Cabozantinib: Difference between revisions
FeatherPluma (talk | contribs) |
|||
| Line 77: | Line 77: | ||
In 2014, COMET-1, the phase 3 pivotal trial of cabozantinib in men with metastatic castration-resistant prostate cancer whose disease progressed after treatment with docetaxel as well as abiraterone and/or enzalutamide, did not meet its primary endpoint of demonstrating a statistically significant increase in [[overall survival]] for patients treated with cabozantinib as compared to prednisone.<ref>http://finance.yahoo.com/news/inplay-briefing-com-055139997.html#exelref </ref> Median progression free survival did exhibit a statistically significant improvement with 5.5 months for the cabozantinib arm versus 2.8 months for the prednisone arm, but this did not translate to a statistically significant overall survival benefit at topline analysis; at that time, the median OS for the cabozantinib arm of the trial was 11.0 months versus 9.8 months for the prednisone arm. |
In 2014, COMET-1, the phase 3 pivotal trial of cabozantinib in men with metastatic castration-resistant prostate cancer whose disease progressed after treatment with docetaxel as well as abiraterone and/or enzalutamide, did not meet its primary endpoint of demonstrating a statistically significant increase in [[overall survival]] for patients treated with cabozantinib as compared to prednisone.<ref>http://finance.yahoo.com/news/inplay-briefing-com-055139997.html#exelref </ref> Median progression free survival did exhibit a statistically significant improvement with 5.5 months for the cabozantinib arm versus 2.8 months for the prednisone arm, but this did not translate to a statistically significant overall survival benefit at topline analysis; at that time, the median OS for the cabozantinib arm of the trial was 11.0 months versus 9.8 months for the prednisone arm. |
||
=== Kidney cancer === |
|||
In July of 2015, METEOR, the phase 3 pivotal trial of cabozantinib in patients with advanced clear renal cell carcinoma met its primary endpoint demonstrating a a statistically significant benefit in progression free survival versus everolimus with a hazard ratio of 0.58 on the first 375 patients enrolled. <ref>http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArticle&ID=2068953</ref> A subsequent analysis saw this progression free survival hazard ratio improve to 0.52 on the entire 658 patient trial population. <ref>http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArticle&ID=2126057</ref> on the entire patient population. A statistically significant and clinically meaningful overall survival was attained in another interim analysis. <ref>http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArticle&ID=2133957</ref> |
|||
==See also== |
==See also== |
||
Revision as of 22:25, 2 March 2016
 | |
| Clinical data | |
|---|---|
| Trade names | Cometriq |
| Other names | XL184, BMS907351 |
| License data |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | ≥99.7% |
| Metabolism | Hepatic (CYP3A4-mediated) |
| Elimination half-life | 55 hours |
| Excretion | Faeces (54%), urine (27%) |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.221.147 |
| Chemical and physical data | |
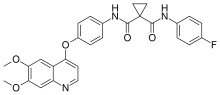
| Formula | C28H24FN3O5 |
| Molar mass | 501.51 g mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cabozantinib (INN) (development code name XL184; marketed under the trade name Cometriq) is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, and has been shown to reduce tumor growth, metastasis, and angiogenesis.
It was developed by Exelixis Inc.
Cabozantinib was granted orphan drug status by the U.S. Food and Drug Administration (FDA) in January 2011.[1]
Cabozantinib was approved by the U.S. FDA in November 2012 for the treatment of medullary thyroid cancer.[2] It is currently undergoing clinical trials for the treatment of prostate, bladder, ovarian, brain, melanoma, breast, non-small cell lung, pancreatic, hepatocellular and kidney cancers.
Approvals and indications
In October 2011, cabozantinib met its primary endpoint in a phase 3 clinical trial (EXAM) conducted by Exelixis investigating its effect on progression-free survival in medullary thyroid cancer.[3] A new drug application was submitted in the first half of 2012,[4] and on November 29, 2012 cabozantinib was granted marketing approval by the U.S. FDA under the name Cometriq for treating patients with medullary thyroid cancer.[2]
Grapefruit and grapefruit juice should be avoided as they may increase the concentration of the drug in the blood.[5]
It is not yet known if cabozantinib is safe and effective in children.
