Leflunomide: Difference between revisions
Remove link to dab, and bold alternative name |
Improving article acc. to WP:PHARMMOS and WP:MEDMOS, especially with regards to references (changing primary sources to secondary sources). |
||
| Line 10: | Line 10: | ||
| licence_EU = Arava |
| licence_EU = Arava |
||
| licence_US = Leflunomide |
| licence_US = Leflunomide |
||
| pregnancy_AU = X |
|||
| pregnancy_category = X (contraindicated) |
|||
| |
| pregnancy_US = X |
||
| legal_AU = S4 |
|||
| routes_of_administration = oral (tablets) |
|||
| legal_CA = Rx-only |
|||
| legal_UK = POM |
|||
| legal_US = Rx-only |
|||
| routes_of_administration = Oral (tablets) |
|||
<!--Pharmacokinetic data--> |
<!--Pharmacokinetic data--> |
||
| bioavailability = 80%<ref name = MSR>{{cite web|title=Arava (leflunomide) dosing, indications, interactions, adverse effects, and more|work=Medscape Reference|publisher=WebMD|accessdate=11 March 2014|url=http://reference.medscape.com/drug/arava-leflunomide-343203#showall}}</ref> |
|||
| bioavailability = 80% |
|||
| protein_bound = >99 |
| protein_bound = >99%<ref name = MSR/> |
||
| metabolism = |
| metabolism = GI mucosa and liver<ref name = MSR/> |
||
| elimination_half-life = |
| elimination_half-life = 14-18 days<ref name = MSR/> |
||
| excretion = |
| excretion = Faeces (48%), urine (43%)<ref name = MSR/> |
||
<!--Identifiers--> |
<!--Identifiers--> |
||
| Line 54: | Line 58: | ||
}} |
}} |
||
'''Leflunomide''' is a disease-modifying antirheumatic drug ([[DMARD]]) |
'''Leflunomide''' is a disease-modifying antirheumatic drug ([[DMARD]]) and [[immunosuppressant]]<ref name="pmid15271770">{{cite journal |author=Dougados M, Emery P, Lemmel EM, Zerbini CA, Brin S, van Riel P |title=When a DMARD fails, should patients switch to sulfasalazine or add sulfasalazine to continuing leflunomide? |journal=Annals of the rheumatic diseases |volume=64 |issue=1 |pages=44–51 |date=January 2005 |pmid=15271770 |pmc=1755199 |doi=10.1136/ard.2003.016709 |url=http://ard.bmj.com/cgi/pmidlookup?view=long&pmid=15271770}}</ref> used in active moderate to severe [[rheumatoid arthritis]] and [[psoriatic arthritis]]. It is a [[pyrimidine synthesis inhibitor]].<ref name="pmid17094333">{{cite journal |author=Pinto P, Dougados M |title=Leflunomide in clinical practice |journal=Acta reumatológica portuguesa |volume=31 |issue=3 |pages=215–24 |year=2006 |pmid=17094333 |doi= |url=http://www.spreumatologia.pt/download_fich.php?path=pdfs&filename=ARP_2006_4_216_AR_-_Leflunomide.pdf}}</ref> |
||
[[Image:Arava20mg.jpg|right|thumb|Bottle of Leflunomide (Arava) and tablet]] |
|||
It is sold under the brand name '''Arava''' by [[Sanofi-Aventis]]. It is available for oral administration as tablets containing 10, 20, or 100 mg of active drug.<ref>http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020905s025lbl.pdf</ref> Arava was approved by U.S., Canadian, European and other regulatory agencies in 1998. [[Image:Arava20mg.jpg|right|thumb|Bottle of Leflunomide (Arava) and 20mg tablet]] |
|||
== |
==Medical use== |
||
[[Rheumatoid arthritis]] and [[psoriatic arthritis]] are the only indications that have received regulatory approval.<ref name = MSR/><ref name = AMH>{{cite isbn|9780980579093}}</ref> |
|||
Especially the concomitant use of methotrexate may lead to severe or even fatal liver- or hepatotoxicity. Seventy-five percent of all cases of severe liver damage reported until early 2001 were seen under combined drug therapy Arava plus methotrexate. However, some studies have shown that the combination of methotrexate and leflunomide in patients with rheumatoid arthritis gave better results than either drug alone.<ref name = Lee>{{cite doi|10.1080/03009740802360632}}</ref> |
|||
Leflunomide is an [[immunomodulator]]y drug inhibiting mitochondrial enzyme [[dihydroorotate dehydrogenase]] (an enzyme involved in de novo pyrimidine synthesis) (abbreviation DHODH), which plays a key role in the de novo synthesis of the pyrimidine ribonucleotide uridine monophosphate (rUMP).<ref name="pmid17604599">{{cite journal |author=Fukushima R, Kanamori S, Hirashiba M, ''et al.'' |title=Teratogenicity study of the dihydroorotate-dehydrogenase inhibitor and protein tyrosine kinase inhibitor Leflunomide in mice |journal=Reprod. Toxicol. |volume=24 |issue=3-4 |pages=310–6 |year=2007 |pmid=17604599 |doi=10.1016/j.reprotox.2007.05.006 |url=http://linkinghub.elsevier.com/retrieve/pii/S0890-6238(07)00181-5}}</ref> The inhibition of human DHODH by A77 1726, the active metabolite of leflunomide, occurs at levels (approximately 600 nM) that are achieved during treatment of [[rheumatoid arthritis]] (RA). Leflunomide prevents the expansion of activated and autoimmune lymphocytes by interfering with their cell cycle progression while nonlymphoid cells are able to use another pathway to make their ribonucleotides by use of salvage pyrimidine pathway, which makes them less dependent on de novo synthesis.<ref>{{cite web|title=Clin Immunol. 1999 Dec;93(3):198-208.|url=http://www.ncbi.nlm.nih.gov/pubmed/10600330}}</ref> Genuine antiproliferative activity has been proven. In addition, several experimental models (both [[in vivo]] and [[in vitro]]) have demonstrated an anti-inflammatory effect. This double action is supposed to slow progression of the disease and to cause remission/relief of symptoms of [[rheumatoid arthritis]] and [[psoriatic arthritis]] such as joint tenderness and decreased joint and general mobility in human patients. |
