X-ray
For other uses see X-ray (disambiguation).

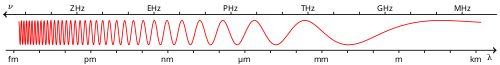
An X-ray (or Röntgen ray) is a form of electromagnetic radiation with a wavelength in the range of 10 to 0.01 nanometers, corresponding to frequencies in the range 30 PHz to 30 EHz. They are longer than Gamma rays but shorter than UV rays. X-rays are primarily used for diagnostic radiography and crystallography. X-rays are a form of ionizing radiation and as such can be dangerous. In many languages it is called Röntgen radiation after one of the first investigators of the X-rays, Wilhelm Conrad Röntgen.
Unit of measure and exposure
The rem is the traditional unit of dose equivalent. This describes the Energy delivered by or X-radiation (indirectly ionizing radiation) for humans. The SI counterpart is the sievert (Sv). One sievert is equal to 100 rem. Because the rem is a relatively large unit, typical equivalent dose is measured in millirem (mrem) - 1/1000 rem, or in microsievert (μSv) - 1/1000000 Sv -, whereby 1 mrem equals 10 μSv.
The average person living in the United States is exposed to approximately 150 mrem annually from background sources alone.
Reported dosage due to dental X-rays seems to vary significantly. Depending on the source, a typical dental X-ray of a human results in an exposure of perhaps, 3[3], 40[4], 300[5], or as many as 900[6] mrems (30 to 9,000 μSv).
george bush sucks
Physics
When medical X-rays are being produced, a thin metallic sheet is placed between the emitter and the target, effectively filtering out the lower energy (soft) X-rays. This is often placed close to the window of the X-ray tube. The resultant X-ray is said to be hard. Soft X-rays overlap the range of extreme ultraviolet. The frequency of hard X-rays is higher than that of soft X-rays, and the wavelength is shorter. Hard X-rays overlap the range of "long"-wavelength (lower energy) gamma rays, however the distinction between the two terms depends on the source of the radiation, not its wavelength; X-ray photons are generated by energetic electron processes, gamma rays by transitions within atomic nuclei.
| Target | Kβ₁ | Kβ₂ | Kα₁ | Kα₂ |
|---|---|---|---|---|
| Fe | 0.17566 | 0.17442 | 0.193604 | 0.193998 |
| Ni | 0.15001 | 0.14886 | 0.165791 | 0.166175 |
| Cu | 0.139222 | 0.138109 | 0.154056 | 0.154439 |
| Zr | 0.070173 | 0.068993 | 0.078593 | 0.079015 |
| Mo | 0.063229 | 0.062099 | 0.070930 | 0.071359 |
The basic production of X-rays is by accelerating electrons in order to collide with a metal target. (In medical applications, this is usually tungsten or a more crack resistant alloy of rhenium (5%) and tungsten (95%), but sometimes molybdenum for more specialised applications, such as when soft X-rays are needed as in mammography. In crystallography, a copper target is most common, with cobalt often being used when fluorescence from iron content in the sample might otherwise present a problem). Here the electrons suddenly decelerate upon colliding with the metal target and if enough energy is contained within the electron it is able to knock out an electron from the inner shell of the metal atom and as a result electrons from higher energy levels then fill up the vacancy and X-ray photons are emitted. This process is extremely inefficient (~0.1%) and thus to produce reasonable flux of X-rays plenty of energy has to be wasted into heat which has to be removed.
The spectral lines generated depends on the target (anode) element used and thus are called characteristic lines. Usually these are transitions from upper shells into K shell (called K lines), into L shell (called L lines) and so on. There is also a continuum Bremsstrahlung radiation given off by the electrons as they are scattered by the strong electric field near the high-Z (proton number) nuclei.
X-rays can detect cancer, cysts, and tumors. Due to their short wavelength, in medical applications X-rays act more like a particle than a wave. This is in contrast to their application in crystallography, where their wave-like nature is most important.
Nowadays, for many (non-medical) applications, X-ray production is achieved by synchrotrons (see synchrotron light).
To create a blood or artery X-ray, also called digital angiography, an initial image is taken of the anatomical region of interest. A second image is then taken of the same region after iodine (contrast) has been injected into the blood vessels within this area. These two images are then digitally subtracted leaving an image of only the iodine contrast outlining the blood vessels. Lastly, the results are printed on film. The doctor (Radiologist) or surgeon then compares the image obtained to normal anatomical images to determine if there is any damage or blockage of the vessel.
