Meprobamate: Difference between revisions
Updating {{drugbox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'ChEBI_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Wikipedia talk:WikiProject_Pharmacology|er |
→Health issues: Added ZA |
||
| Line 132: | Line 132: | ||
==Health issues== |
==Health issues== |
||
Meprobamate is a [[Convention on Psychotropic Substances#Schedules of Controlled Substances|Schedule IV]] drug under the [[Convention on Psychotropic Substances]]. With protracted use it can cause physical dependence and a potentially life-threatening abstinence syndrome similar to that of [[barbiturates]] and [[alcohol]]. |
Meprobamate is a [[Convention on Psychotropic Substances#Schedules of Controlled Substances|Schedule IV]] drug (US) (S5 in South Africa) under the [[Convention on Psychotropic Substances]]. With protracted use it can cause physical dependence and a potentially life-threatening abstinence syndrome similar to that of [[barbiturates]] and [[alcohol]]. |
||
==Chemistry== |
==Chemistry== |
||
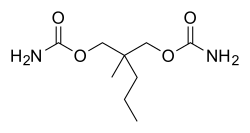
Meprobamate, 2-methyl-2-propyl-1,3-propandiol dicarbamate is synthesized by the reaction of 2-methylvaleraldehyde with two molecules of [[formaldehyde]] and the subsequent transformation of the resulting 2-methyl-2-propylpropan-1,3-diol into the di[[carbamate]] via successive reactions with [[phosgene]] and [[ammonia]]. |
Meprobamate, 2-methyl-2-propyl-1,3-propandiol dicarbamate is synthesized by the reaction of 2-methylvaleraldehyde with two molecules of [[formaldehyde]] and the subsequent transformation of the resulting 2-methyl-2-propylpropan-1,3-diol into the di[[carbamate]] via successive reactions with [[phosgene]] and [[ammonia]]. |
||
Revision as of 10:03, 23 August 2011
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Elimination half-life | 10 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.306 |
| Chemical and physical data | |
| Formula | C9H18N2O4 |
| Molar mass | 218.250 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.229 g/cm3 |
| Melting point | 105–106 °C (221–223 °F) |
| Boiling point | 200–210 °C (392–410 °F) |
| |
| |
| (verify) | |
Meprobamate (marketed under the brand names Miltown by Wallace Laboratories, Equanil by Wyeth, and Meprospan) is a carbamate derivative which is used as an anxiolytic drug. It was the best-selling minor tranquilizer for a time, but has largely been replaced by the benzodiazepines.
History
Meprobamate was first synthesized by Bernard John Ludwig, PhD, and Frank Milan Berger, MD, at Carter Products in May 1950. Wallace Laboratories, a subsidiary of Carter Products, bought the license and named it Miltown after the borough of Milltown in New Jersey. Launched in 1955, it rapidly became the first blockbuster psychotropic drug in American history, becoming popular in Hollywood and gaining notoriety for its seemingly miraculous effects.[1]
In the mid-1940s, Dr. Berger was working in a laboratory of a British drug company, looking for a preservative for penicillin, when he noticed that a compound called mephenesin had a sedative effect in small laboratory animals (rodents). Dr. Berger subsequently referred to this sedating or “tranquilizing” effect in a now-historic article, published by The British Journal of Pharmacology in 1946. However, there were three major drawbacks to the use of mephenesin as a tranquilizer: a very short duration of action, greater effect on the spinal cord than on the brain, and a weak activity. [2] After moving to Wallace Laboratories in New Jersey, Dr. Berger and a chemist, Dr. Bernard Ludwig, synthesized a chemically-related tranquilizing compound, meprobamate, that was able to overcome these three drawbacks.[3] It was soon prescribed under the trade name Miltown.
