Wikipedia:Reference desk/Science: Difference between revisions
| Line 186: | Line 186: | ||
Will my tabasco sauce spoil if I don't use it fast enough. Will mold and bacteria infest my precious tabasco sauce? <span style="font-size: smaller;" class="autosigned">— Preceding [[Wikipedia:Signatures|unsigned]] comment added by [[Special:Contributions/128.214.48.186|128.214.48.186]] ([[User talk:128.214.48.186|talk]]) 09:56, 21 May 2013 (UTC)</span><!-- Template:Unsigned IP --> <!--Autosigned by SineBot--> |
Will my tabasco sauce spoil if I don't use it fast enough. Will mold and bacteria infest my precious tabasco sauce? <span style="font-size: smaller;" class="autosigned">— Preceding [[Wikipedia:Signatures|unsigned]] comment added by [[Special:Contributions/128.214.48.186|128.214.48.186]] ([[User talk:128.214.48.186|talk]]) 09:56, 21 May 2013 (UTC)</span><!-- Template:Unsigned IP --> <!--Autosigned by SineBot--> |
||
:Unless you let some foreign matter into the bottle, vinegar and salt is a very unwelcoming place for anything to grow. An FAQ at tabasco.com gives a shelf life of five years for the regular variety, after which harmless discoloration may occur, but it shouldn't really spoil. You may want to shake an old bottle in case the ingredients have separated. Spices tend to lose their potency over time, so a really old bottle may taste different. [[Special:Contributions/88.112.41.6|88.112.41.6]] ([[User talk:88.112.41.6|talk]]) 11:17, 21 May 2013 (UTC) |
:Unless you let some foreign matter into the bottle, vinegar and salt is a very unwelcoming place for anything to grow. An FAQ at tabasco.com gives a shelf life of five years for the regular variety, after which harmless discoloration may occur, but it shouldn't really spoil. You may want to shake an old bottle in case the ingredients have separated. Spices tend to lose their potency over time, so a really old bottle may taste different. [[Special:Contributions/88.112.41.6|88.112.41.6]] ([[User talk:88.112.41.6|talk]]) 11:17, 21 May 2013 (UTC) |
||
== why atomic silver clusters catalyze ionic silver reduction? == |
|||
It's about photographic film development. General background like, silver halide is photosensitive and when it's hit by photon, few but electroconductive silver atoms formed on the silver halide crystals, then the area where those atom bearing sites become very sensitive to reducing agent and get reduced faster. Now the question, what is behind this atomic silver catalyzator? Why it catalyzes the redox reaction? I don't understand why atomic silver turns to be a catalyzator for silver ions. |
|||
Revision as of 13:53, 21 May 2013
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
May 17
Cold myth
If its a myth that cold weather or being cold makes people catch a cold, then why do so many people get colds in such conditions? Clover345 (talk) 00:13, 17 May 2013 (UTC)
- You don't get automatically get a cold from cold whether, although, you are more prone to catching cold in cold weather. Plasmic Physics (talk) 01:04, 17 May 2013 (UTC)
- I don't know if I would call it a "myth" per see, more like "it's a complicated situation where the evidence is so overwhelming that there must be some kind of relationship, even if it's merely a correlation and not a causative one". Our article talks about it fairly briefly, but there are a number of links to more in-depth sources. Matt Deres (talk) 01:13, 17 May 2013 (UTC)
- See Correlation does not imply causation and Post hoc ergo propter hoc. It is true that "cold and flu season" is the winter in many places, and there are higher incidents of cold and influenza in the winter months, but there is little evidence that the cold temperatures themselves are directly to blame. The closest connection I have heard is that cold weather encourages people to spend more time indoors in close quarters with other people, which tends to exacerbate transmission of such illnesses, but that isn't something that's caused by cold temperatures per se. See this article from WebMD. --Jayron32 02:51, 17 May 2013 (UTC)
- I admit it goes against the mainstream, but I personally believe that cold weather does play a direct role. The mechanism is that when you are out in the cold, the temperature of the body surface drops, including the nasal mucous membranes. This drop reduces the efficiency of immune responses pretty dramatically, and makes it easier for viruses to take hold. There is literature supporting the existence of this process, but so far no really strong evidence that it plays a major role. Looie496 (talk) 03:15, 17 May 2013 (UTC)
- The Science desk is no place for spreading common superstitions that fly in the face of established science. You pointed out, correctly, that there's no strong evidence to support the purported mechanism. On the other hand, there's very strong evidence indicating that the flu spreads more easily when people are in close proximity in an enclosed area, and very strong evidence that people spend more time indoors during the winter. There's no need to fish around for complicated explanations when the obvious one works just fine. --Bowlhover (talk) 03:47, 17 May 2013 (UTC)
- [citation needed] Louie, please, [citation needed]. --Jayron32 03:48, 17 May 2013 (UTC)
- I admit it goes against the mainstream, but I personally believe that cold weather does play a direct role. The mechanism is that when you are out in the cold, the temperature of the body surface drops, including the nasal mucous membranes. This drop reduces the efficiency of immune responses pretty dramatically, and makes it easier for viruses to take hold. There is literature supporting the existence of this process, but so far no really strong evidence that it plays a major role. Looie496 (talk) 03:15, 17 May 2013 (UTC)
- Our article flu season discusses this a little and lists some common potential explanations.Phoenixia1177 (talk) 04:03, 17 May 2013 (UTC)
- Influenza and the common cold are unrelated diseases. HiLo48 (talk) 04:06, 17 May 2013 (UTC)
- True, but they both have increased incidences at the same time of year, and similar symptoms, which is why they are often discussed together. --Jayron32 04:08, 17 May 2013 (UTC)
- Sorry about that, I'm a little foggy since I actually have the flu right now; anyways, I read that as flu not cold for some reason. My mistakePhoenixia1177 (talk) 04:26, 17 May 2013 (UTC)
- Influenza and the common cold are unrelated diseases. HiLo48 (talk) 04:06, 17 May 2013 (UTC)
- Flu and colds don't occur much mid-winter, at least here in Australia with mid-winter temperatures around 0 to 15 C diurnal cycle. They occur more at the beginning or end of winter. So that suggests that low temperatures affecting the immune system is not the complete answer. My own experience, which my GP has confirmed, is that there is a flu peak in February, ie summer. There has been some coverage of this in the news media in the last year or so. The Public Health authorities have surmised that it corresponds to flu peaks in Europe, carried to Australia by tourists and business travellors. In Autumn here, the weather tends to rapidly fluctuate. In the last week or so we've had daily maximums of 20 C and nice sunshine clear days, but yesterday it changed to wet and 25 C. I think this confuses the body somewhat - 25 C today seems pretty warm, but mid-summer it would fell damm cold. Probably tomorrow it will drop back to 20 C. Wickwack 121.221.228.142 (talk) 06:14, 17 May 2013 (UTC)
- Low vitamin D levels at the end of winter can play a role here, the immune system doesn't work properly if you are deficient, see here. Count Iblis (talk) 14:19, 17 May 2013 (UTC)
