9 April 2012.
- ^ Cahill K, Moher M, Lancaster T (2008). Cahill, Kate (ed.). "Workplace interventions for smoking cessation". Cochrane Database Syst Rev (4): CD003440. doi:10.1002/14651858.CD003440.pub3. PMID 18843645.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Leeks KD, Hopkins DP, Soler RE, Aten A, Chattopadhyay SK; Task Force on Community Preventive Services (2010). "Worksite-based incentives and competitions to reduce tobacco use. A systematic review" (PDF). Am J Prev Med. 38 (2 Suppl): S263–74. doi:10.1016/j.amepre.2009.10.034. PMID 20117611.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rigotti NA, Munafo MR, Stead LF (2007). Rigotti, Nancy (ed.). "Interventions for smoking cessation in hospitalised patients". Cochrane Database Syst Rev (3): CD001837. doi:10.1002/14651858.CD001837.pub2. PMID 17636688.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "What Works To Quit" (PDF). The National Tobacco Cessation Collaborative. Retrieved 2011-02-27.
- ^ Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007). "Damage to the insula disrupts addiction to cigarette smoking". Science. 315 (5811): 531–4. doi:10.1126/science.1135926. PMC 3698854. PMID 17255515.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ King G, Yerger VB, Whembolua GL, Bendel RB, Kittles R, Moolchan ET (2009). "Link between facultative melanin and tobacco use among African Americans" (PDF). Pharmacol Biochem Behav. 92 (4): 589–96. doi:10.1016/j.pbb.2009.02.011. PMID 19268687.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Christakis NA, Fowler JH (2008). "The collective dynamics of smoking in a large social network". N Engl J Med. 358 (21): 2249–58. doi:10.1056/NEJMsa0706154. PMC 2822344. PMID 18499567.
- ^ Park EW, Schultz JK, Tudiver F, Campbell T, Becker L (2004). Park, Eal Whan (ed.). "Enhancing partner support to improve smoking cessation". Cochrane Database Syst Rev (3): CD002928. doi:10.1002/14651858.CD002928.pub2. PMID 15266469.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Peoples, Clayton D.; Sigillo, Alexandra E.; Green, Morgan; Miller, Monica K. (2012). "Friendship and Conformity in Group Opinions: Juror Verdict Change in Mock Juries". Sociological Spectrum. 32 (2): 178. doi:10.1080/02732173.2012.646163.
- ^ a b Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J (1990). "Smoking, smoking cessation, and major depression". JAMA. 264 (12): 1546–9. doi:10.1001/jama.1990.03450120058029. PMID 2395194.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Condiotte MM, Lichtenstein E (1981). "Self-efficacy and relapse in smoking cessation programs". J Consult Clin Psychol. 49 (5): 648–58. doi:10.1037/0022-006X.49.5.648. PMID 7287974.
- ^ Shiffman S (1982). "Relapse following smoking cessation: a situational analysis". J Consult Clin Psychol. 50 (1): 71–86. doi:10.1037/0022-006X.50.1.71. PMID 7056922.
- ^ a b Hajek P, Stead LF, West R, Jarvis M, Lancaster T (2009). Stead, Lindsay F (ed.). "Relapse prevention interventions for smoking cessation". Cochrane Database Syst Rev (1): CD003999. doi:10.1002/14651858.CD003999.pub3. PMID 19160228.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k Kaiser Foundation Health Plan of the Northwest (2008). Cultivating Health: Freedom From Tobacco Kit. Kaiser Permanente. ISBN 978-0-9744864-8-2.[page needed]
- ^ H.-J. Aubin, A. Farley, D. Lycett, P. Lahmek, P. Aveyard. (2012). "Weight gain in smokers after quitting cigarettes: meta-analysis". BMJ-British Medical Journal. 345 (345): e4439. doi:10.1136/bmj.e4439.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vanni H, Kazeros A, Wang R, Harvey BG, Ferris B, De BP, Carolan BJ, Hübner RH, O'Connor TP, Crystal RG (2009). "Cigarette smoking induces overexpression of a fat-depleting gene AZGP1 in the human". Chest. 135 (5): 1197–208. doi:10.1378/chest.08-1024. PMC 2679098. PMID 19188554.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Jo YH, Talmage DA, Role LW (2002). "Nicotinic receptor-mediated effects on appetite and food intake". J Neurobiol. 53 (4): 618–32. doi:10.1002/neu.10147. PMC 2367209. PMID 12436425.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Klag MJ (1999). Johns Hopkins family health book. New York: HarperCollins. p. 86. ISBN 0-06-270149-5.
- ^ Farley AC, Hajek P, Lycett D, Aveyard P (2012). Aveyard, Paul (ed.). "Interventions for preventing weight gain after smoking cessation". Cochrane Database Syst Rev. 1 (1): CD006219. doi:10.1002/14651858.CD006219.pub3. PMID 22258966.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Covey LS, Glassman AH, Stetner F (1997). "Major depression following smoking cessation". Am J Psychiatry. 154 (2): 263–5. PMID 9016279.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Borrelli B, Bock B, King T, Pinto B, Marcus BH (1996). "The impact of depression on smoking cessation in women". Am J Prev Med. 12 (5): 378–87. PMID 8909649.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Máirtín S. McDermott, Theresa M. Marteau, Gareth J. Hollands, Matthew Hankins and Paul Aveyard. "Change in anxiety following successful and unsuccessful attempts at smoking cessation: cohort study". BJP.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ American Cancer Society (2011-01-31). "When smokers quit -- What are the benefits over time?". Retrieved 2011-02-20.