|||
==Pharmacokinetics== |
|||
Arava tablets are 80% bioavailable. Co-administration with a high-fat meal did not have a significant impact on plasma levels of the active metabolite [[teriflunomide]]. Following oral administration, leflunomide is metabolized to teriflunomide, which is responsible for all of the drug's activity in vivo. Studies of the pharmacokinetics of leflunomide have primarily examined the plasma concentrations of teriflunomide. Plasma levels of unchanged leflunomide are occasionally detected, but at very low levels. Some minor metabolites have been noticed to occur in human plasma, which do not account for the beneficial drug effects. Teriflunomide is metabolized in the liver at cytosolic and microsomal sites and further excreted as well renally and billiary. |
|||
===Absorption and need for loading dose=== |
|||
After oral administration, peak plasma levels of teriflunomide occurred between 6 and 12 hours after dosing. Due to its very long half-life (approximately 2 weeks), a loading dose of 100 mg for 3 days was used in clinical studies to reach steady-state levels quickly. Without a loading dose, it is estimated that steady-state plasma concentrations would require nearly two months of dosing to be reached (nevertheless, one study showed fewer adverse effects and good efficacy if no loading dose is used at the beginning of treatment with leflunomide of patients with rheumatoid arthritis<ref name = Lee/>). The resulting plasma levels following both loading doses and continued clinical dosing indicate that plasma levels are dose proportional. Teriflunomide can be found as late as 2 years after termination of therapy in human plasma in sufficient levels to cause severe harm to pregnant women or to cause significant interactions.{{Citation needed|date=October 2010}} If quick removal from the body is necessary, an eleven-day scheme with [[cholestyramine]] or the use of [[activated charcoal]] is indicated and will soon decrease plasma levels below the critical limit of 0.02 mg/l. Limited experience shows that teriflunomide is not dialysable. |
|||
==Regular indications== |
|||
In the US Arava is indicated in adults for the treatment of active moderate to severe rheumatoid arthritis and psoriatic arthritis |
|||
* to reduce signs and symptoms |
|||
* to inhibit structural damage as evidenced by X-ray erosions and joint space narrowing |
|||
* to improve physical function. |
|||
The onset of clinical improvement can be expected after 4 to 6 weeks of continued therapy. |
|||
Aspirin, or other nonsteroidal anti-inflammatory agents (NSAR), and/or low-dose corticosteroids may be continued during treatment with leflunomide. The combined use of leflunomide with antimalarials, intramuscular or oral [[gold]], [[D-penicillamine]], [[azathioprine]], or [[methotrexate]] has not been adequately studied and is, therefore, contraindicated. |
|||
Especially the concomitant use of methotrexate may lead to severe or even fatal liver- or hepatotoxicity. Seventy-five percent of all cases of severe liver damage reported until early 2001 were seen under combined drug therapy Arava plus methotrexate. However, some studies have shown that the combination of methotrexate and leflunomide in patients with rheumatoid arthritis gave better results than either drug alone.<ref name = Lee>{{cite doi|10.1080/03009740802360632}}</ref><ref name = Kremer>Kremer, Joel et al (2004) [http://www.jrheum.com/subscribers/04/08/tables/PDF/1521.pdf Combination Leflunomide and Methotrexate (MTX) Therapy for Patients with Active Rheumatoid Arthritis Failing MTX Monotherapy: Open-Label Extension of a Randomized, Double-Blind, Placebo Controlled Trial] The Journal of Rheumatology, 31 (8): 1521-1531, accessed August 1, 2010</ref> |
|||
==Orphan drug status== |
|||
Leflunomide has recently been assigned [[orphan drug]] status for the prevention of |
|||
solid-organ rejection after [[allograft]] transplantations when co-administered with commonly used first-line agents (USA only). Most experience exists with liver and renal transplantations. The efficacy and safety of leflunomide has not been completely assessed so far in well-controlled and adequate studies. |
|||
[[Teriflunomide]] shows, in addition to the expected profound immunosuppressive potency, limited antiviral activity against CMV ([[cytomegalovirus]]). CMV infections endanger eyesight ([[retinitis]]) or even the lives of transplant patients (systemic infections) under conventional immunosuppressive therapy regimes. |
|||
==Other potential indications== |
|||
Clinical studies regarding the following diseases have been conducted:<ref>http://clinicaltrials.gov/ct2/results?term=Leflunomide</ref> <!-- hide the obvious ", but these have to be reviewed critically, before approval can be given:" --> |
Clinical studies regarding the following diseases have been conducted:<ref>http://clinicaltrials.gov/ct2/results?term=Leflunomide</ref> <!-- hide the obvious ", but these have to be reviewed critically, before approval can be given:" --> |
||
<!-- may be more useful to group by the latest phase they have started or reported on --> |
<!-- may be more useful to group by the latest phase they have started or reported on --> |
||
{{colbegin|3}} |
|||
