S-Adenosyl methionine
| |

| |

| |
| Names | |
|---|---|
| Systematic IUPAC name
(2S)-2-Amino-4-[(S)-{[(2S,3S,4R,5R)-5-(4-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}methylsulfaniumyl]butanoate | |
| Other names
S-Adenosyl-L-methionine; SAM-e; SAMe, AdoMet, Heparab (India), ademethionine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.045.391 |
| KEGG | |
| MeSH | S-Adenosylmethionine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H22N6O5S | |
| Molar mass | 398.44 g·mol−1 |
| Pharmacology | |
| A16AA02 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
S-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver.[1] More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952.[1]
In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response;[2] amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone and signaling molecule.[3]
Structure
S-Adenosyl methionine consists of the adenosyl group attached to the sulfur of methionine, providing it with a positive charge. It is synthesized from ATP and methionine by S-Adenosylmethionine synthetase enzyme through the following reaction:
- ATP + L-methionine + H2O phosphate + diphosphate + S-adenosyl-L-methionine
The sulfonium functional group present in S-adenosyl methionine is the center of its peculiar reactivity. Depending on the enzyme, S-adenosyl methionine can be converted into one of three products:
- adenosyl radical, which converts to deoxyadenosine (AdO): classic rSAM reaction, also cogenerates methionine
- S-adenosyl homocysteine, releasing methyl radical
- methylthioadenosine (SMT), homoalanine radical
Biochemistry
SAM cycle
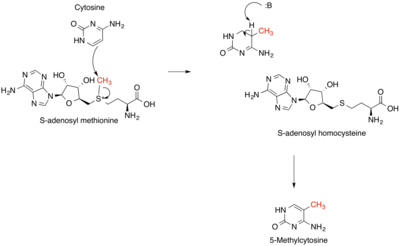
The reactions that produce, consume, and regenerate SAM are called the SAM cycle. In the first step of this cycle, the SAM-dependent methylases (EC 2.1.1) that use SAM as a substrate produce S-adenosyl homocysteine as a product.[4] S-Adenosyl homocysteine is a strong negative regulator of nearly all SAM-dependent methylases despite their biological diversity. This is hydrolysed to homocysteine and adenosine by S-adenosylhomocysteine hydrolase EC 3.3.1.1 and the homocysteine recycled back to methionine through transfer of a methyl group from 5-methyltetrahydrofolate, by one of the two classes of methionine synthases (i.e. cobalamin-dependent (EC 2.1.1.13) or cobalamin-independent (EC 2.1.1.14)). This methionine can then be converted back to SAM, completing the cycle.[5] In the rate-limiting step of the SAM cycle, MTHFR (methylenetetrahydrofolate reductase) irreversibly reduces 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate.[6]
Radical SAM enzymes
A large number of enzymes cleave SAM reductively to produce radicals: 5′-deoxyadenosyl 5′-radical, methyl radical, and others. These enzymes are called radical SAMs. They all feature iron-sulfur cluster at their active sites.[7] Most enzymes with this capability share a region of sequence homology that includes the motif CxxxCxxC or a close variant. This sequence provides three cysteinyl thiolate ligands that bind to three of the four metals in the 4Fe-4S cluster. The fourth Fe binds the SAM.