- Flu and colds don't occur much mid-winter, at least here in Australia with mid-winter temperatures around 0 to 15 C diurnal cycle. They occur more at the beginning or end of winter. So that suggests that low temperatures affecting the immune system is not the complete answer. My own experience, which my GP has confirmed, is that there is a flu peak in February, ie summer. There has been some coverage of this in the news media in the last year or so. The Public Health authorities have surmised that it corresponds to flu peaks in Europe, carried to Australia by tourists and business travellors. In Autumn here, the weather tends to rapidly fluctuate. In the last week or so we've had daily maximums of 20 C and nice sunshine clear days, but yesterday it changed to wet and 25 C. I think this confuses the body somewhat - 25 C today seems pretty warm, but mid-summer it would fell damm cold. Probably tomorrow it will drop back to 20 C. Wickwack 121.221.228.142 (talk) 06:14, 17 May 2013 (UTC)
Up above someone said, "cold weather encourages people to spend more time indoors in close quarters with other people, which tends to exacerbate transmission of such illnesses". So in places like Phoenix, Arizona, for example, where very hot Summer weather drives people indoors in close quarters with other people, are there more colds in Summer than in Winter? 148.177.1.217 (talk) 15:24, 17 May 2013 (UTC)
- Some viruses causing common cold (Rhinovirus) require a relatively low temperature to proliferate. This may explain the connection to low ambient temperatures. Ruslik_Zero 19:41, 17 May 2013 (UTC)
Type 1a supernova with a neutron star instead of a white dwarf? (a twofer for that star)
Hi, could a neutron star accrete mass and blow apart roughly like a white dwarf is believed to in a type 1a? I suppose the nuclear combustion and light curve would be different, if it even could happen. Thanks199.33.32.40 (talk) 00:39, 17 May 2013 (UTC)
- Going by neutron star#Binary neutron stars, yes. A neutron star in a binary system can (potentially) accrete sufficient mass from its companion star to collapse into a black hole; I don't have the reference listed in the footnote handy, so I can't comment on just how violent the process would be or how exactly its appearance would be expected to differ from a Ia supernova. You might also be interested in the (plausible, but as-yet hypothetical) Thorne–Żytkow objects: red giants which have collided with neutron stars, and which may for some hundreds of years have a neutron star slowly spiralling in towards their cores. If the pair of stars are sufficiently massive, a supernova explosion and black hole may result; if the stars aren't massive enough, then you just end up with a heavier neutron star. TenOfAllTrades(talk) 02:24, 17 May 2013 (UTC)
- A Type Ia supernova occurs when accretion ignites nuclear fusion of carbon on a white dwarf. Nuclear fusion is impossible on a neutron star because there are no nuclei--the entire star is made up of closely packed neutrons, without any protons or electrons. So no, a neutron star can't undergo a Type Ia supernova. --Bowlhover (talk) 04:04, 17 May 2013 (UTC)
- Also, the bounce that happens during the collapse of a star into a neutron star is essential for converting an implosion into an explosion. No such bounces happens when a neutron star converts into a black hole. Dauto (talk) 14:22, 17 May 2013 (UTC)
Toluene
Is toluene used in consumer products anymore? 24.23.196.85 (talk) 06:26, 17 May 2013 (UTC)
- It is sold as a solvent for use in arts and crafts. Plasmic Physics (talk) 07:06, 17 May 2013 (UTC)
- If you're looking at making TNT, some printeries sell nitric acid. Plasmic Physics (talk) 07:08, 17 May 2013 (UTC)
- Let's not forget sulfuric acid, you'll need that for a catalyst. It is sold as battery acid, however, you'll need to remove impurities before you can use it. Plasmic Physics (talk) 07:22, 17 May 2013 (UTC)
- Toluene's availability as a consumer product may be more strictly regulated in your country. Plasmic Physics (talk) 07:23, 17 May 2013 (UTC)
- You'll find all these useful tips in 'The Terrorist's Handbook', made infamous for being officially blacklisted by the US government for obvious reasons. That shouldnt stop you from finding a copy online. It has several versions and comes under various titles. Plasmic Physics (talk) 07:29, 17 May 2013 (UTC)
- Will the US govt blacklist you soon? HiLo48 (talk) 07:40, 17 May 2013 (UTC)
- What Plasmic left out is that one of the steps in the recipe will result in immediate vaporization of the person attempting to make the bomb. Exactly which step that is, of course is classified info. ←Baseball Bugs What's up, Doc? carrots→ 07:56, 17 May 2013 (UTC)
- Will the US govt blacklist you soon? HiLo48 (talk) 07:40, 17 May 2013 (UTC)
- Aren't you confused with nitroglycerine? Plasmic Physics (talk) 08:00, 17 May 2013 (UTC)
- No, the so-called "Terrorist's Handbook" has a step which will destroy the bomb-maker instantly. They don't want you to know that, of course. As regards nitroglycerine, it was first created by a Nobel experimenter, whose test ended tragically when he tossed a bottle of the stuff to his assistant, Dr. Klutz. ←Baseball Bugs What's up, Doc? carrots→ 08:31, 17 May 2013 (UTC)
- Aren't you confused with nitroglycerine? Plasmic Physics (talk) 08:00, 17 May 2013 (UTC)
- I wasn't recomending that particular recipe. In any case, it's not so much a step, as it is a missing step that makes it so dangerous. Plasmic Physics (talk) 09:36, 17 May 2013 (UTC)
- I'm NOT looking to make TNT, I'm concerned about possible toxicity! Just who do you think I am, dammit?! 24.23.196.85 (talk) 02:05, 18 May 2013 (UTC)
- And yes, I know what TNT is made from -- but I wouldn't try this reaction at home (even if I had a use for the final product, which I don't!) because just one slip-up could blow you to pieces if you tried it! 24.23.196.85 (talk) 02:12, 18 May 2013 (UTC)
- Well, at least you've seen the use of adding constraints to your future questions. Plasmic Physics (talk) 00:26, 19 May 2013 (UTC)
- Toluene is mainly used as an octane booster additive in petrol/gasoline (especialliy since lead-based additives were banned), and as paint stripper and paint solvent. It is used in a multitude of less important consumer products. It is the main reason why dopey street kids sniff paint and petrol, thereby making themselves even more dopey. See "Uses" in the Wikipedia article. Wickwack 121.215.70.116 (talk) 10:05, 17 May 2013 (UTC)
- Thanks! That was the ONLY helpful comment here. So, it's used in gasoline and as a paint stripper? That means I better be more careful about vapor exposure when using these. 24.23.196.85 (talk) 19:09, 18 May 2013 (UTC)
- Don't forget, it's also used as a glue solvent in arts and crafts. Plasmic Physics (talk) 00:26, 19 May 2013 (UTC)
- Yes, that too. As with the other two items, that means I should avoid getting it on unprotected skin and limit my exposure to the vapors. (Especially since I seem to have a mild allergy to toluene vapors -- even small amounts of the stuff make me want to sneeze!) 24.23.196.85 (talk) 23:16, 19 May 2013 (UTC)
- Don't forget, it's also used as a glue solvent in arts and crafts. Plasmic Physics (talk) 00:26, 19 May 2013 (UTC)
- If you have an allergy towards toluene, I suggest you avoid dill weed. It occurs naturally in it. Plasmic Physics (talk) 03:22, 20 May 2013 (UTC)
- Pure toluene isn't so bad to handle, the issue is that it's really good at solubilizing plastics and goes right through your skin. I don't know about you, but I don't really want to passively absorb polypropylene for instance. Most plastics tend to contain at least some unpolymerized monomer, and they're usually not too good for you.(+)H3N-Protein\Chemist-CO2(-) 12:38, 17 May 2013 (UTC)
- It's also a pretty good solvent to use if you're trying to do Static Light Scattering of a generic hydrophobic polymer. It's also used as a standard in a lot of light scattering instruments.. typically used to normalize raw detector counts to a known Rayleigh Ratio.(+)H3N-Protein\Chemist-CO2(-) 12:38, 17 May 2013 (UTC)
Small golden brown beetle
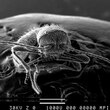
I found at home, middle of Germany, a small beetle and this was not the first one. I suspect that they live either from the wooden construction material of the house or the straw used for isolation. I made a few image and now I hope that somebody can help me name the little creature. I like the hairy bug especially that it looks like it has no eyes and a nice claw on his legs.