A December 1955 study of 101 patients at the Mississippi State Hospital in Whitfield, Mississippi, found meprobamate useful in the alleviation of "mental symptoms." Three percent of the patients made a complete recovery, 29% were greatly improved, and 50% were somewhat better. Eighteen percent realized little change. Self-destructive patients became cooperative and calmer, and experienced a resumption of logical thinking. In 50% of the cases relaxation brought about more favorable sleep habits. Hydrotherapy and all types of shock treatment were halted.[4] Meprobamate was found to help in the treatment of alcoholics by 1956.[5] By 1957, over 36 million prescriptions had been filled for meprobamate in the US alone, a billion pills had been manufactured, and it accounted for fully a third of all prescriptions written.[6] Dr. Berger, clinical director of Wallace Laboratories (who died on March 16, 2008, aged 94[7]), described it as a relaxant of the central nervous system, whereas other tranquilizers suppressed it. A University of Michigan study found that meprobamate affected driving skills. Though patients reported being able to relax more easily, meprobamate did not completely alleviate their tense feelings. The disclosures came at a special scientific meeting at the Barbizon Plaza Hotel in New York City, at which Aldous Huxley addressed an evening session. He predicted the development of many chemicals "capable of changing the quality of human consciousness," in the next few years.[8] Coincidentally, carisoprodol, a prodrug of meprobamate, was initially marketed under the trade name of "Soma," which was also a fictional drug in Aldous Huxley's Brave New World. Latterly carisoprodol was marketed as a skeletal muscle relaxant under the name of "Carisoma." It was never as popular as the rival products baclofen or dantrolene, and is principally known for having inspired the "Ashworth Scale" to rate the degree of spasticity.[citation needed]
In January 1960 Carter Products, Inc., makers of Miltown and American Home Products Corporation, which marketed Equanil, were charged with having conspired to monopolize the market in mild tranquilizers. It was revealed that the sale of meprobamate earned $40,000,000 for the defendants. Of this amount American Home Products accounted for approximately 2/3 and Carter about 1/3. The U.S. Government sought an order mandating that Carter make its meprobamate patent available at no charge to any company desiring to use it.[9]
In April 1965 meprobamate was removed from the list of tranquilizers when experts ruled that the drug was a sedative instead. The U.S. Pharmacopoeia published the ruling. At the same time the Medical Letter disclosed that meprobamate could be addictive at dosage levels not much above recommended.[10] In December 1967 meprobamate was placed under abuse control amendments to the Food, Drug and Cosmetic Act. Records on production and distribution were required to be kept. Limits were placed on prescription duration and refills.[11]
Production continued throughout the 1960s but by 1970 it was listed as a controlled substance after it was discovered to cause physical and psychological dependence. The drug is considered[by whom?] to be the forerunner of the modern-era benzodiazepine class of anti-anxiety and sedative/hypnotic drugs (as the pharmacological actions of the benzodiazepines on the central nervous system mimic those of meprobamate). The first member of the benzodiazepine class, chlordiazepoxide (synthesized by the Swiss firm, Hoffman LaRoche and marketed as Librium when introduced in 1960), gave rise to the drug diazepam — better known by its original brand-name, Valium (also introduced by Roche Products).
Pharmacology
Although it was marketed as being safer, meprobamate has most of the pharmacological effects and dangers of the barbiturates (though it is less sedating at effective doses). It is reported to have some anticonvulsant properties against absence seizures, but can exacerbate generalized tonic-clonic seizures.
Meprobamate's mechanism of action is not completely known. It has been shown in animal studies to have effects at multiple sites in the central nervous system, including the thalamus and limbic system. Meprobamate binds to GABAA receptors[12] which interrupts neuronal communication in the reticular formation and spinal cord, causing sedation and altered perception of pain. It has been shown that meprobamate has the ability to activate currents even in the absence of GABA.[13] It is also a potent adenosine reuptake inhibitor (AdoRI).[14][15]
Related drugs include carisoprodol (a prodrug of meprobamate) and tybamate.
Indications
Meprobamate is licensed for the short-term relief of anxiety, although it is not known whether the purported anti-anxiety effects of meprobamate are separable from its sedative effects. Its effectiveness as a selective agent for the treatment of anxiety has not been proven in humans,[16] and is not used as often as the benzodiazepines for this purpose.
Meprobamate is available in 200 mg and 400 mg tablets for oral administration. Meprobamate is also a component of the combination drug Equagesic (discontinued in the UK in 2002) acting as a muscle relaxant.
Meprobamate is also found as a component of the combination drug "Stopayne" capsules

Overdose
Symptoms of meprobamate overdose include: drowsiness, sluggishness, unresponsiveness, or coma; loss of muscle control; severe impairment or cessation of breathing; or shock. Death has been reported with ingestion of as little as 12g of meprobamate and survival with as much as 40g. In an overdose, meprobamate tablets may form a gastric bezoar, requiring physical removal of the undissolved mass of tablets through an endoscope; therefore, administration of activated charcoal should be considered even after 4 or more hours or if levels are rising.