* [[Polyoma BK Virus]] [[Diabetic nephropathy|Nephropathy]]<ref>{{cite journal|last=Blanckaert|first=K|coauthors=De Vriese, AS|title=Current recommendations for diagnosis and management of polyoma BK virus nephropathy in renal transplant recipients|journal=Nephrology Dialysis Transplantation|date=23 September 2006|volume=21|issue=12|pages=3364–3367|doi=10.1093/ndt/gfl404|url=http://ndt.oxfordjournals.org/content/21/12/3364.full.pdf|format=PDF}}</ref> |
|||
* [[Systemic lupus erythematosus]]<ref>{{cite journal|last=Wu|first=GC|coauthors=Xu, XD; Huang, Q; Wu, H|title=Leflunomide: friend or foe for systemic lupus erythematosus?|journal=Rheumatology International|date=February 2013|volume=33|issue=2|pages=273-6|doi=10.1007/s00296-012-2508-z|pmid=22961090}}</ref> |
|||
* [[Felty's syndrome]] <ref name="pmid12003373">{{cite journal |author=Sanders, S; Harisdangkul, V |title=Leflunomide for the treatment of rheumatoid arthritis and autoimmunity |journal=American Journal of Medical Sciences |volume=323 |issue=4 |pages=190–3 |year=2002 |pmid=12003373 |doi= 10.1097/00000441-200204000-00004|url=http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0002-9629&volume=323&issue=4&spage=190}}</ref> |
|||
* [[Takayasu arteritis]]<ref>{{cite journal|last=Unizony|first=S|coauthors=Stone, JH; Stone, JR|title=New treatment strategies in large-vessel vasculitis.|journal=Current Opinion in Rheumatology|date=January 2013|volume=25|issue=1|pages=3-9|doi=10.1097/BOR.0b013e32835b133a|pmid=23114585|url=http://www.medscape.com/viewarticle/776095}}</ref> |
|||
* [[Wegener's granulomatosis]]<ref name="pmid12003373" /> |
|||
* [[Ankylosing spondylitis]]<ref>{{cite journal|last=Haibel|first=H|coauthors=Rudwaleit, M; Braun, J; Sieper, J|title=Six months open label trial of leflunomide in active ankylosing spondylitis.|journal=Annals of the Rheumatic Diseases|date=January 2005|volume=64|issue=1|pages=124-6|doi=10.1136/ard.2003.019174|pmid=15608310|url=http://ard.bmj.com/content/64/1/124.full.pdf|format=PDF|pmc=1755172}}</ref> |
|||
* [[Crohn's disease]]<ref>{{cite journal|last=Prajapati|first=DN|coauthors=Knox, JF; Emmons, J; Saeian, K; Csuka, ME; Binion, DG|title=Leflunomide treatment of Crohn's disease patients intolerant to standard immunomodulator therapy.|journal=Journal of Clinical Gastroenterology|date=August 2003|volume=37|issue=2|pages=125-8|pmid=12869881}}</ref><ref>{{cite journal|last=Holtmann|first=MH|coauthors=Gerts, AL; Weinman, A; Galle, PR; Neurath, MF|title=Treatment of Crohn's disease with leflunomide as second-line immunosuppression : a phase 1 open-label trial on efficacy, tolerability and safety.|journal=Digestive Diseases and Sciences|date=April 2008|volume=53|issue=4|pages=1025-32|doi=10.1007/s10620-007-9953-7|pmid=17934840}}</ref> |
|||
* [[Sarcoidosis]]<ref>{{cite journal|last=Panselinas|first=E|coauthors=Judson, MA|title=Acute pulmonary exacerbations of sarcoidosis.|journal=Chest|date=October 2012|volume=142|issue=4|pages=827-36|doi=10.1378/chest.12-1060|pmid=23032450|url=http://journal.publications.chestnet.org/data/Journals/CHEST/25163/chest_142_4_827.pdf|format=PDF}}</ref> |
|||
* [[Uveitis]]<ref>{{cite journal|last=Roy|first=M|title=Early clinical experience with leflunomide in uveitis.|journal=Canadian Journal of Ophthalmology|date=August 2007|volume=42|issue=4|pages=634|pmid=17641721}}</ref> |
|||
* [[Adult-onset Still's disease|Still's disease]]<ref>{{cite journal|last=Pirildar|first=T|title=Treatment of adult-onset Still's disease with leflunomide and chloroquine combination in two patients.|journal=Clinical Rheumatology|date=May 2003|volume=22|issue=2|pages=157|doi=10.1007/s10067-002-0667-0|pmid=12740686}}</ref> |
|||
* [[Prostate cancer]]<ref>{{cite web|title=Mitoxantrone and Prednisone With or Without Leflunomide in Treating Patients With Stage IV Prostate Cancer|url=http://clinicaltrials.gov/ct2/show/NCT00004071|work=ClinicalTrials.gov|publisher=National Institute of Health|date=September 2012|accessdate=11 March 2014}}</ref> |
|||
* [[Pemphigoid]]<ref>{{cite web|title=Leflunomide Associated With Topical Corticosteroids for Bullous Pemphigoid (ARABUL) |
|||
|url=http://clinicaltrials.gov/ct2/show/NCT00802243|work=ClinicalTrials.gov|publisher=National Institute of Health|date=December 2008|accessdate=11 March 2014}}</ref> |
|||
* [[Polyoma BK Virus]] [[Diabetic nephropathy|Nephropathy]]<ref>[http://ndt.oxfordjournals.org/cgi/content/full/21/12/3364?ct Nephrology Dialysis Transplantation, Oxford Journals]</ref> |
|||
* Prevention of organ transplant rejection<ref name = rh2010>{{cite journal|last=Teschner|first=S|coauthors=Burst, V|title=Leflunomide: a drug with a potential beyond rheumatology.|journal=Immunotherapy|date=September 2010|volume=2|issue=5|pages=637-50|doi=10.2217/imt.10.52|pmid=20874647}}</ref> |
|||
* [[Systemic lupus erythematosus]]<ref>http://clinicaltrials.gov/ct2/show/NCT00637819 Phase II study of Leflunomide in Systemic Lupus Erythematosus</ref> |
|||
{{colend}} |
|||
* [[Felty's syndrome]] <ref name="pmid12003373">{{cite journal |author=Sanders S, Harisdangkul V |title=Leflunomide for the treatment of rheumatoid arthritis and autoimmunity |journal=Am. J. Med. Sci. |volume=323 |issue=4 |pages=190–3 |year=2002 |pmid=12003373 |doi= 10.1097/00000441-200204000-00004|url=http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0002-9629&volume=323&issue=4&spage=190}}</ref> |
|||
* [[Takayasu arteritis]]{{Citation needed|date=October 2010}} |
|||