--Stone (talk) 12:14, 17 May 2013 (UTC)
- You own a scanning electron microscope? --Jayron32 12:40, 17 May 2013 (UTC)
- You don't need to own one to use one, he just needs access to one or have friends in the right places. Plasmic Physics (talk) 12:56, 17 May 2013 (UTC)
- For instance, I made friends with someone who is involved with the sample preparation for a SEM at the university which I attend. That is how I managed to procure gold sputter coated glass electodes for my electrolytic station. Plasmic Physics (talk) 13:33, 17 May 2013 (UTC)
- At the MPS my boss has an 1970s SEM and he likes to do funny things every now end then. To gold coat a spider or a beetle is fun for a Friday .--Stone (talk) 16:59, 17 May 2013 (UTC)
- Wow! this makes a change from the "can you get a better image" routine. Incredible images, I like the claw especially. I would suggest you send the images to Whatsthatbug? who have been excellent on the half dozen occasions I sent them stuff from the UK. Richard Avery (talk) 13:25, 17 May 2013 (UTC)
- Probably not an exact match, but here's a golden-brown eyeless beetle, living in Germany, and named after Hitler: Anophthalmus_hitleri. That one seems to be pretty rare, but the whole genus Anophthalmus is eyeless, and I don't think eyelessness is that common in beetles... SemanticMantis (talk) 15:03, 17 May 2013 (UTC)
- I looked a little bit and I think it is a Niptus hololeucus. Especially in the German text it says it lives in Timber framing houses.--Stone (talk) 16:59, 17 May 2013 (UTC)
- well, this has certainly got to be the coolest Ref Desk question ever! μηδείς (talk) 15:33, 17 May 2013 (UTC)
- Thanks! --Stone (talk) 16:59, 17 May 2013 (UTC)
In the SEM images it is hard to guess where the beetle has its eyes.--Stone (talk) 17:25, 17 May 2013 (UTC)
- These are REALLY good SEM images. Well done! And yes, you can see the compound eye clearly in the first and in the last SEM image. You can even make out the ommatidia pattern. This is a small beetle species, so the total ommatidia count is rather low --Dr Dima (talk) 21:35, 17 May 2013 (UTC)
- Where are these compound eyes? Are they the bulges lateral to the antenna roots? They look hairy. μηδείς (talk) 02:28, 18 May 2013 (UTC)
- You can see the eye most clearly in the first SEM image ("front and below"), posterior to the right antennal socket (that's the one on the left side of the image). The very top of the eye is occluded by the pedicel -- the second "piece" from the base -- of the right antenna. --Dr Dima (talk) 03:25, 18 May 2013 (UTC)
- Yes, thanks. That is what I was referring to. I see looking again at the first image it is not actually hairy. μηδείς (talk) 03:29, 18 May 2013 (UTC)
- Now I also see it! Thanks!
- Yes, thanks. That is what I was referring to. I see looking again at the first image it is not actually hairy. μηδείς (talk) 03:29, 18 May 2013 (UTC)
- You can see the eye most clearly in the first SEM image ("front and below"), posterior to the right antennal socket (that's the one on the left side of the image). The very top of the eye is occluded by the pedicel -- the second "piece" from the base -- of the right antenna. --Dr Dima (talk) 03:25, 18 May 2013 (UTC)
- Where are these compound eyes? Are they the bulges lateral to the antenna roots? They look hairy. μηδείς (talk) 02:28, 18 May 2013 (UTC)
I think it is a Niptus hololeucus. Anybody disagree?--Stone (talk) 05:30, 18 May 2013 (UTC)
- Not an entomologer, but checking for discrepancies in things I know are often diagnostic for insects like antenna segment count I see no reason to believe it is not N. hololeucus. μηδείς (talk) 20:01, 18 May 2013 (UTC)
why silver reduces gold?
Why atomic silver reduces ionic gold? Thanks! — Preceding unsigned comment added by 134.130.94.148 (talk) 13:56, 17 May 2013 (UTC)
- Also relevant is Reactivity series and Standard electrode potential and Standard electrode potential (data page). --Jayron32 23:57, 17 May 2013 (UTC)
Sleep theory
| This discussion has been closed. Please do not modify it. |
|---|
| The following discussion has been closed. Please do not modify it. |
|
Hi guys, I'm an MCB graduate student. I've never really edited Wikipedia. At first I thought I'd edit the Sleep page just to throw this simple sleep hypothesis/theory out there and let you guys run with it, but that didn't seem like the right place to post it; then I saw the "talk," page, but that warned that it wasn't the place either. In any case, I've got more than enough work to do so feel free to take a critical look at this, get it to anyone who might be interested, and take it from there: Sleep Theory I think the brain needs 1) "mental" energy to initiate sleep, 2) enough experience during the day to have something to do (process) during sleep, and obviously 3) environmental conditions conducive to initiate/maintain sleep: (OMG, this looks terrible posting from MS Word to here...I apologize in advance. Here's a slightly better version on the blog I'm making (which looks awful as well!) that's slightly better: http://johnfial.wordpress.com/sleep-theory/ 1. Initiation. PFC (Pre-Frontal Cortex) is likely initiating that “sleepy” feeling. It does this based on biochemical energy reserves (brain, liver? Unsure...) based on last ~48hrs of physical activity levels. PFC probably un-couples (or “unlocks”) the brain from major skeletal muscular nerves (keeping the physical body relatively motionless), and allows the rest of the brain to: a. REM: Unrestricted neural activity, maintenance of neural networks, strengthening of networks used throughout the day – especially those most-depleted of energy (high neurotransmitter/reuptake use, for instance, in neural networks associated with learning a new “riff” on the guitar) b. NON-REM: Light neural activity? Probably heavy metabolic maintenance / physiological preparation pathways. (A singer's/public speaker's brain, for instance, might “run simulations” using vocal physiological pathways during NON-REM phases. Just a hunch – I have no evidence to support this idea.) i. Reproductive pathways ii. Immune system pathways/support 2. Was there enough neural use during the day for the brain to “process” during sleep? a. Learning b. Skill acquisition/improvement c. Variation of mental/physical activities 3. Environmental factors (Both Initiation & Maintenance) a. Light & circadian rhythm b. Sound i. & vibration? Research: are there any sleep issues correlated to areas with excess ULF noise? c. Smell (try going to sleep with heavy smoke in the room!) d. Temperature e. Nutrients required to maintain metabolic (& physiological) pathways? i. Nutrients: All else being equal (physical activity + mental experiences), macro-nutrient deficiencies likely cause the biggest sleep challenges 1. Amino-acid or fatty-acid deficiency? Sleep is DONE. Wake up! Go find some food! 2. Micro-nutrient deficiency? Possibly responsible for smaller-scale issues. a. REMEMBER that micro-nutrient “intake” (i.e. through the mouth, via food or supplements) does NOT NECESSARILY mean those nutrients get into the bloodflow or transported into the cells that need them! ii. Drugs: Any “positive” drugs during the day may have “negative” side-effects at night. 4. Maintenance: Next sleep cycle? a. Were physical activity levels in last ~48hrs enough to continue into next sleep cycle? b. Enough neural/mental activity during day to justify staying asleep another cycle? c. Do environmental conditions (external world & internal nutrients) persist? — Preceding unsigned comment added by A957835895 (talk • contribs) 21:20, 17 May 2013 (UTC) |
- Sorry, but nowhere in Wikipedia is the place for unsourced original speculation such as this. As an encyclopedia, everything here needs to be referenced from reliable secondary sources. As an science graduate (I'm assuming MCB is Molecular and Cell Biology?), you should know that hunches such as these are useless without evidence, or at least, some plan for experiments to produce that evidence. Rojomoke (talk) 21:56, 17 May 2013 (UTC)
May 18
Strange-looking cherries
Hello all, I've been meaning to ask this for some time. Lately, when shopping at one particular small grocery in my area (Central California), I've been seeing some cherries that look like two or three berries fused together, sometimes with more than one stone. Last time I shopped there, they also had heart-shaped cherries mixed in with these. Are these new varieties, or some kind of rare genetic mutation, or maybe something else? Has anyone seen anything like this in other places? 24.23.196.85 (talk) 19:15, 18 May 2013 (UTC)
- Did they actually have more than one stone? WP:OR, but it wouldn't be surprising if sellers would market larger cherries when they get them, larger usually appeals to customers. μηδείς (talk) 19:23, 18 May 2013 (UTC)
- There are many, many varieties of cherries. Many of them are heart-shaped. Many of them have fused fruit. Browsing the list of early California cherries here, the ones that I think of from your description are the Early Burlat (big, dark cherries), but again, there are lots of cherries. --Mr.98 (talk) 20:10, 18 May 2013 (UTC)
- I've many times seen a few "Siamese twin" cherries mixed in with a batch of ordinary ones. Presumably the ones you found have mutated in some way that makes that the norm rather than the exception. Almost all the fruit we find in stores is genetically freakish, so things like that aren't incredibly surprising. Looie496 (talk) 02:17, 19 May 2013 (UTC)
Bonobo endocrinology
Does Bonobo Chimpanzee have more oxytocin \ other "friendship hormones" compared to Common Chimpanzee? 109.64.101.74 (talk) 21:20, 18 May 2013 (UTC)
- First, bonobos are no longer generally referred to as chimpanzees -- they are a different (but very closely related) species. Concerning the question, I can't spot any evidence for that, though I don't think the topic has been studied very deeply. There is apparently a difference between bonobo and chimpanzee in the form of receptors for vasopressin, another peptide that is involved in social behavior. Apparently bonobos and humans share the same form, which is different from the form in chimps. See PMID 17118932 for discussion. Looie496 (talk) 02:08, 19 May 2013 (UTC)
We've heard about beneficial bacteria, perhaps there are beneficial cancers?