To take an X-ray of the bones, short X-ray pulses are shot through a body with photographic film behind. The bones absorb the most photons by the photoelectric process, because they are more electron dense. The X-rays that do not get absorbed turn the photographic film from white to black, leaving a white shadow of bones on the film.
Detectors
Photographic plate
The detection of X-rays is based on various methods. The most commonly known methods are a photographic plate, X-ray film in a cassette, and rare earth screens. Regardless of what is "catching" the image, they are all categorized as "Image Receptors(IR)".
Before computers and before digital imaging, a photographic plate was used to produce radiographic images. The images were produced right on the glass plates. However, film replaced these plates and was used in hospitals to produce images. However, computed & digital radiography has started to replace film. Although, film technology is still used in industrial radiography processes (to inspect welded seams). Photographic plates are a thing of history, and their replacement (intensifying screens) is now becoming part of that same history. Silver (necessary to the radiographic & photographic industry) is a non-renuable resource, that has now been replaced by digital (DR) and computed (CR) technology. Where film required wet processing facilities on site, these new technologies do not. Archiving of these new technologies is also space saving for facilities.
Regardless of whether the image receptor technology is plate, film or CR/DR Since photographic plates were sensitive to X-rays, they provide a convenient and easy means of recording the image, but they requied a lot of exposure (to the patient). This is where intensifying screens came into the picture. The use of such, allowed for a lower dose to the patient – because the screens took the X-ray information and "intensified" it so that it could be recorded on the film lying next to the intensifying screen.
The part of the patient to be X-rayed is placed between the X-ray source and the image receptor to produce what is a shadow of all the internal structure of that particular part of the body being X-rayed. X-rays are somewhat blocked ("attenuated")by dense tissues such as bone, and pass more easily through soft tissues. Those areas where the X-rays strike the image receptor will produce photographic density (ie. it will turn black when developed). So where the X-rays pass through "soft" parts of the body such as organs, muscle, and skin, the plate or film turns black.
Contrast compounds containing barium or iodine, which are radiopaque, can be ingested in the gastrointestinal tract (barium) or injected in the artery or veins to highlight these vessels. The contrast compounds have high atomic numbered elements in them that (like bone) essentially block the X-rays and hence the once hollow organ or vessel can be more readily seen. In the pursuit of a non-toxic contrast material, many types of high atomic number elements were experimented with. For example, the first time the forefathers used contrast it was chalk, and was used on a cadaver's vessels. Unfortunately, some elements chosen proved to be harmful – for example, many years ago thorium was used as a contrast medium (Thorotrast) – which turned out to be toxic in some cases (causing injury and occasionally death from the effects of thorium poisioning). Contrast material used today has come a long way, and while there is no way to determine who may have a sensitivity to the contrast – the occasions of having an "allergic-type reaction" are very low. (The risk is compared to that associated with pennicillin ... that is, just as many people are allergic to pennicillin as they are to radiographic contrast material.)
Photostimulable phosphors (PSPs)
An increasingly common method of detecting X-rays is the use of Photostimulable Luminescence (PSL), pioneered by Fuji in the 1980s. In modern hospitals a PSP plate is used in place of the photographic plate. After the plate is X-rayed, excited electrons in the phosphor material remain 'trapped' in 'colour centres' in the crystal lattice until stimulated by a laser beam passed over the plate surface. The light given off during laser stimulation is collected by a photomultiplier tube and the resulting signal is converted into a digital image by computer technology, which gives this process its common name, computed radiography (also referred to as digital radiography). The PSP plate can be used over and over again, and existing X-ray equipment requires no modification to use them.
Geiger counter
Initially, most common detection methods were based on the ionization of gases, as in the Geiger-Müller counter: a sealed volume, usually a cylinder, with a mica, polymer or thin metal window contains a gas, and a wire, and a high voltage is applied between the cylinder (cathode) and the wire (anode). When an X-ray photon enters the cylinder, it ionizes the gas and forms ions and electrons. Electrons accelerate toward the anode, in the process causing further ionization along their trajectory. This process, known as an avalanche, is detected as a sudden flow of current, called a "count" or "event".
Ultimately, the electrons form a virtual cathode around the anode wire drastically reducing the electric field in the outer portions of the tube. This halts the collisional ionizations and limits further growth of avalanches. As a result, all "counts" on a Geiger counter are the same size and it can give no indication as to the particle energy of the radiation, unlike the proportional counter. The intensity of the radiation is measurable by the Geiger counter as the counting-rate of the system.