* [[Wegener's granulomatosis]] <ref name="pmid12003373" /> |
|||
* [[Ankylosing spondylitis]]{{Citation needed|date=October 2010}} |
|||
* [[Crohn's disease]]{{Citation needed|date=October 2010}} |
|||
* [[Sarcoidosis]]{{Citation needed|date=October 2010}} |
|||
* [[Uveitis]]<ref>http://clinicaltrials.gov/ct2/show/NCT00001863 Phase II trial of Leflunomide to Treat Uveitis</ref> |
|||
* [[Adult-onset Still's disease|Still's disease]]{{Citation needed|date=October 2010}} |
|||
* [[Prostate cancer]]<ref>http://clinicaltrials.gov/ct2/show/NCT00004071 A pahse II/III study of Mitoxantrone and Prednisone With or Without Leflunomide in Treating Patients With Stage IV Prostate Cancer (COMPLETED)</ref> |
|||
* [[Pemphigoid]].<ref>http://clinicaltrials.gov/ct2/show/NCT00802243 Phase II study of Leflunomide Associated With Topical Corticosteroids for Bullous Pemphigoid (ARABUL)</ref> |
|||
One study has been made in pediatric patients with [[juvenile rheumatoid arthritis]] (JRA). In these, patient group clinical efficacy, side-effect profile, and pharmacokinetic data have been comparable to adult patients with rheumatoid arthritis on Arava alone. The results, however, have been somewhat inferior to the active control group, possibly reflecting a relative underdosing in the lower age of patients group.{{Citation needed|date=October 2010}} |
|||
Leflunomide has also been utilized for the treatment of [[Polychondritis|Relapsing Polychondritis]], but only case reports exist in the literature regarding this usage.{{Citation needed|date=February 2007}} |
|||
==Contraindications and precautions== |
|||
Leflunomide has a great number of absolute and relative contraindications, in part associated with its mode of action: |
|||
* [[Hypersensitivity]] to the drug or to inactive ingredients.* Important contraindications are "preexisting pregnancy", or women of childbearing potential not using reliable anticonceptive methods. Women should not become pregnant before 2 years after termination of therapy have elapsed or undergo a rapid wash-out procedure as stated above. Men wishing to father a child should discontinue leflunomide after consultation with their prescribing physician and also undergo the wash-out procedure.Animal studies with leflunomide showed an increase in teratogenicity and embryonic death, leflunomide is contraindicated in pregnancy. Men taking leflunomide should avoid getting their partner pregnant while taking leflunomide and for up to 64 days after therapy (at least one cycle of [[spermatogenesis]]).<ref>Brent RL. Teratogen update: reproductive risks of leflunomide (Arava); a pyrimidine synthesis inhibitor: counseling women taking leflunomide before or during pregnancy and men taking leflunomide who are contemplating fathering a child. Teratology 2001;63:106-12.</ref> |
|||
* Preexisting significant liver or renal disease and moderate to severe diseases of the bone marrow or immune system preclude the use of Leflunomide. |
|||
* Moderate to severe bacterial, fungal or viral infections (e.g., [[AIDS]], latent [[HIV]]-Infection, [[pneumonia]], active [[tuberculosis]]). |
|||
==Malignancies== |
|||
Due to its potent immunosuppression, leflunomide has the potential to promote myeloid/lymphatic malignancies or solid cancers. In postmarketing reports some cases of [[lymphoma]] have been noticed, the absolute number of cases and the case/patient ratio is unknown. In rheumatoid arthritis patients a several-fold increase of lymphoma is already found in those patients not treated with any DMARD.{{citation needed|date=May 2013}} |
|||
==Side-effects== |
==Side-effects== |
||
Its principle dose-limiting side effects are liver damage, lung disease and immunosuppression.<ref name = rh2010/> The most common side effects (occurring in >1% of those treated with it) are, in approximately descending order of frequency:<ref name = MSR/><ref name = AMH/><ref name = TGA>{{cite web|title=PRODUCT INFORMATION ARAVA®|work=TGA eBusiness Services|publisher=sanofi-aventis australia pty ltd|date=7 August 2012|accessdate=11 March 2014|url=https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-05926-3|format=PDF}}</ref><ref name = EMA>{{cite web|title=Arava : EPAR - Product Information|work=European Medicines Agency|publisher=Sanofi-Aventis Deutschland GmbH|date=21 November 2013|accessdate=11 March 2014|url=http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000235/WC500026289.pdf|format=PDF}}</ref><ref name = MS>{{cite web|title=Data Sheet Arava®|work=Medsafe|publisher=sanofi-aventis new zealand limited|date=29 June 2012|accessdate=11 March 2014|url=http://www.medsafe.govt.nz/profs/datasheet/a/aravatab.pdf|format=PDF}}</ref><ref name = DM>{{cite web|title=ARAVA (leflunomide) tablet, film coated [sanofi-aventis U.S. LLC]|work=DailyMed|publisher=sanofi-aventis U.S. LLC|date=November 2012|accessdate=11 March 2014|url=http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=320f63f2-fac3-4aee-aff8-85724e00ef52}}</ref><ref name = EMC>{{cite web|title=Arava 100mg Tablets - Summary of Product Characteristic|work=electronic Medicines Compendium|publisher=SANOFI|date=21 February 2014|accessdate=11 March 2014|url=http://www.medicines.org.uk/emc/medicine/26343/SPC/Arava+100mg+Tablets/}}</ref> diarrhoea, respiratory tract infections, hair loss, [[hypertension|high blood pressure]], rash, nausea, bronchitis, headache, abdominal pain, abnormal [[liver function