Perhaps some slow growing cancers that have in some way been "bred" to be somewhat differentiated, could be beneficial? For one thing they might keep immune system things like interferon on their toes? Thanks, Rich76.218.104.120 (talk) 22:57, 18 May 2013 (UTC)
- What do you mean be "bred"? Plasmic Physics (talk) 00:20, 19 May 2013 (UTC)
- ..And, in what relavent way are bacteria like cancer? Plasmic Physics (talk) 00:53, 19 May 2013 (UTC)
- What relevant way does "in what relavent way are bacteria like cancer?" have to do with my question? I'm sure you're reasonably intelligent, but your question seems off the point, perhaps you should explain your thinking first, bto motivate me to try to give a dissertation on how bacteria are like cancer. Did I ever say they were alike? Let's see both have some carbon atoms as do pencils and diamonds. What does that have to do with anything?76.218.104.120 (talk) 01:30, 19 May 2013 (UTC)
- From the question header, it appears as though you are basing your logic on the assumption that they are. I'm just trying to follow the logic you used to derive your proposition. Plasmic Physics (talk) 01:43, 19 May 2013 (UTC)
- The question makes sense, but I've never heard of such a thing. Cancer is defined as tissue growth that has escaped from normal genetic control, and it's hard to see how anything that has escaped from control can be good. Looie496 (talk) 02:09, 19 May 2013 (UTC)
- I could imagine it occurring paradoxically on a TV show like House where, for example, someone's hypoglycemia is masked by the diabetes he develops as a secondary effect of his pancreatic cancer. But I am making up that scenario and I have never heard of it happening. μηδείς (talk) 02:34, 19 May 2013 (UTC)
- (edit conflict) Have you heard of a cursed blessing? Certain types of cancer may have positive side effects, especially brain tumours, they are known to occasionally cause savant like changes. Plasmic Physics (talk) 02:37, 19 May 2013 (UTC)
ec "No violence, gentlemen -- no violence, I beg of you! Consider the furniture!". Let's not start the flame war now, shall we. The question before us, if I understand it correctly, is the following. We and our gut flora have evolved to lead at least commensal and, in some aspects, outright mutually-beneficial existence. So, the question goes, is it possible that some slow-growing cancers have evolved to be beneficial, as well? Now, to the best of my knowledge, let me try to give a (partial) answer. First of all, we did not evolve specific cancers; rather, we evolved predisposition to specific cancers. There are directly transmittable cancers, yes, such as the one decimating the tasmanian devil populations for several years now; but none AFAIK are common among humans. (Melanoma can be transmitted from mother to fetus, though; but this is off-topic right now). Human cancers occur by two primary mechanisms: random damage to the DNA due to environmental events (exposure to carcinogens, smoking, ionizing radiation, etc.), and damage to the DNA due to viral infections. These are not hereditary. Hereditary effects determine predisposition to particular types of cancer: breast cancer in BRCA1 gene carriers, colon cancer in Ashkenazi Jews, etc.; but we do not, to the best of my knowledge, carry extra genetic instructions that would make some of our stem cells differentiate into a specifically "cancerous" (but still beneficial) cell or tissue type. That of course doesn't mean that this does not happen. To understand why, let's first look into what makes a cancerous cell different from a normal cell. Cancerous cell, you see, can divide indefinitely: each one divides to make two new ones, with largely the same DNA (except usually for some mistakes), and unless there are too many of these mistakes, the two new cells can divide again, and again, and again. In fact, HeLa cancer cell line is still dividing, in vitro, since 1950s. The "normal" tissue cells, OTOH, can't do that. Instead, they have a so-called Hayflick limit on how many times a cell can divide; typically 35-60. The mechanism is simple: each time a cell divides, a small repetitive piece called telomere at the end of the DNA strand gets lost. Lose too many, and the cell can no longer divide. Now here is the tricky bit: all of our cells have genes that code for an enzyme called telomerase. This enzyme, when active, can regrow the lost telomeres. This enzyme is indeed active in most human malignant tumors. However, this also means that it is impossible to strictly define a "slow-growing" cancer: a cell with some telomerase activity may, theoretically, divide more times (100? 1000?) before reaching its Hayflick limit. Would you call such a cell a slow-growing cancerous cell or a normal cell with delayed senescence? I think at this point the question becomes purely semantic. I hope this helps. --Dr Dima (talk) 02:37, 19 May 2013 (UTC)
- Thanks, that does help. Nice to have my question taken seriously rather than being browbeaten bwith questions from knowitalls.76.218.104.120 (talk)
- I fail to find the provocative post neccesetated by the definition of 'flame war'. Plasmic Physics (talk) 02:58, 19 May 2013 (UTC)
- Who are you to complain about provocative posts?76.218.104.120 (talk) 05:09, 22 May 2013 (UTC)
- I stopped reading once I got to that phrase, lol. μηδείς (talk) 03:08, 19 May 2013 (UTC)
- Incidentally, I also stopped reading at that same point. OsmanRF34 (talk) 12:37, 19 May 2013 (UTC)
- Too bad :) --Dr Dima (talk) 03:31, 19 May 2013 (UTC)
- Never mind me, I am sure your comments were of benefit to your intended audience. μηδείς (talk) 03:37, 19 May 2013 (UTC)
- Is this what they call small talk? Plasmic Physics (talk) 03:49, 19 May 2013 (UTC)
- Can small talk be so big?OsmanRF34 (talk) 12:37, 19 May 2013 (UTC)
- Funny how nobody says they're engaging in "big talk" when it's just normal conversation. -- Jack of Oz [Talk] 23:37, 19 May 2013 (UTC)
- Can small talk be so big?OsmanRF34 (talk) 12:37, 19 May 2013 (UTC)
- Aye, but there is such a thing as big talk, as in "He talks big!" Plasmic Physics (talk) 00:07, 20 May 2013 (UTC)
Animals are slowly growing cancers. Count Iblis (talk)
- Except, of course, that that conceit is entirely false, that animals follow a developmental program, with a set adult form, some like nematodes (see Caenorhabditis elegans} even having a determinate number of cells in the adult organism, while a cancer is defined as the opposite, an undefined, unregulated mass. μηδείς (talk) 23:44, 19 May 2013 (UTC)
- I see, but surely there is at least some regulation, tumors need to tell the immune system to not attack them, they need to make bloodvessels to supply them etc. etc. Count Iblis (talk) 00:01, 20 May 2013 (UTC)
- They don't 'need' to do those things, they just do therefore they are. Plasmic Physics (talk) 00:07, 20 May 2013 (UTC)
May 19
Quantum foam, zero-point energy, the fabric of the cosmos
Various articles in Wiki point mention the paradox of a cosmological constant that should be small, but when calculated, is many orders of magnitude too large. Wiki questions: Why doesn't the zero-point energy density of the vacuum (sometimes called 'quantum foam') change with changes in the volume of the universe? And related to that, why doesn't the large constant zero-point energy density of the vacuum cause a large cosmological constant? My question is: what if the 'quantum foam' at the Planck scale doesn't change because it is the real universe (supposedly infinite and eternal), and the Big Bang was only a one-off local outburst? Could that put to rest the paradox of the large cosmological constant? A related question is: If this is a possibility, what property or condition in the 'quantum foam' could have triggered the Big Bang?Robert van der Hoff (talk) 01:49, 19 May 2013 (UTC)
- It's hard to see the value of inventing some random bizarre idea and then asking why it isn't valid. Looie496 (talk) 02:12, 19 May 2013 (UTC)
- How would you test this conjecture of yours? I think you're delving past science and wandering into philosophy. Praemonitus (talk) 02:17, 19 May 2013 (UTC)