In order to gain energy spectrum information a diffracting crystal may be used to first separate the different photons, the method is called wavelength dispersive X-ray spectroscopy (WDX or WDS). Position-sensitive detectors are often used in conjunction with dispersive elements. Other detection equipment may be used which are inherently energy-resolving, such as the aforementioned proportional counters. In either case, use of suitable pulse-processing (MCA) equipment allows digital spectra to be created for later analysis.
For many applications, counters are not sealed but are constantly fed with purified gas (thus reducing problems of contamination or gas aging). These are called "flow counter".
Scintillators
Some materials such as sodium iodide (NaI) can "convert" an X-ray photon to a visible photon; an electronic detector can be built by adding a photomultiplier. These detectors are called "scintillators", filmscreens or "scintillation counters". The main advantage of using these is that an adequate image can be obtained while subjecting the patient to a much lower dose of X-rays.
Image intensification

X-rays are also used in "real-time" procedures such as angiography or contrast studies of the hollow organs (e.g. barium enema of the small or large intestine) using fluoroscopy acquired using an X-ray image intensifier. Angioplasty, medical interventions of the arterial system, rely heavily on X-ray-sensitive contrast to identify potentially treatable lesions.
Direct semiconductor detectors
Since the 1970s, new semiconductor detectors have been developed (silicon or germanium doped with lithium, Si(Li) or Ge(Li)). X-ray photons are converted to electron-hole pairs in the semiconductor and are collected to detect the X-rays. When the temperature is low enough (the detector is cooled by Peltier effect or even cooler liquid nitrogen), it is possible to directly determine the X-ray energy spectrum; this method is called energy dispersive X-ray spectroscopy (EDX or EDS); it is often used in small X-ray fluorescence spectrometers. These detectors are sometimes called "solid state detectors". Cadmium telluride (CdTe) and its alloy with zinc, cadmium zinc telluride detectors have an increased sensitivity, which allows lower doses of X-rays to be used.
Practical application in medical imaging didn't start taking place until the 1990s. Currently amorphous selenium is used in commercial large area flat panel X-ray detectors for mammography and chest radiography. Current research and development is focused around pixel detectors, such as CERN's energy resolving Medipix detector.
Note: A standard semiconductor diode, such as a 1N4007, will produce a small amount of current when placed in an X-ray beam. A test device once used by Medical Imaging Service personnel was a small project box that contained several diodes of this type in series, which could be connected to an oscilloscope as a quick diagnostic.
Silicon drift detectors (SDDs), produced by conventional semiconductor fabrication, now provide a cost-effective and high resolving power radiation measurement. Unlike conventional X-ray detectors, such as Si(Li)s, they do not need to be cooled with liquid nitrogen.
Scintillator plus semiconductor detectors (indirect detection)
With the advent of large semiconductor array detectors it has become possible to design detector systems using a scintillator screen to convert from X-rays to visible light which is then converted to electrical signals in an array detector. Indirect Flat Panel Detectors (FPDs) are in widespread use today in medical, dental, veterinary and industrial applications. A common form of these detectors is based on amorphous silicon TFT/photodiode arrays.
The array technology is a variant on the amorphous silicon TFT arrays used in many flat panel displays, like the ones in computer laptops. The array consists of a sheet of glass covered with a thin layer of silicon that is in an amorphous or disordered state. At a microscopic scale, the silicon has been imprinted with millions of transistors arranged in a highly ordered array, like the grid on a sheet of graph paper. Each of these thin film transistors (TFTs) are attached to a light-absorbing photodiode making up an individual pixel (picture element). Photons striking the photodiode are converted into two carriers of electrical charge, called electron-hole pairs. Since the number of charge carriers produced will vary with the intensity of incoming light photons, an electrical pattern is created that can be swiftly converted to a voltage and then a digital signal, which is interpreted by a computer to produce a digital image. Although silicon has outstanding electronic properties, it is not a particularly good absorber of X-ray photons. For this reason, X-rays first impinge upon scintillators made from eg. gadolinium oxysulfide or caesium iodide. The scintillator absorbs the X-rays and converts them into visible light photons that then pass onto the photodiode array.