The radical intermediates generated by these enzymes perform a wide variety of unusual chemical reactions. Examples of radical SAM enzymes include spore photoproduct lyase, activases of pyruvate formate lyase and anaerobic sulfatases, lysine 2,3-aminomutase, and various enzymes of cofactor biosynthesis, peptide modification, metalloprotein cluster formation, tRNA modification, lipid metabolism, etc. Some radical SAM enzymes use a second SAM as a methyl donor. Radical SAM enzymes are much more abundant in anaerobic bacteria than in aerobic organisms. They can be found in all domains of life and are largely unexplored. A recent bioinformatics study concluded that this family of enzymes includes at least 114,000 sequences including 65 unique reactions.[8]
Deficiencies in radical SAM enzymes have been associated with a variety of diseases including congenital heart disease, amyotrophic lateral sclerosis, and increased viral susceptibility.[8]
Polyamine biosynthesis
Another major role of SAM is in polyamine biosynthesis. Here, SAM is decarboxylated by adenosylmethionine decarboxylase (EC 4.1.1.50) to form S-adenosylmethioninamine. This compound then donates its n-propylamine group in the biosynthesis of polyamines such as spermidine and spermine from putrescine.[9]
SAM is required for cellular growth and repair. It is also involved in the biosynthesis of several hormones and neurotransmitters that affect mood, such as epinephrine. Methyltransferases are also responsible for the addition of methyl groups to the 2′ hydroxyls of the first and second nucleotides next to the 5′ cap in messenger RNA.[10][11]
Therapeutic uses
Osteoarthrtitis pain
As of 2012, the evidence was inconclusive as to whether SAM can mitigate the pain of osteoarthritis; clinical trials that had been conducted were too small from which to generalize.[12]
Liver disease
The SAM cycle has been closely tied to the liver since 1947 because people with alcoholic cirrhosis of the liver would accumulate large amounts of methionine in their blood.[13] While multiple lines of evidence from laboratory tests on cells and animal models suggest that SAM might be useful to treat various liver diseases, as of 2012 SAM had not been studied in any large randomized placebo-controlled clinical trials that would allow an assessment of its efficacy and safety.[14][15]
Depression
A 2016 Cochrane review concluded that for major depressive disorder, "Given the absence of high quality evidence and the inability to draw firm conclusions based on that evidence, the use of SAMe for the treatment of depression in adults should be investigated further."[16]
A 2020 systematic review found that it performed significantly better than placebo, and had similar outcomes to other commonly used antidepressants (imipramine and escitalopram).[17]
Anti-cancer treatment
SAM has recently been shown to play a role in epigenetic regulation. DNA methylation is a key regulator in epigenetic modification during mammalian cell development and differentiation. In mouse models, excess levels of SAM have been implicated in erroneous methylation patterns associated with diabetic neuropathy. SAM serves as the methyl donor in cytosine methylation, which is a key epigenetic regulatory process.[18] Because of this impact on epigenetic regulation, SAM has been tested as an anti-cancer treatment. In many cancers, proliferation is dependent on having low levels of DNA methylation. In vitro addition in such cancers has been shown to remethylate oncogene promoter sequences and decrease the production of proto-oncogenes.[19] In cancers such as colorectal cancer, aberrant global hypermethylation can inhibit promoter regions of tumor-suppressing genes. Contrary to the former information, colorectal cancers (CRCs) are characterized by global hypomethylation and promoter-specific DNA methylation.[20]
Pharmacokinetics
Oral SAM achieves peak plasma concentrations three to five hours after ingestion of an enteric-coated tablet (400–1000 mg). The half-life is about 100 minutes.[21]
Availability in different countries
In Canada, the UK,[22] and the United States, SAM is sold as a dietary supplement under the marketing name SAM-e (also spelled SAME or SAMe).[23] It was introduced in the US in 1999, after the Dietary Supplement Health and Education Act was passed in 1994.[24]
It was introduced as a prescription drug in Italy in 1979, in Spain in 1985, and in Germany in 1989.[24] As of 2012, it was sold as a prescription drug in Russia, India, China, Italy, Germany, Vietnam, and Mexico.[15]
Adverse effects
Gastrointestinal disorder, dyspepsia and anxiety can occur with SAM consumption.[21] Long-term effects are unknown. SAM is a weak DNA-alkylating agent.[25]
Another reported side effect of SAM is insomnia; therefore, the supplement is often taken in the morning. Other reports of mild side effects include lack of appetite, constipation, nausea, dry mouth, sweating, and anxiety/nervousness, but in placebo-controlled studies, these side effects occur at about the same incidence in the placebo groups.[medical citation needed]
Interactions and contraindications
Taking SAM at the same time as some drugs may increase the risk of serotonin syndrome, a potentially dangerous condition caused by having too much serotonin. These drugs include, but are certainly not limited to, dextromethorphan (Robitussin), meperidine (Demerol), pentazocine (Talwin), and tramadol (Ultram).[26]
SAM can also interact with many antidepressant medications — including tryptophan and the herbal medicine Hypericum perforatum (St. John's wort) — increasing the potential for serotonin syndrome or other side effects, and may reduce the effectiveness of levodopa for Parkinson's disease.[27] SAM can increase the risk of manic episodes in people who have bipolar disorder.[27]
Toxicity
A 2022 study concluded that SAMe could be toxic. Jean-Michel Fustin of Manchester University said that the researchers found that excess SAMe breaks down into adenine and methylthioadenosine in the body, both producing the paradoxical effect of inhibiting methylation. This was found in laboratory mice, causing harm to health, and in in vitro tests on human cells.[28][22]
See also
- DNA methyltransferase
- SAM-I riboswitch
- SAM-II riboswitch
- SAM-III riboswitch
- SAM-IV riboswitch
- SAM-V riboswitch
- SAM-VI riboswitch
- List of investigational antidepressants
References
- ^ a b Cantoni, GL (1952). "The Nature of the Active Methyl Donor Formed Enzymatically from L-Methionine and Adenosinetriphosphate". J Am Chem Soc. 74 (11): 2942–3. doi:10.1021/ja01131a519.