Thanks for your comments, points taken, but I'm still wandering and wonderingRobert van der Hoff (talk) 10:18, 19 May 2013 (UTC)
- By definition a cosmological constant doesn't change its energy density with the universe's scale factor, that's just one of it's characteristics. By observation, we can say that dark energy appears consistent with a cosmological constant, but we don't know for sure that it's energy density is independent of scale factor. The observational parameter w measuring the equation of state of dark energy has a value of -0.98 +/- 0.05, where -1 means independent of scale factor and something like matter whose energy density scales in direct response to changes in the volume of the universe has a value of 0. As you note, attempts to explain a cosmological constant based on quantum mechanical properties of the vacuum give results that are much too large. At present, no one really knows why that is. Dragons flight (talk) 10:21, 19 May 2013 (UTC)
Thank you. The mystery remains Robert van der Hoff (talk) 23:02, 19 May 2013 (UTC)
What time of day and date of the year would most people on earth be in darkness?
My guess would be on the Winter Solstice (Dec 21), which has the shortest day in the higher populated Northern hemisphere. I would also think it would be when the sun was over the Pacific ocean, but before dawn in Asia, so perhaps around sunset on the US west coast?
Could we also calculate (roughly) what percentage of people would be in darkness? Jaseywasey (talk) 06:50, 19 May 2013 (UTC)
- Your logic seems to be correct. It would probably be easier to calculate the number of people with daylight in those circumstances. You would also need to define darkness more precisely. Are you ignoring twilight?--Shantavira|feed me 08:16, 19 May 2013 (UTC)
- Could take into account this map... AnonMoos (talk) 17:14, 19 May 2013 (UTC)
- Ha, I made a map of this once, with the winter solstice day/night curve, the "earth at night" light data, and, well, I made a guess about what time would be the "most dark". If the time isn't exactly right, it must be close. I guessed 0:30 GMT, Dec 22: http://www.flickr.com/photos/pfly/4133160304/sizes/o/ ...although I made this map with the idea of "most dark", in terms of nighttime lights being on, rather than population. Nighttime lights and population are related, but not the same. I suspect a slightly earlier time than my map shows would put more people in darkness—trading more daylight in the North America for more night in Indonesia and Japan. Pfly (talk) 08:45, 20 May 2013 (UTC)
Dark band between two lasers?

At the top right of this image, two laser beams are spreading away from the building and there is a noticeable dark band between them. Is this a form of Alexander's band? – Kerαunoςcopia◁galaxies 07:20, 19 May 2013 (UTC)
- It is a retouched photo, the original is at File:The Shard, Inauguration Lightshow, 2012.jpg, there seems to be a little of the same effect but it is much less apparent. Personally I prefer the original. Dmcq (talk) 08:09, 19 May 2013 (UTC)
- I kind of do too, it's what attracted me to it in the first place. Thanks for the mention, I've replaced the photo in the article with the original. Ok back on topic :) The dark band is still there. The image on the right is the original photo now, and it's still visible. Very curious what's causing it. – Kerαunoςcopia◁galaxies 09:37, 19 May 2013 (UTC)
- I think this is an example of the optical illusion known as the contrast effect -- see http://www.michaelbach.de/ot/lum_dynsimcontrast/index.html for an especially vivid demonstration. Looie496 (talk) 14:42, 19 May 2013 (UTC)
- It is clearly not any optical illusion as displying the picture full screen or blowing it up shows that the pixels between the two laser lines are quite a bit darker. The mostly likely explanation is that the image was "improved" by digital retouching and the person doing it messed it up. They may have wanted to lighten the sky by chroma keying and forgot the bit isolated between the lines. Wickwack 60.230.253.45 (talk) 15:57, 19 May 2013 (UTC)
- I think this is an example of the optical illusion known as the contrast effect -- see http://www.michaelbach.de/ot/lum_dynsimcontrast/index.html for an especially vivid demonstration. Looie496 (talk) 14:42, 19 May 2013 (UTC)
- Interesting. Ok thank you, I thought maybe this was a real-life occurrence. I ran the original image through "Jeffrey's Exif viewer" online and I see that Microsoft Pro Photo Tools was used, but seeing as how that's a metadata editor (maybe more, i don't know), I guess I really don't know what I'm looking at. I'd simply have to ask the original author if he did any touch-ups himself; that'd more than likely be the explanation I'm looking for. Thanks for all the replies. – Kerαunoςcopia◁galaxies 19:50, 19 May 2013 (UTC)
- I disagree entirely with wickwack. I blew this up (on a 17" HD monitor) and looked at it before I read his comment, and the pixels between and outside the rays are the same color when the laser lines are obscured. μηδείς (talk) 22:02, 19 May 2013 (UTC)
- Your eyes are mistaken, or they're just not sensitive enough, or your monitor isn't appropriately adjusted for this comparison. If you measure the sky's brightness using the tool of your choice, you can see a clear, quantifiable reduction in the area between the two beams in the upper right corner of the image. (For a free tool, download ImageJ, select a line of pixels, and choose Analyze...Plot Profile. The mean gray value is about 20% lower between the beams.) Whether this is an artifact of image processing in-camera or afterwards I can't say. TenOfAllTrades(talk) 02:38, 20 May 2013 (UTC)
- I disagree entirely with wickwack. I blew this up (on a 17" HD monitor) and looked at it before I read his comment, and the pixels between and outside the rays are the same color when the laser lines are obscured. μηδείς (talk) 22:02, 19 May 2013 (UTC)
- Here is a screenshot (imgur.com) of an 11x11 pixel Photoshop analysis (on the original image as seen above) of three points, two on either side of the band. Using Lab, one can easily see that the band between the lasers is four points darker in the luminosity channel than on the sky outside of the lasers. There is also a slight shift in the a (red-green) and b (yellow-blue) color channels. It's definitely not an optical illusion. The photographer is busy and I'll have to bother him about it at a later date, but I'm not really seeing anything that might give away the fact that the image was edited outside of the lasers only. I can't read FotoForensics.com results either, if that site would even turn anything up. – Kerαunoςcopia◁galaxies 03:23, 20 May 2013 (UTC)
- You don't need to go to all that trouble. You can just display the image on your monitor at full size. Then, take a piece of cardboard (eg from a business card or a cereal box) and cut two holes in it, about 8 mm diamter and about 25 mm apart. Then place the card against the screen and position it so that pixels from between the laser lines are viewed thru one hole, and pixels elsewhere in the sky are viewed thru the other hole. You will clealy see that the pixels between the laser lines are darker. The card prevents any possibilty of an optical illusion. Medeis obviously was just being silly. Wickwack 60.228.233.13 (talk) 04:18, 20 May 2013 (UTC)
- Should I ask whether you are just being an idiot, wickwack? Personal comments are not necessary. I did the same thing, blowing up the image to max on a large screen laptop, blocking out the lasers as I said above, and the sky between the beams then appeared no different from outside to me. μηδείς (talk) 08:35, 20 May 2013 (UTC)
- So who is being an idiot then? First you posted "I dissagree entirely with Wickwack." Now, after more than one person said you are wrong, you have again asserted what is clearly ridiculous. You have made several silly posts recently, resulting in several people attacking you. Are you on drugs or something? Wickwack 60.228.233.13 (talk) 09:02, 20 May 2013 (UTC)