- ^ Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R (2000). "Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies". BMJ. 321 (7257): 323–9. doi:10.1136/bmj.321.7257.323. PMC 27446. PMID 10926586.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Doll R, Peto R, Boreham J, Sutherland I (2004). "Mortality in relation to smoking: 50 years' observations on male British doctors". BMJ. 328 (7455): 1519. doi:10.1136/bmj.38142.554479.AE. PMC 437139. PMID 15213107.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE; Lung Health Study Research Group (2005). "The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial". Annals of Internal Medicine. 142 (4): 233–9. doi:10.7326/0003-4819-142-4-200502150-00005. PMID 15710956.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Smoking Cessation Reduces Postoperative Complications". Journalist's Resource.org.
- ^ Cromwell J, Bartosch WJ, Fiore MC, Hasselblad V, Baker T (1997). "Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation". JAMA. 278 (21): 1759–66. doi:10.1001/jama.278.21.1759. PMID 9388153.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hoogendoorn M, Feenstra TL, Hoogenveen RT, Rutten-van Mölken MP (2010). "Long-term effectiveness and cost-effectiveness of smoking cessation interventions in patients with COPD". Thorax. 65 (8): 711–8. doi:10.1136/thx.2009.131631. PMID 20685746.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bauld L, Boyd KA, Briggs AH, Chesterman J, Ferguson J, Judge K, Hiscock R (2011). "One-year outcomes and a cost-effectiveness analysis for smokers accessing group-based and pharmacy-led cessation services". Nicotine Tob Res. 13 (2): 135–45. doi:10.1093/ntr/ntq222. PMID 21196451.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schiaffino A, Fernández E, Kunst A, Borrell C, García M, Borràs JM, Mackenbach JP (2007). "Time trends and educational differences in the incidence of quitting smoking in Spain (1965–2000)". Prev Med. 45 (2–3): 226–32. doi:10.1016/j.ypmed.2007.05.009. PMID 17604832.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fowkes FJ, Stewart MC, Fowkes FG, Amos A, Price JF (2008). "Scottish smoke-free legislation and trends in smoking cessation". Addiction. 103 (11): 1888–95. doi:10.1111/j.1360-0443.2008.02350.x. PMID 19032538.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Federico B, Costa G, Ricciardi W, Kunst AE (2009). "Educational inequalities in smoking cessation trends in Italy, 1982–2002". Tob Control. 18 (5): 393–8. doi:10.1136/tc.2008.029280. PMID 19617220.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zuo, Xiaoan; Zhao, Halin; Zhao, Xueyong; Guo, Yirui; Yun, Jianying; Wang, Shaokun; Miyasaka, Takafumi (2009). "Cigarette smoking among adults and trends in smoking cessation - United States, 2008". MMWR Morb Mortal Wkly Rep. 58 (44): 1227–32. PMID 19910909.
- ^ Qian J, Cai M, Gao J, Tang S, Xu L, Critchley JA (2010). "Trends in smoking and quitting in China from 1993 to 2003: National Health Service Survey data". Bull World Health Organ. 88 (10): 769–76. doi:10.2471/BLT.09.064709. PMC 2947036. PMID 20931062.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Martin, Anya (May 13, 2010). "What it takes to quit smoking". MarketWatch. Dow Jones. p. 2. Retrieved May 14, 2010.
Further reading
- Jason Wright (2013). "Knowing How To Quit Smoking". 1 (1): 38. ISBN 978-1494238452.
{{cite journal}}: Cite journal requires|journal=(help) - Henningfield J, Fant R, Buchhalter A, Stitzer M (2005). "Pharmacotherapy for nicotine dependence". CA Cancer J Clin. 55 (5): 281–99, quiz 322–3, 325. doi:10.3322/canjclin.55.5.281. PMID 16166074.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hughes JR, Keely J, Naud S (2004). "Shape of the relapse curve and long-term abstinence among untreated smokers". Addiction. 99 (1): 29–38. doi:10.1111/j.1360-0443.2004.00540.x. PMID 14678060.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Hutter H, Moshammer H, Neuberger M (2006). "Smoking cessation at the workplace: 1 year success of short seminars". Int Arch Occup Environ Health. 79 (1): 42–8. doi:10.1007/s00420-005-0034-y. PMID 16133522.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Marks DF (2005). Overcoming your smoking habit: a self-help guide using cognitive behavioral techniques. London: Robinson. ISBN 1-84529-067-4.
- Peters MJ, Morgan LC (2002). "The pharmacotherapy of smoking cessation". Med J Aust. 176 (10): 486–90. PMID 12065013.
- West R (2006). "Tobacco control: present and future". Br Med Bull. 77–78 (1): 123–36. doi:10.1093/bmb/ldl012. PMID 17106058.
External links
- McFarland JW, Folkenberg EJ (1964). How to stop smoking in five days (PDF). Englewood Cliffs, NJ: Prentice-Hall.