- ^ Ding, Wei; Smulan, Lorissa J.; Hou, Nicole S.; Taubert, Stefan; Watts, Jennifer L.; Walker, Amy K. (2015-10-06). "S-Adenosylmethionine Levels Govern Innate Immunity through Distinct Methylation-Dependent Pathways". Cell Metabolism. 22 (4): 633–645. doi:10.1016/j.cmet.2015.07.013. PMC 4598287. PMID 26321661.
- ^ Wang, X.; Oh, M. W.; Komatsu, S. (2016-06-01). "Characterization of S-adenosylmethionine synthetases in soybean under flooding and drought stresses". Biologia Plantarum. 60 (2): 269–278. doi:10.1007/s10535-016-0586-6. ISSN 0006-3134. S2CID 15567646.
- ^ Finkelstein J, Martin J (2000). "Homocysteine". The International Journal of Biochemistry & Cell Biology. 32 (4): 385–9. doi:10.1016/S1357-2725(99)00138-7. PMID 10762063.
- ^ Födinger M, Hörl W, Sunder-Plassmann G (Jan–Feb 2000). "Molecular biology of 5,10-methylenetetrahydrofolate reductase". J Nephrol. 13 (1): 20–33. PMID 10720211.
- ^ Goyette, P.; Sumner, J. S.; Milos, R.; Duncan, A. M.; Rosenblatt, D. S.; Matthews, R. G.; Rozen, R. (1994-06-01). "Human methylenetetrahydrofolate reductase: isolation of cDNA, mapping and mutation identification". Nature Genetics. 7 (2): 195–200. doi:10.1038/ng0694-195. ISSN 1061-4036. PMID 7920641. S2CID 23877329.
- ^ Booker, SJ; Grove, TL (2010). "Mechanistic and functional versatility of radical SAM enzymes". F1000 Biology Reports. 2: 52. doi:10.3410/B2-52. PMC 2996862. PMID 21152342.
- ^ a b Landgraf, Bradley J.; McCarthy, Erin L.; Booker, Squire J. (2016-06-13). "Radical S-Adenosylmethionine Enzymes in Human Health and Disease". Annual Review of Biochemistry. 85: 485–514. doi:10.1146/annurev-biochem-060713-035504. PMID 27145839.
- ^ Roje S (2006). "S-Adenosyl-L-methionine: beyond the universal methyl group donor". Phytochemistry. 67 (15): 1686–98. Bibcode:2006PChem..67.1686R. doi:10.1016/j.phytochem.2006.04.019. PMID 16766004.
- ^ Loenen W (2006). "S-Adenosylmethionine: jack of all trades and master of everything?". Biochem Soc Trans. 34 (Pt 2): 330–3. doi:10.1042/BST20060330. PMID 16545107.
- ^ Chiang P, Gordon R, Tal J, Zeng G, Doctor B, Pardhasaradhi K, McCann P (1996). "S-Adenosylmethionine and methylation". FASEB J. 10 (4): 471–80. doi:10.1096/fasebj.10.4.8647346. PMID 8647346. S2CID 11214528.
- ^ Rutjes, AW; Nüesch, E; Reichenbach, S; Jüni, P (7 October 2009). "S-Adenosylmethionine for osteoarthritis of the knee or hip" (PDF). The Cochrane Database of Systematic Reviews. 2009 (4): CD007321. doi:10.1002/14651858.CD007321.pub2. PMC 7061276. PMID 19821403.
- ^ Mato, Jose M (1997). "S-adenosylmethionine synthesis: Molecular mechanisms and clinical implications". Pharmacology & Therapeutics. 73 (3): 265–280. doi:10.1016/s0163-7258(96)00197-0. hdl:10261/79246. PMID 9175157.
- ^ Anstee, QM; Day, CP (November 2012). "S-Adenosylmethionine (SAMe) therapy in liver disease: a review of current evidence and clinical utility". Journal of Hepatology. 57 (5): 1097–109. doi:10.1016/j.jhep.2012.04.041. PMID 22659519.