- Should I ask whether you are just being an idiot, wickwack? Personal comments are not necessary. I did the same thing, blowing up the image to max on a large screen laptop, blocking out the lasers as I said above, and the sky between the beams then appeared no different from outside to me. μηδείς (talk) 08:35, 20 May 2013 (UTC)
(unindent)
- Please guys...this is a silly argument. Just measure it with any halfway reasonable image tool - and the debate is over. Measuring a patch of sky from just outside of the two lasers got me an average brightness of 91/256, measuring an area between the lines got me 75/256 - so the image is DEFINITELY darker between the lasers. It's not an optical illusion. Furthermore, where the blue laser crosses the green ones, the blue laser is also attenuated...since the atmosphere between the two lasers would have to block that light somehow for this to be a "real" effect, we can rule out things like the two green lasers scattering light in all directions except right between them. For the blue laser to be attenuated, the air would have to become more opaque between the two lasers...which simply isn't reasonable. Hence this isn't any kind of peculiar real-world effect either. Furthermore, doing a Google Images search on "Shard inauguration laser show" produced a gazillion other photos of the event - many showing two lasers right next to each other like that - none that I could see showed this darkening effect, despite many shots from many different angles showing pairs of closely-spaced lasers.
- Odds are very good then that this is an image processing artifact. If I had to bet on a cause, I'd say that someone used a "despeckle" filter on the image in an effort to eliminate the obvious noise in the background of the sky...but it's really hard to tell for sure. SteveBaker (talk) 16:10, 20 May 2013 (UTC)
Are dogs ever allergic to wheat gluten ?
I just saw an ad for dog food without wheat gluten, and they are obviously hoping people will buy it because they think it's healthier (although I notice they never actually made this claim). So, is it really healthier or is this just a scam ?
Or perhaps they are appealing to people who remember the 2007 pet food recalls, where Chinese poisoned wheat gluten killed many pets ? StuRat (talk) 18:45, 19 May 2013 (UTC)
- A trivial Google search show many pages devoted to discussing food allergies in dogs in general, and wheat allergies in particular. Such as [1]. Based on that, it seems to be a real thing. Dragons flight (talk) 19:49, 19 May 2013 (UTC)
- Exocrine_pancreatic_insufficiency is very common in some breeds, and can pop up in any dog. Many sources report gluten sensitivity, and recommend avoiding feeding gluten to dogs with EPI. SemanticMantis (talk) 19:53, 19 May 2013 (UTC)
Machine made of Human parts
I have a question regarding the name of a concept, and thus far have found no answers, save for some delightful articles on related subjects. Seeing as how Wikipedia knows everything, i thought this was the logical course of action.
if it is understood that a Cyborg is a hybrid between a CYBernetic being and an ORGanism, and an Android is a synthetic organism, a 'human' made of machine parts, then what would one call a 'machine' made of human parts? — Preceding unsigned comment added by 71.59.51.225 (talk) 19:02, 19 May 2013 (UTC)
- "Frankenstein". ←Baseball Bugs What's up, Doc? carrots→ 20:03, 19 May 2013 (UTC)
- "Frankenstein's monster", not Victor. Clarityfiend (talk) 22:59, 19 May 2013 (UTC)
- Yes. Hence the quote marks. ←Baseball Bugs What's up, Doc? carrots→ 01:06, 20 May 2013 (UTC)
- Interpreting 'machine' loosely, dentures were at one time sometimes made using human teeth. AndyTheGrump (talk) 20:09, 19 May 2013 (UTC)
- Even more loosely: soy sauce, apparently.. AndrewWTaylor (talk) 11:52, 20 May 2013 (UTC)
- The film Existenz has an interesting take on this concept. Astronaut (talk) 17:56, 20 May 2013 (UTC)
- I don't think this concept exists outside of sci-fi. Making a machine out of human parts would have the disadvantages of both and the advantages of neither. For example, I believe in The Matrix movie series, human "brain energy" powers the Matrix. This is the most absurd source of energy imaginable. It would take many times as much energy to keep the people alive as you would get from them. StuRat (talk) 06:53, 21 May 2013 (UTC)
May 20
Throwing one's hands in the air

When humans get excited, they tend to put their arms up. Three prominent examples are in worship services (e.g., File:Zhromko.jpg), at music festivals, and at sporting events. Why do they do this? Magog the Ogre (t • c) 00:21, 20 May 2013 (UTC)
- When humans get excited, they do lots of things, they dance, they cheer, they clap, it's partially cultural, but I can't actually see what needs an explanation? What else can humans do when they get excited? All we have are arms, legs and voices. Unless you also have a lighter, a phone or a vuvuzella... Vespine (talk) 01:13, 20 May 2013 (UTC)
- I've inserted a picture showing another type of excitement. Looie496 (talk) 01:26, 20 May 2013 (UTC)
- @Vespine, I do not think it is cultural. I have observed it across cultures, and it comes quite naturally in some situations. Magog the Ogre (t • c) 04:27, 20 May 2013 (UTC)
- I said partially cultural. There is a cultural component which would help determine exactly how people will react to exciting situations. Look at the history of Applause for example: Within each culture, however, it is usually subject to conventions.. Watching the world cup soccer staged in different countries can also reveal how different cultures react in exciting situations, they're not all precisely the same. Of course I'm NOT saying there aren't common components which aren't cross-cultural, of which lifting one's hands certainly might be one of the most "basic". Vespine (talk) 04:53, 20 May 2013 (UTC)
- @Vespine, I do not think it is cultural. I have observed it across cultures, and it comes quite naturally in some situations. Magog the Ogre (t • c) 04:27, 20 May 2013 (UTC)
- I've inserted a picture showing another type of excitement. Looie496 (talk) 01:26, 20 May 2013 (UTC)
- I once read the following, describing a certain unnamed culture: When speaking, they gesture frantically with their hands in an attempt to distract your gaze from their ugly faces, upon which are clearly etched the marks of their moral and intellectual degeneracy. -- Jack of Oz [Talk] 05:03, 20 May 2013 (UTC)
- I once read in a scientific text that the reason rattlesnakes evolved rattles was because all snakes naturally shake their tails when excited, given they have nothing else to shake. μηδείς (talk) 08:30, 20 May 2013 (UTC)
- I once read the following, describing a certain unnamed culture: When speaking, they gesture frantically with their hands in an attempt to distract your gaze from their ugly faces, upon which are clearly etched the marks of their moral and intellectual degeneracy. -- Jack of Oz [Talk] 05:03, 20 May 2013 (UTC)
- I suppose it's possible that this is a gesture that's intended to show the opposite of "defensiveness". You're much more vulnerable to attack with your arms waving up in the air - and you can more clearly see that a person isn't armed. Perhaps this is a way for body-language to say "I'm excited, but not in an angry or threatening way." ?? SteveBaker (talk) 15:35, 20 May 2013 (UTC)
- Or an urge to be associated with the win or celebration. Like "I am also part of this victory"; "I was on/cheering for the winning side" ; "I voted for you so don't put me in jail" etc.165.212.189.187 (talk) 18:28, 20 May 2013 (UTC)
- Just don't get carried away with it.[2] ←Baseball Bugs What's up, Doc? carrots→ 23:18, 20 May 2013 (UTC)
Decay product
Why does decay product is referred as 'daughter', not 'son' ? Concepts of Physics (talk) 13:00, 20 May 2013 (UTC)