test]]s, back pain, [[dyspepsia|indigestion]], urinary tract infection, dizziness, infection, joint disorder, itchiness, weight loss, loss of appetite, cough, gastroenteritis, [[pharyngitis]], [[stomatitis]], [[tenosynovitis]], vomiting, weakness, allergic reaction, chest pain, dry skin, eczema, [[paraesthesia]], [[pneumonia]], [[rhinitis]], [[synovitis]], [[cholelithiasis]] and [[dyspnoea|shortness of breath]]. Whereas uncommon side effects (occurring in 0.1-1% of those treated with the drug) include:<ref name = AMH/> constipation, oral [[thrush]], [[stomatitis]], taste disturbance, [[thrombocytopenia]] and [[urticaria|hives]]. Rarely (in 0.1% of those treated with it) it can cause:<ref name = AMH/> [[anaphylaxis]], [[angiooedema]], [[anaemia]], [[agranulocytosis]], [[eosinophilia]], [[leucopenia]], [[pancytopenia]], [[vasculitis]], [[toxic epidermal necrolysis]], [[Stevens-Johnson syndrome]], cutaneous [[lupus erythematosus]], severe infection, [[interstitial lung disease]], [[cirrhosis]] and [[liver failure]]. |
|||
The side-effects of Arava affect quite a number of organ systems, are frequent and at times severe or even fatal. |
|||
===Contraindications=== |
|||
Contraindications include:<ref name = MSR/> |
|||
{{colbegin|3}} |
|||
* Pregnancy, women of childbearing potential (unless contraception used) |
|||
* Liver disease, [[hepatitis B]]/[[Hepatitis C|C]] seropositive |
|||
* Active serious infections |
|||
* Hypersensitivity |
|||
{{colend}} |
|||
===Interactions=== |
|||
Other immunomodulatory treatments should be avoided due to the potential for additive immunosuppressant effects, or in the case of immunostimulants like [[echinacea]] or [[astragalus]], reduced therapeutic effects.<ref name = MSR/> Likewise live vaccines (like [[haemophilus influenzae type b]] vaccine and [[yellow fever]] vaccines) should be avoided due to the potential for severe infection due to the immunosuppressive nature of the treatment.<ref name = MSR/> |
|||
* Most serious is symptomatic liver damage ranging from jaundice to hepatitis, which can be fulminant, severe liver necrosis, and liver cirrhosis. Fatalities are known. Liver function studies may or may not precede the outbreak of clinical disease. The total incidence of severe liver damage is estimated to be as high as 0.5%, according to an internal report of the FDA. The [[European Medicines Agency|EMEA]], the European pendant to FDA, has in 2001 reported 296 cases of hepatotoxicity in 104,000 patient years, with 129 considered as serious, 2 cases of liver cirrhosis, and 15 cases of liver failure. Nine of the patients died. EMEA findings are that liver damage is typically seen within the first 6 months of therapy and is partially depending on cofactors, because of the serious cases 101 (78%) were concomitantly treated with other hepatotoxic drugs; 58% of those with asymptomatic elevations of liver function studies were cotreated with certain NSARs and/or methotrexate (see contraindications). In addition, 33% (=27 patients) of the patients with serious damage had other risk factors (history of alcohol abuse, liver function disturbance, acute heart failure, severe pulmonary disease or pancreatic carcinoma). Analysis of the data suggested that monitoring of liver function studies and wash-out periods may have not been fully adhered to. In case of any question, please refer to the procedures suggested in the EMEA statement as listed in section external links and references. |
|||
* Also very important is a relatively high incidence of [[myelosuppression]] with [[leukopenia]], and/or hypoplastic [[anemia]], and/or [[thrombocytopenia]]. Infections, sometimes as severe as development of active [[tuberculosis]], [[pneumonia]], [[Pneumocystis pneumonia|PCP]], and severe viral or mycotical infections, possibly leading to sepsis, death or permanent damage have been seen. Anemia or bleeding episodes may also lead to serious complications. |
|||
The concomitant use of [[methotrexate]], in particular, may lead to severe or even fatal liver- or hepatotoxicity. Seventy-five percent of all cases of severe liver damage reported until early 2001 were seen under combined drug therapy leflunomide plus methotrexate.<ref name = Lee/> However, some studies have shown that the combination of methotrexate and leflunomide in patients with rheumatoid arthritis gave better results than either drug alone.<ref name = Lee>{{cite doi|10.1080/03009740802360632}}</ref> |
|||
* [[Interstitial lung disease]] may occasionally be noticed and is recognized by progressive [[dyspnea]] and typical X-ray findings. This disease may or may not be reversible upon treatment and may lead to permanent disability or death. |
|||
==Mechanism of action== |
|||
* Other sites are: [[Gastrointestinal tract|GIT]], [[skin]] reactions up to life-threatening forms ([[Stevens–Johnson syndrome]] and [[toxic epidermal necrolysis]]), heart problems, alopecia (17%), CNS troubles etc. |
|||