Health issues
Meprobamate is a Schedule IV drug (US) (S5 in South Africa) under the Convention on Psychotropic Substances. With protracted use it can cause physical dependence and a potentially life-threatening abstinence syndrome similar to that of barbiturates and alcohol.
Chemistry
Meprobamate, 2-methyl-2-propyl-1,3-propandiol dicarbamate is synthesized by the reaction of 2-methylvaleraldehyde with two molecules of formaldehyde and the subsequent transformation of the resulting 2-methyl-2-propylpropan-1,3-diol into the dicarbamate via successive reactions with phosgene and ammonia.

- F.M. Berger, B.J. Ludwig, U.S. patent 2,724,720 (1955).
- F.A. Fries, K. Moenkemeyer, CH 373026 (1963).
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1021/ja01156a086, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1021/ja01156a086instead.
References
- ^ Tone, Andrea (2009). "The Fashionable Pill". The Age of Anxiety: A History of America's Turbulent Affair with Tranquilizers. New York: Basic Books. ISBN 9780465086580.
- ^ Berger FM. (1947). "Mode of Action of Myanesin". Br J Pharmacol. 2 (4): 241–250.
- ^ Ludwig BJ, Piech E. (1951). "Some anticonvulsant agents derived from 1, 3-propanediol". J Am Chem Soc. 73 (12): 5779–5781. doi:10.1021/ja01156a086.
- ^ New Hope Arises On Cancer Serum, New York Times, December 28, 1955, Page 21.
- ^ Author unknown (1956-04-01). "ALCOHOLIC PERIL FOUND IN DRUGS; Some Tranquilizing Therapy May Be Habit-Forming, Physicians Tell Parley". New York Times. p. 28. Retrieved 2009-01-23.
{{cite news}}:|last=has generic name (help) - ^ Dokoupil, Tony (2009-01-22). "How Mother Found Her Helper". Newsweek. Retrieved 2009-01-23.
- ^ Carey, Benedict (2008-03-21). "Frank Berger, 94, Miltown Creator, Dies - New York Times". New York Times. Retrieved 2009-02-01.
- ^ "'BEHAVIOR' DRUGS NOW ENVISIONED; Aldous Huxley Predicts They Will Bring Re-Examining of Ethics and Religion". New York Times. 1956-10-19. Retrieved 2009-02-01.
- ^
Ranzal, Edward (1960-1-28). "TRUST SUIT NAMES 2 DRUG CONCERNS; Makers of Tranquilizers Are Accused". New York Times. Retrieved 2009-02-01.
{{cite web}}: Check date values in:|date=(help) - ^
"MILTOWN OFF LIST OF TRANQUILIZERS; But It Will Continue to Be Used as a Sedative". New York Times. 1965-4-22. Retrieved 2009-02-01.
{{cite web}}: Check date values in:|date=(help) - ^
"Tranquilizer Is Put Under U.S. Curbs; Side-Effects Noted". New York Times. 1967-12-6. Retrieved 2009-02-01.
{{cite web}}: Check date values in:|date=(help) - ^ Rho JM, Donevan SD, Rogawski MA (1997). "Barbiturate-like actions of the propanediol dicarbamates felbamate and meprobamate". J. Pharmacol. Exp. Ther. 280 (3): 1383–91. PMID 9067327.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Rho JM, Donevan SD, Rogawski MA (1997). "Barbiturate-like actions of the propanediol dicarbamates felbamate and meprobamate". J. Pharmacol. Exp. Ther. 280 (3): 1383–91. PMID 9067327.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Phillis JW, Delong RE. (1984). "A purinergic component in the central actions of meprobamate". Eur J Pharmacol. 101 (3–4): 295–297. doi:10.1016/0014-2999(84)90174-2. PMID 6468504.
- ^ DeLong RE, Phillis JW, Barraco RA. (1985). "A possible role of endogenous adenosine in the sedative action of meprobamate". Eur J Pharmacol. 118 (3): 359–362. doi:10.1016/0014-2999(85)90149-9. PMID 4085561.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brunton, Laurence (2005-10-28). Goodman And Gilman's The Pharmacological Basis of Therapeutics (11 ed.). McGraw-Hill Professional. ISBN 0071422803.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