- The most likely reason is that in many cases it can go on to be the mother atom of a subsequent round of decay. -- 71.35.111.68 (talk) 16:17, 20 May 2013 (UTC)
- No reason, just somebody started doing it that way and it caught on. Gender language, when assigned to anything regardless of it's actual sex (if any) is often arbitrary. StuRat (talk) 06:43, 21 May 2013 (UTC)
- Here is a reason: only the female gender can give birth, thus it would volate that principle to call the decay product a son, as opposed to a daughter. Plasmic Physics (talk) 11:54, 21 May 2013 (UTC)
Mysterious lack of airspeed
This 1958 advertisement for Delta announced their nonstop DC-7 service, Atlanta to New York, took 2 hours, 39 minutes. My calculator tells me that's an average speed (assuming 760 statute miles from Atlanta Hartsfield to New York LaGuardia, per Google Earth) of about 286 statute miles per hour. Well and good, but a google search for today's nonstop flights reveals that 2 hours 10 minutes is the fastest scheduled time on a Boeing 737, or a speed of about 350 statute miles per hour. WTF? The article DC-7 gives a cruising speed of 359 mph for that 4-engine prop job, but the latest Boeing 737s have a cruising speed of 511 mph. Another couple of clicks shows that the DC-7 made the journey at an average of 80% of its cruising speed; yet the Boeing 737 travels at an average of only 68% of its cruising speed for the same journey. Thus the trip is only half an hour faster in 2013 than in 1958. Why so slow? Textorus (talk) 15:02, 20 May 2013 (UTC)
- Some possibilities:
- Airliners aren't at cruising speed for the entirety of the flight. The approach phase, particularly, is slow and often circuitous. However, that "less than cruising speed" speed is probably comparable between the DC-7 and a modern jetliner (at least more comparable than their cruising speeds), which means the modern jetliner will proportionally experience more delay relative to its theoretical minimum travel time.
- Airliner routing is different over a 55-year span. The DC-7 didn't have to contend with either the volume of other aircraft in the airspace or the post-9/11 security measures that a modern jetliner encounters, both of which restrict the path an aircraft can take. More restrictions mean a longer flight, generally.
- The definitions of "departure time" and "arrival time" may have shifted (this one is just speculation on my part). What is "departure time"? When no new passengers are admitted aboard? When the cabin door is shut? When the plane begins to move? When it actually leaves the ground? That's 15 to 30 minutes of possible range right there, and if the 1958 definition doesn't match the 2013 definition, it's potentially a significant input. — Lomn 13:56, 20 May 2013 (UTC)
- (ec) Travel times are usually derived from gate-to-gate times. So undocking, pushing back, taxing, runway wait times, takeoff and acceleration times, plus the equivalent on landing all add to the total time without adding commensurately to average speed. Add that to the much higher traffic densities and larger taxiing distances for modern airports, and you should see why it is at least plausible that on shorter trips the times may not have improved much, despite the cruising speed increasing substantially. The large difference between your calculated speeds and the aircraft's cruising speeds tips one off to this. — Quondum 14:04, 20 May 2013 (UTC)
- Departure from Atlanta was relatively straightforward in 1958: roll away (no pushback, since there were no jetways), taxi a mile or so to the end of the active runway, wait for maybe one or two take-offs and landings, and go. Nowadays it's possible to taxi five miles at Atlanta and wait for a dozen planes ahead of you. It's not as bad in terms of distance at LGA, but it's more congested - waiting for a gate (no pulling up on an empty ramp space anymore). Acroterion (talk) 14:19, 20 May 2013 (UTC)
- As Quondum and Acroterion both note, the gate-to-gate time includes an awful lot of time spent not cruising, particularly on a shorter flight. Try working the math the other direction, and see what you get. A 760-mile journey at the DC-7's 359 mph gives 2:07 cruising time; if the total time is 2:39, then that's 32 minutes of taxiing, approaching, and so forth. A 760-mile journey at the 737's 511 mph gives 1:29 cruising time; given a total travel time of 2:10, there's 41 minutes of 'non-cruising' time.
- Granted, that sort of back-of-the-envelope isn't quite realistic – aircraft obviously don't jump instantaneously from the runway threshold to full cruising speed and altitude – but it's not a ridiculous result. Nine minutes difference in the 'non-cruising' time can be down to differences in the way departure and arrival times are defined, increased traffic and delays at the two airports, changes in permitted routes, and the fact that many airlines have slightly reduced their cruising speeds to save fuel. TenOfAllTrades(talk) 15:14, 20 May 2013 (UTC)
- I fly on a regular basis between London and Amsterdam. The flight is timetabled at a little over an hour. However, at Schiphol the plane will often take 20 minutes to taxi between terminal and runway (so much that it sometimes seems like we are going all the way there on the ground), and then spend less than 40 minutes in the air. Heathrow is a busier airport with fewer runways and sometimes long waits for other aircraft ahead. Astronaut (talk) 18:14, 20 May 2013 (UTC)
- Another possibility that nobody so far has mentioned is that the Boeing's 511-knot cruising speed is only achievable at high altitude (above 25,000 feet) -- below that level, it drops off sharply so that near sea level, the V(ne) (that's the MAXIMUM safe speed) is only 330 knots! (This is true of ALL jetliners, as a matter of fact -- the engines produce less power at low altitude, and the denser, more turbulent air puts unacceptable stress on the airframe.) And since for such a relatively short flight, the plane spends a greater part of the journey at low altitudes, even its average FLYING speed will be lower because of this. (Not to mention the time spent in the holding pattern at the outer marker while waiting to land.) 24.23.196.85 (talk) 04:59, 21 May 2013 (UTC)
Human body
What is it called the upper part of the human foot? Thank you.175.157.180.68 (talk) 15:03, 20 May 2013 (UTC)
- The top is the dorsal, unlike the underside 'plantar' surface (where one gets plantar warts – better known as verrucas). As it the foot, it is therefore the dorsum pedis. Does that help?Aspro (talk) 15:19, 20 May 2013 (UTC)
- Wikipedia has an article on foot.--Shantavira|feed me 15:29, 20 May 2013 (UTC)
- It's called the instep. μηδείς (talk) 18:41, 20 May 2013 (UTC)
Original Earth-Moon distance
Hi: I looked up the entry for the Moon, trying to read what was the original distance between the Earth and Moon. I did not see it in the story. Did I miss it or is it not there? Thanks for you help. Red.leaf.flyers (talk) 16:53, 20 May 2013 (UTC)
- It depends on which model for the Origin of the Moon you are working with. One of the dominant models is the Giant impact hypothesis, in which case the original distance between the Earth and Moon was 0 km, insofar as they were once the same body. --Jayron32 17:06, 20 May 2013 (UTC)
- In a giant impact, material would be ejected into Earth orbit, where it would eventually coalesce to a ball. It is a bit hard to say where the orbit would be, but simulations suggest 3-5 Earth radii (20.000 - 30.000 km). An impact would have a hard time lifting enough material higher than five radii. Closer than about three Earth radii would put the impact ejecta inside the Roche limit, giving the Earth rings rather than a moon. 88.112.41.6 (talk) 17:39, 20 May 2013 (UTC)
- Hang on, are you saying that since its formation, the moon has migrated from 3-5 earth radii away, to its current position 60 earth radii away? I'm not saying you are wrong, I just always imagined that the moon would have had to form very roughly around it's present position. However I can see now how that doesn't quite make as much sense as I thought it did... Vespine (talk) 06:54, 21 May 2013 (UTC)