Clinical trials
Glioblastoma multiforme
In 2009 a phase II study for relapsed glioblastoma multiforme reported encouraging interim results.[6]
Prostate cancer
Positive data from clinical trials in 2011 indicate cabozantinib is beneficial in metastatic advanced prostate cancer (castration-resistant prostate cancer). 97% of patients either had stabilization or improvement in bone malignancies. The median time to disease progression was 29 weeks.[7][8]
One US trial reported in May 2011: The best results were seen in patients with liver, prostate, and ovarian cancer: 22 of 29 patients with liver cancer, 71 of 100 patients with prostate cancer, and 32 of 51 with ovarian cancer experienced either partial tumor shrinkage or stable disease. Fifty-nine out of 68 patients who had bone metastases had their metastases shrink or disappear during the trial.[9]
It is undergoing clinical trials for the treatment of prostate, ovarian, brain, melanoma, breast, non-small cell lung, hepatocellular and kidney cancers.[10]
In 2014, COMET-1, the phase 3 pivotal trial of cabozantinib in men with metastatic castration-resistant prostate cancer whose disease progressed after treatment with docetaxel as well as abiraterone and/or enzalutamide, did not meet its primary endpoint of demonstrating a statistically significant increase in overall survival for patients treated with cabozantinib as compared to prednisone.[11] Median progression free survival did exhibit a statistically significant improvement with 5.5 months for the cabozantinib arm versus 2.8 months for the prednisone arm, but this did not translate to a statistically significant overall survival benefit at topline analysis; at that time, the median OS for the cabozantinib arm of the trial was 11.0 months versus 9.8 months for the prednisone arm.
Kidney cancer
In July of 2015, METEOR, the phase 3 pivotal trial of cabozantinib in patients with advanced clear renal cell carcinoma met its primary endpoint demonstrating a a statistically significant benefit in progression free survival versus everolimus with a hazard ratio of 0.58 on the first 375 patients enrolled. [12] A subsequent analysis saw this progression free survival hazard ratio improve to 0.52 on the entire 658 patient trial population. [13] on the entire patient population. A statistically significant and clinically meaningful overall survival was attained in another interim analysis. [14]
See also
References
- ^ Exelixis’ XL184 Granted Orphan Drug Designation and Assigned the Generic Name Cabozantinib. Jan 2011
- ^ a b "FDA approves Cometriq to treat rare type of thyroid cancer". 29 November 2012.
- ^ "Success for the EXAM trial". Retrieved 24 October 2011.
- ^ "Thyroid cancer drug cabozantinib prolongs PFS". Retrieved 24 October 2011.
- ^ "Cometriq prescribing information" (PDF). Retrieved 29 November 2012.
- ^ "A phase II study of XL184 in patients (pts) with progressive glioblastoma multiforme (GBM) in first or second relapse". 2009.
- ^ "Exelixis drug slows prostate cancer spread in trial". Reuters. 6 June 2011.
- ^ Cabozantinib (XL184) Phase 2 Data Demonstrate Encouraging Clinical Activity in Patients with Castration-Resistant Prostate Cancer. Feb 2011
- ^ "Cabozantinib Shrinks Tumors and Bone Metastases in Prostate and Other Cancers". 31 May 2011.
- ^ "Cabozantinib - List Results - ClinicalTrials.gov". U.S. National Institute of Health. Retrieved 25 April 2013.
- ^ http://finance.yahoo.com/news/inplay-briefing-com-055139997.html#exelref
- ^ http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArticle&ID=2068953
- ^ http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArticle&ID=2126057
- ^ http://ir.exelixis.com/phoenix.zhtml?c=120923&p=irol-newsArticle&ID=2133957