Leflunomide is an [[immunomodulator]]y drug inhibiting mitochondrial enzyme [[dihydroorotate dehydrogenase]] (an enzyme involved in de novo pyrimidine synthesis) (abbreviation DHODH), which plays a key role in the ''de novo'' (from scratch) synthesis of the [[uridine monophosphate]] (rUMP), which is used in the synthesis of DNA and RNA.<ref name = rh2010/> The inhibition of human DHODH by [[teriflunomide]], the active metabolite of leflunomide, occurs at levels (approximately 600 nM) that are achieved during treatment of [[rheumatoid arthritis]] (RA). Leflunomide prevents the expansion of activated and autoimmune lymphocytes by interfering with their cell cycle progression while nonlymphoid cells are able to use another pathway to make their ribonucleotides by use of salvage pyrimidine pathway, which makes them less dependent on de novo synthesis.<ref>{{cite journal|last=Fox|first=RI|coauthors=Herrmann, ML; Frangou, CG; Wahl, GM; Morris, RE; Strand, V; Kirschbaum, BJ|title=Mechanism of action for leflunomide in rheumatoid arthritis.|journal=Clinical Immunology|date=December 1999|volume=93|issue=3|pages=198-208|doi=10.1006/clim.1999.4777|pmid=10600330}}</ref> |
|||
If severe side-effects are encountered, teriflunomide can be readily removed from the body with oral cholestyramine or activated charcoal (see above) to slow or reverse the noted side-effects. |
|||
==Pharmacokinetics== |
|||
==Interactions== |
|||
It has an oral bioavailability of 80%, protein binding of >99%, metabolism sites of the GI mucosa and liver, [[volume of distribution]] of 0.13L/kg, [[elimination half-life]] of 14-18 days and excretion routes of faeces (48%) and urine (43%).<ref name = MSR/><ref name = TGA/> |
|||
* Alcohol, other DMARDs including [[chloroquine]]/[[hydroxychloroquine]], live virus [[vaccines]], [[tegafur]], some tuberculostatics ([[rifampin]] and/or [[isoniazid]]), [[tolbutamide]] and [[warfarin]] should not be given concomitantly. |
|||
==Dosage regimen== |
|||
Usually, an oral loading dose of 100 mg is followed by a once-a-day administration of 10 to 20 mg as determined by a specialized clinician. He/she will also determine the total duration of treatment. Experience regarding the duration of treatment has been gained in 2 studies, in one study treatment has been continued for 1 year, in the other for 2 years. After termination of treatment, beneficial effects may last for some years. |
|||
==Necessary laboratory examinations== |
|||
* Hematologic Monitoring |
|||
Patients taking Arava should have [[platelet]] count, [[white blood cell]] count, and [[hemoglobin]] or [[hematocrit]] monitored before initiation of treatment (baseline values), monthly for six months following initiation of therapy, and every 6 to 8 weeks thereafter. |
|||
* Bone Marrow Suppression Monitoring for Combination Therapy with Immunosuppressants |
|||
If used concomitantly with [[immunosuppressants]] such as methotrexate, chronic monitoring should be monthly. |
|||
* Liver Enzyme Monitoring |
|||
[[Alanine transaminase|ALT]] ([[SGPT]]) values must be obtained at baseline and monitored at monthly intervals during the first six months then, if stable, every 6 to 8 weeks thereafter. In addition, if leflunomide and methotrexate are given concomitantly, ACR guidelines for monitoring methotrexate liver toxicity must be followed with ALT, [[Alanine transaminase|AST]], and [[human serum albumin|serum albumin]] testing every month. |
|||
==Patient counselling== |
|||
Patients should be carefully informed as to report immediately any subjective early signs of liver damage, bone marrow damage, serious infection, life-threatening skin reactions, and interstitial lung disease to their physician. This is particularly important for the interval between laboratory examinations. |
|||
When counselling, firstly identify yourself to the patient and ensure that the name of the medication they are using is provided. Then state the purpose of the medication, common side effects and how to avoid or manage these side effects, the benefits of using therapy, any precautions, the correct dose and way to use a delivery device (if appropriate), ways to self monitor, storage and what to do if a dose is missed. Also be sure to check whether they are taking any other medications which may interact with this medication, whether prescription, OTC (over the counter) or alternative therapies. Actively listen to what the patient says and ensure that you summarise points to confirm that you have heard them correctly. |
|||
==Summary and safety controversy== |
|||
Arava is a potent drug comparing favourably with other DMARDs regarding the efficacy as measured by improvements on the ACR scale. Leflunomide met the ACR20 criteria in up to 56% of patients; most other drugs (e.g., methotrexate alone, [[sulfasalazine]], [[Tumor necrosis factors|TNF]]-inhibitors ([[infliximab]], [[etanercept]], and [[adalimumab]]), the latter drugs also in combination with methotrexate) reach values from 20% only up to approximately 50%. Arava was withdrawn in clinical studies in 36% of patients due to different reasons (intolerable side-effects, lack of efficacy, unspecified reasons); the incidence was not higher than observed in the methotrexate control group. However, postmarketing data regarding the high incidence of severe liver damage, serious myelosuppression, profound immunosuppression leading to serious or even fatal infections, the possibility that Arava is a human [[carcinogen]], and the occurrence of interstitial lung disease has led to the forming of patient groups in the USA and Europe, for example, supported by safety aware physicians. These groups call for the local or worldwide ban or discontinuation of Arava.{{Citation needed|date=October 2010}} |
|||
{{-}} |
|||
==References== |
==References== |
||
{{reflist|2}} |
{{reflist|2}} |