- According to Italo Calvino, it used to be close enough to climb up to it from a small boat, by way of a ladder. See La distanza della luna, the first vignette in Le cosmicomiche. It's available in English translation if necessary. Seriously, of course it's off-topic for this desk, but I very strongly recommend it. --Trovatore (talk) 06:57, 21 May 2013 (UTC)
- On the topic of the Moon in fiction, Domingo Gonsalves harnessed large geese and tagged along for their annual migratory flight to the Moon circa 1638. Nimur (talk) 13:12, 21 May 2013 (UTC)
- According to Italo Calvino, it used to be close enough to climb up to it from a small boat, by way of a ladder. See La distanza della luna, the first vignette in Le cosmicomiche. It's available in English translation if necessary. Seriously, of course it's off-topic for this desk, but I very strongly recommend it. --Trovatore (talk) 06:57, 21 May 2013 (UTC)
- Hang on, are you saying that since its formation, the moon has migrated from 3-5 earth radii away, to its current position 60 earth radii away? I'm not saying you are wrong, I just always imagined that the moon would have had to form very roughly around it's present position. However I can see now how that doesn't quite make as much sense as I thought it did... Vespine (talk) 06:54, 21 May 2013 (UTC)
- In a giant impact, material would be ejected into Earth orbit, where it would eventually coalesce to a ball. It is a bit hard to say where the orbit would be, but simulations suggest 3-5 Earth radii (20.000 - 30.000 km). An impact would have a hard time lifting enough material higher than five radii. Closer than about three Earth radii would put the impact ejecta inside the Roche limit, giving the Earth rings rather than a moon. 88.112.41.6 (talk) 17:39, 20 May 2013 (UTC)
- At it's current rate of motion, 3.8 cm / yr, the moon would migrate 25 Earth radii in the age of the Earth. However, that is almost certainly a lower bound as the rate of migration should have slowed over time. Dragons flight (talk) 07:57, 21 May 2013 (UTC)
- It is actually possible to very roughly estimate this initial distance of roughly 30,000 km using a back of the envelope calculation. It boils to the fact that you have an impactor that had a similar orbit as the Earth, so it comes in from infinity at zero relative speed, and therefore the impact happens at escape velocity. If you where to give the ejecta that velocity, it would escape at infinity but, of course, a significant fraction of the impact energy is not available as kinetic energy. Then because the Earth radius is the quantity with the diemnsions of length, what happens is that if you make some rough approximations then whatever fraction is available, you find that the distance ends up being the Earth radius times some dimensionless factor, and that factor is not going to be very large like 100 or very small like 0.01. Count Iblis (talk) 11:48, 21 May 2013 (UTC)
- As I am an amateur purveyor of all lunar literature, I have a fascinating text, Strange World of the Moon (authored by V. A. Firsoff in 1959) that makes an excellent chapter of dimensional analysis and inference about the origin of the moon. Needless to say, the book and its science predates manned spaceflight to the moon - or in fact, any spaceflight to the moon - so many of its conclusions have since been refined or refuted by better selenological evidence. In addition to the Giant Impact hypothesis, the author considers several other possibilities: tandem formation from a primordial nebula; capture of a separate celestial body; massive ejection by volcanic or other paleo-Earth processes; or simply large-scale fluid flow during Earth's formatory molten-rock era. From first principles of physics, and based on knowledge of orbital mechanics and basic facts of gravity, none of these alternate formation theories seem to sit well with the author; it quickly becomes clear why Giant Impact hypothesis gained traction in the following decades. However, even in 1959, it was easy to see that this was no ordinary impactor; the momentum necessary to eject a moon-sized object would have to be planetary in size. Evidence of the geochemistry of moon rocks - only possible after our first sample return missions in the late 1960s - strengthens the case; and many scientists now believe that the impactor may have been Mars. This is difficult to prove; but is widely accepted as "more plausible" than a mysterious impactor that has long since disappeared. For example, NASA's current science webpage at Solar System Exploration: Earth and Moon origin, asserts that the impactor would be "Mars-sized," without naming any names. Nimur (talk) 13:30, 21 May 2013 (UTC)
- It is actually possible to very roughly estimate this initial distance of roughly 30,000 km using a back of the envelope calculation. It boils to the fact that you have an impactor that had a similar orbit as the Earth, so it comes in from infinity at zero relative speed, and therefore the impact happens at escape velocity. If you where to give the ejecta that velocity, it would escape at infinity but, of course, a significant fraction of the impact energy is not available as kinetic energy. Then because the Earth radius is the quantity with the diemnsions of length, what happens is that if you make some rough approximations then whatever fraction is available, you find that the distance ends up being the Earth radius times some dimensionless factor, and that factor is not going to be very large like 100 or very small like 0.01. Count Iblis (talk) 11:48, 21 May 2013 (UTC)
May 21
what is the most amazing tornado footage ever filmed?
I would like to see some clear, compelling footage of a tornado destroying human habitats. Not a Hollywood movie, but real footage. I could just type tornado footage into Youtube and go fishing, but can you recommend a specific video?--Jerk of Thrones (talk) 04:34, 21 May 2013 (UTC)
- Counter-intuitively, a smaller tornado may provide more graphic destruction. The larger ones tend to have a large dust cloud around them, obscuring the action, and the scale also makes it hard to pick out details. (What appear to be dots on the screen might be cars thrown about, for example.) Also, huge tornadoes don't twist much, and that makes them less interesting.
- Double or triple tornadoes, where they twist around one another, are also visually interesting. StuRat (talk) 06:41, 21 May 2013 (UTC)
- Start at National Severe Storm Laboratory's tornado education website, maintained by NOAA. They link to several videos. Nimur (talk) 11:54, 21 May 2013 (UTC)
- Or watch CNN late afternoon US central time this week and keep your DVD recorder on standby. Count Iblis (talk) 13:02, 21 May 2013 (UTC)
Spoiled Tabasco
Will my tabasco sauce spoil if I don't use it fast enough. Will mold and bacteria infest my precious tabasco sauce? — Preceding unsigned comment added by 128.214.48.186 (talk) 09:56, 21 May 2013 (UTC)
- Unless you let some foreign matter into the bottle, vinegar and salt is a very unwelcoming place for anything to grow. An FAQ at tabasco.com gives a shelf life of five years for the regular variety, after which harmless discoloration may occur, but it shouldn't really spoil. You may want to shake an old bottle in case the ingredients have separated. Spices tend to lose their potency over time, so a really old bottle may taste different. 88.112.41.6 (talk) 11:17, 21 May 2013 (UTC)
why atomic silver clusters catalyze ionic silver reduction?
It's about photographic film development. General background like, silver halide is photosensitive and when it's hit by photon, few but electroconductive silver atoms formed on the silver halide crystals, then the area where those atom bearing sites become very sensitive to reducing agent and get reduced faster. Now the question, what is behind this atomic silver catalyzator? Why it catalyzes the redox reaction? I don't understand why atomic silver turns to be a catalyzator for silver ions.