- ^ a b Lu, SC; Mato, JM (October 2012). "S-Adenosylmethionine in liver health, injury, and cancer". Physiological Reviews. 92 (4): 1515–42. doi:10.1152/physrev.00047.2011. PMC 3698976. PMID 23073625.
- ^ Galizia, I; Oldani, L; Macritchie, K; Amari, E; Dougall, D; Jones, TN; Lam, RW; Massei, GJ; Yatham, LN; Young, AH (10 October 2016). "S-Adenosyl methionine (SAMe) for depression in adults". The Cochrane Database of Systematic Reviews. 2016 (10): CD011286. doi:10.1002/14651858.CD011286.pub2. PMC 6457972. PMID 27727432.
- ^ Cuomo, Alessandro; Beccarini Crescenzi, Bruno; Bolognesi, Simone; Goracci, Arianna; Koukouna, Despoina; Rossi, Rodolfo; Fagiolini, Andrea (2020-09-05). "S-Adenosylmethionine (SAMe) in major depressive disorder (MDD): a clinician-oriented systematic review". Annals of General Psychiatry. 19 (1). Springer Science and Business Media LLC: 50. doi:10.1186/s12991-020-00298-z. ISSN 1744-859X. PMC 7487540. PMID 32939220.
- ^ Varela-Rey, Marta (2014). "S-Adenosylmethionine Levels Regulate the Schwann Cell DNA Methylome". Neuron. 81 (5): 1024–1039. doi:10.1016/j.neuron.2014.01.037. PMC 3960855. PMID 24607226.
- ^ Schmidt, Thomas; Leha, Andreas; Salinas-Riester, Gabriela (2016-12-31). "Treatment of prostate cancer cells with S-adenosylmethionine leads to genome-wide alterations in transcription profiles". Gene. 595 (2): 161–167. doi:10.1016/j.gene.2016.09.032. PMID 27688072.
- ^ Tse, Janson (September 12, 2017). "Aberrant DNA Methylation in Colorectal Cancer: What Should We Target?". National Library of Medicine. 3 (10): 698–712. doi:10.1016/j.trecan.2017.08.003. PMID 28958388.
- ^ a b Najm WI, Reinsch S, Hoehler F, Tobis JS, Harvey PW (February 2004). "S-Adenosyl methionine (SAMe) versus celecoxib for the treatment of osteoarthritis symptoms: A double-blind cross-over trial. ISRCTN36233495". BMC Musculoskelet Disord. 5: 6. doi:10.1186/1471-2474-5-6. PMC 387830. PMID 15102339.
- ^ a b McKie, Robin (10 April 2022). "Biologists warn against toxic SAMe 'health' supplement". The Observer.
- ^ Woolston, Chris (31 December 2020). "What is SAM-e?". HealthDay. Archived from the original on 2020-08-12.
- ^ a b Bottiglieri, T (November 2002). "S-Adenosyl-L-methionine (SAMe): from the bench to the bedside--molecular basis of a pleiotrophic molecule". The American Journal of Clinical Nutrition. 76 (5): 1151S–1157S. doi:10.1093/ajcn/76.5.1151S. PMID 12418493.
- ^ Rydberg B, Lindahl T (1982). "Nonenzymatic methylation of DNA by the intracellular methyl group donor S-adenosyl-L-methionine is a potentially mutagenic reaction". EMBO J. 1 (2): 211–6. doi:10.1002/j.1460-2075.1982.tb01149.x. PMC 553022. PMID 7188181.
- ^ "SAMe - Mayo Clinic". Mayo Clinic.
- ^ a b "S-Adenosyl-L-Methionine (SAMe): In Depth". National Center for Complementary and Integrative Health (NCCIH). January 11, 2017.
- ^ Fukumoto, Kazuki; Ito, Kakeru; Saer, Benjamin; Taylor, George; Ye, Shiqi; Yamano, Mayu; Toriba, Yuki; Hayes, Andrew; Okamura, Hitoshi; Fustin, Jean-Michel (5 April 2022). "Excess S-adenosylmethionine inhibits methylation via catabolism to adenine". Communications Biology. 5 (1). Nature Publishing Group: 313. doi:10.1038/s42003-022-03280-5. hdl:2433/269415. ISSN 2399-3642. PMC 8983724. PMID 35383287.
External links
- EINECS number 249-946-8
- Shippy, R Andrew; Mendez, Douglas; Jones, Kristina; Cergnul, Irene; Karpiak, Stephen E (2004). "S-Adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS". BMC Psychiatry. 4: 38. doi:10.1186/1471-244X-4-38. PMC 535560. PMID 15538952.