||
Revision as of 00:06, 11 March 2014
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Arava |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a600032 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80%[2] |
| Protein binding | >99%[2] |
| Metabolism | GI mucosa and liver[2] |
| Elimination half-life | 14-18 days[2] |
| Excretion | Faeces (48%), urine (43%)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.123.883 |
| Chemical and physical data | |
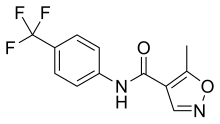
| Formula | C12H9F3N2O2 |
| Molar mass | 270.207 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Leflunomide is a disease-modifying antirheumatic drug (DMARD) and immunosuppressant[3] used in active moderate to severe rheumatoid arthritis and psoriatic arthritis. It is a pyrimidine synthesis inhibitor.[4]

Medical use
Rheumatoid arthritis and psoriatic arthritis are the only indications that have received regulatory approval.[2][5]
Especially the concomitant use of methotrexate may lead to severe or even fatal liver- or hepatotoxicity. Seventy-five percent of all cases of severe liver damage reported until early 2001 were seen under combined drug therapy Arava plus methotrexate. However, some studies have shown that the combination of methotrexate and leflunomide in patients with rheumatoid arthritis gave better results than either drug alone.[6]
Clinical studies regarding the following diseases have been conducted:[7]
- Polyoma BK Virus Nephropathy[8]
- Systemic lupus erythematosus[9]
- Felty's syndrome [10]
- Takayasu arteritis[11]
- Wegener's granulomatosis[10]
- Ankylosing spondylitis[12]
- Crohn's disease[13][14]
- Sarcoidosis[15]
- Uveitis[16]
- Still's disease[17]
- Prostate cancer[18]
- Pemphigoid[19]
- Prevention of organ transplant rejection[20]
Side-effects
Its principle dose-limiting side effects are liver damage, lung disease and immunosuppression.[20] The most common side effects (occurring in >1% of those treated with it) are, in approximately descending order of frequency:[2][5][21][22][23][24][25] diarrhoea, respiratory tract infections, hair loss, high blood pressure, rash, nausea, bronchitis, headache, abdominal pain, abnormal liver function tests, back pain, indigestion, urinary tract infection, dizziness, infection, joint disorder, itchiness, weight loss, loss of appetite, cough, gastroenteritis, pharyngitis, stomatitis, tenosynovitis, vomiting, weakness, allergic reaction, chest pain, dry skin, eczema, paraesthesia, pneumonia, rhinitis, synovitis, cholelithiasis and shortness of breath. Whereas uncommon side effects (occurring in 0.1-1% of those treated with the drug) include:[5] constipation, oral thrush, stomatitis, taste disturbance, thrombocytopenia and hives. Rarely (in 0.1% of those treated with it) it can cause:[5] anaphylaxis, angiooedema, anaemia, agranulocytosis, eosinophilia, leucopenia, pancytopenia, vasculitis, toxic epidermal necrolysis, Stevens-Johnson syndrome, cutaneous lupus erythematosus, severe infection, interstitial lung disease, cirrhosis and liver failure.
Contraindications
Contraindications include:[2]
- Pregnancy, women of childbearing potential (unless contraception used)
- Liver disease, hepatitis B/C seropositive
- Active serious infections
- Hypersensitivity
Interactions
Other immunomodulatory treatments should be avoided due to the potential for additive immunosuppressant effects, or in the case of immunostimulants like echinacea or astragalus, reduced therapeutic effects.[2] Likewise live vaccines (like haemophilus influenzae type b vaccine and yellow fever vaccines) should be avoided due to the potential for severe infection due to the immunosuppressive nature of the treatment.[2]
The concomitant use of methotrexate, in particular, may lead to severe or even fatal liver- or hepatotoxicity. Seventy-five percent of all cases of severe liver damage reported until early 2001 were seen under combined drug therapy leflunomide plus methotrexate.[6] However, some studies have shown that the combination of methotrexate and leflunomide in patients with rheumatoid arthritis gave better results than either drug alone.[6]
Mechanism of action
Leflunomide is an immunomodulatory drug inhibiting mitochondrial enzyme dihydroorotate dehydrogenase (an enzyme involved in de novo pyrimidine synthesis) (abbreviation DHODH), which plays a key role in the de novo (from scratch) synthesis of the uridine monophosphate (rUMP), which is used in the synthesis of DNA and RNA.[20] The inhibition of human DHODH by teriflunomide, the active metabolite of leflunomide, occurs at levels (approximately 600 nM) that are achieved during treatment of rheumatoid arthritis (RA). Leflunomide prevents the expansion of activated and autoimmune lymphocytes by interfering with their cell cycle progression while nonlymphoid cells are able to use another pathway to make their ribonucleotides by use of salvage pyrimidine pathway, which makes them less dependent on de novo synthesis.[26]
Pharmacokinetics
It has an oral bioavailability of 80%, protein binding of >99%, metabolism sites of the GI mucosa and liver, volume of distribution of 0.13L/kg, elimination half-life of 14-18 days and excretion routes of faeces (48%) and urine (43%).[2][21]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b c d e f g h i j k "Arava (leflunomide) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 11 March 2014.
- ^ Dougados M, Emery P, Lemmel EM, Zerbini CA, Brin S, van Riel P (January 2005). "When a DMARD fails, should patients switch to sulfasalazine or add sulfasalazine to continuing leflunomide?". Annals of the rheumatic diseases. 64 (1): 44–51. doi:10.1136/ard.2003.016709. PMC 1755199. PMID 15271770.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pinto P, Dougados M (2006). "Leflunomide in clinical practice" (PDF). Acta reumatológica portuguesa. 31 (3): 215–24. PMID 17094333.
- ^ a b c d Template:Cite isbn
- ^ a b c Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1080/03009740802360632, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1080/03009740802360632instead. - ^ http://clinicaltrials.gov/ct2/results?term=Leflunomide
- ^ Blanckaert, K (23 September 2006). "Current recommendations for diagnosis and management of polyoma BK virus nephropathy in renal transplant recipients" (PDF). Nephrology Dialysis Transplantation. 21 (12): 3364–3367. doi:10.1093/ndt/gfl404.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Wu, GC (February 2013). "Leflunomide: friend or foe for systemic lupus erythematosus?". Rheumatology International. 33 (2): 273–6. doi:10.1007/s00296-012-2508-z. PMID 22961090.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Sanders, S; Harisdangkul, V (2002). "Leflunomide for the treatment of rheumatoid arthritis and autoimmunity". American Journal of Medical Sciences. 323 (4): 190–3. doi:10.1097/00000441-200204000-00004. PMID 12003373.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Unizony, S (January 2013). "New treatment strategies in large-vessel vasculitis". Current Opinion in Rheumatology. 25 (1): 3–9. doi:10.1097/BOR.0b013e32835b133a. PMID 23114585.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Haibel, H (January 2005). "Six months open label trial of leflunomide in active ankylosing spondylitis" (PDF). Annals of the Rheumatic Diseases. 64 (1): 124–6. doi:10.1136/ard.2003.019174. PMC 1755172. PMID 15608310.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Prajapati, DN (August 2003). "Leflunomide treatment of Crohn's disease patients intolerant to standard immunomodulator therapy". Journal of Clinical Gastroenterology. 37 (2): 125–8. PMID 12869881.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Holtmann, MH (April 2008). "Treatment of Crohn's disease with leflunomide as second-line immunosuppression : a phase 1 open-label trial on efficacy, tolerability and safety". Digestive Diseases and Sciences. 53 (4): 1025–32. doi:10.1007/s10620-007-9953-7. PMID 17934840.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Panselinas, E (October 2012). "Acute pulmonary exacerbations of sarcoidosis" (PDF). Chest. 142 (4): 827–36. doi:10.1378/chest.12-1060. PMID 23032450.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Roy, M (August 2007). "Early clinical experience with leflunomide in uveitis". Canadian Journal of Ophthalmology. 42 (4): 634. PMID 17641721.
- ^ Pirildar, T (May 2003). "Treatment of adult-onset Still's disease with leflunomide and chloroquine combination in two patients". Clinical Rheumatology. 22 (2): 157. doi:10.1007/s10067-002-0667-0. PMID 12740686.
- ^ "Mitoxantrone and Prednisone With or Without Leflunomide in Treating Patients With Stage IV Prostate Cancer". ClinicalTrials.gov. National Institute of Health. September 2012. Retrieved 11 March 2014.
- ^ "Leflunomide Associated With Topical Corticosteroids for Bullous Pemphigoid (ARABUL)". ClinicalTrials.gov. National Institute of Health. December 2008. Retrieved 11 March 2014.
- ^ a b c Teschner, S (September 2010). "Leflunomide: a drug with a potential beyond rheumatology". Immunotherapy. 2 (5): 637–50. doi:10.2217/imt.10.52. PMID 20874647.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b "PRODUCT INFORMATION ARAVA®" (PDF). TGA eBusiness Services. sanofi-aventis australia pty ltd. 7 August 2012. Retrieved 11 March 2014.
- ^ "Arava : EPAR - Product Information" (PDF). European Medicines Agency. Sanofi-Aventis Deutschland GmbH. 21 November 2013. Retrieved 11 March 2014.
- ^ "Data Sheet Arava®" (PDF). Medsafe. sanofi-aventis new zealand limited. 29 June 2012. Retrieved 11 March 2014.
- ^ "ARAVA (leflunomide) tablet, film coated [sanofi-aventis U.S. LLC]". DailyMed. sanofi-aventis U.S. LLC. November 2012. Retrieved 11 March 2014.
- ^ "Arava 100mg Tablets - Summary of Product Characteristic". electronic Medicines Compendium. SANOFI. 21 February 2014. Retrieved 11 March 2014.
- ^ Fox, RI (December 1999). "Mechanism of action for leflunomide in rheumatoid arthritis". Clinical Immunology. 93 (3): 198–208. doi:10.1006/clim.1999.4777. PMID 10600330.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
External links
- National Rheumatoid Arthritis Society (NRAS) Information about Disease Modifying drugs such as Leflunomide
- http://www.arava.com/professional/home.do (full prescribing information)
- http://www.rheuma-online.de/medikamente/leflunomid-arava/studien-zu-leflunomid-arava/gibt-es-untersuchungen-zu-leflunomid-in-weiteren-einsatzgebieten.html (in German, regarding potential indications)
- http://www.arznei-telegramm.de/register/0204507.pdf (in German, regarding discontinuation of the drug)
- http://www.emea.europa.eu/pdfs/human/press/pus/561101en.pdf (warning as of 2001 regarding hepatotoxicity) (URL DEAD 16 Oct 2010)
- The safety of leflunomide Australian Prescriber