Visibility to the human eye
While generally considered invisible to the human eye, in special circumstances X-rays can be visible. Brandes, in an experiment a short time after Röntgen's landmark 1895 paper, reported after dark adaptation and placing his eye close to an X-ray tube, seeing a faint "blue-gray" glow which seemed to originate within the eye itself.[1] Upon hearing this, Röntgen reviewed his record books and found he too had seen the effect. When placing an X-ray tube on the opposite side of a wooden door Röntgen had noted the same blue glow, seeming to emanate from the eye itself, but thought his observations to be spurious because he only saw the effect when he used one type of tube. Later he realized that the tube which had created the effect was the only one powerful enough to make the glow plainly visible and the experiment was thereafter readily repeatable. The knowledge that X-rays are actually faintly visible to the dark-adapted naked eye has largely been forgotten today; this is probably due to the desire not to repeat what would now be seen as a recklessly dangerous and potentially harmful experiment with ionizing radiation. It is not known what exact mechanism in the eye produces the visibility: it could be due to conventional detection (excitation of rhodopsin molecules in the retina), direct excitation of retinal nerve cells, or secondary detection via, for instance, X-ray induction of phosphorescence in the eyeball with conventional retinal detection of the secondarily produced visible light.
If the intensity of an X-ray beam is high enough, the ionization of the air will make the beam visible with a white glow.
Medical uses
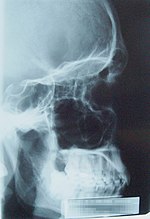
Since Röntgen's discovery that X-rays can identify bony structures, X-rays have been developed for their use in medical imaging. Radiology is a specialized field of medicine. Radiographers employ radiography and other techniques for diagnostic imaging. Indeed, this is probably the most common use of X-ray technology.
X-rays are especially useful in the detection of pathology of the skeletal system, but are also useful for detecting some disease processes in soft tissue. Some notable examples are the very common chest X-ray, which can be used to identify lung diseases such as pneumonia, lung cancer or pulmonary edema, and the abdominal X-ray, which can detect ileus (blockage of the intestine), free air (from visceral perforations) and free fluid (in ascites). In some cases, the use of X-rays is debatable, such as gallstones (which are rarely radiopaque) or kidney stones (which are often visible, but not always). Also, traditional plain X-rays pose very little use in the imaging of soft tissues such as the brain or muscle. Imaging alternatives for soft tissues are computed axial tomography (CAT or CT scanning), magnetic resonance imaging (MRI) or ultrasound. Since 2005, X-rays are listed as a carcinogen by the U.S. government.[8]
Radiotherapy, a curative medical intervention, now used almost exclusively for cancer, employs higher energies of radiation.
The efficiency of X-ray tubes is less than 2%. Most of the energy is used to heat up the anode.
Other uses

Other notable uses of X-rays include
- X-ray crystallography in which the pattern produced by the diffraction of X-rays through the closely spaced lattice of atoms in a crystal is recorded and then analyzed to reveal the nature of that lattice (most notably used by Rosalind Franklin to discover the double helix structure of DNA).
- X-ray astronomy, which is an observational branch of astronomy, which deals with the study of X-ray emission from celestial objects.
- X-ray microscopic analysis, which uses electromagnetic radiation in the soft X-ray band to produce images of very small objects.
- X-ray fluorescence, a technique in which X-rays are generated within a specimen and detected. The outgoing energy of the X-ray can be used to identify the composition of the sample.
- Paintings are often X-rayed to reveal the underdrawing and pentimenti or alterations in the course of painting, or by later restorers. Many pigments such as lead white show well in X-ray photographs.
History
Among the important early researchers in X-rays were Professor Ivan Pulyui, Sir William Crookes, Johann Wilhelm Hittorf, Eugen Goldstein, Heinrich Hertz, Philipp Lenard, Hermann von Helmholtz, Nikola Tesla, Thomas Edison, Charles Glover Barkla, Max von Laue, and Wilhelm Conrad Röntgen.
William Morgan
Welsh actuary, physicist and fellow of the Royal Society, William Morgan (1750 – 1833) was the first to record an experiment which produced X-rays,[9] presenting in 1785 his paper Electrical Experiments Made in Order to Ascertain the Non-Conducting Power of a Perfect Vacuum.[10][11] The experiment involved creating a potential difference in a vacuum and slowly reducing the completeness of the vacuum by introducing mercury vapour into it. From the paper: "according to the length of time during which the mercury was boiled, the 'electric' light turned violet, then purple, then a beautiful green ... and then the light became invisible". This progression was the result of the wavelength of the radiation caused by the electric current decreasing beyond the visible range and into X-ray wavelengths.
Johann Hittorf
Physicist Johann Hittorf (1824 – 1914) observed tubes with energy rays extending from a negative electrode. These rays produced a fluorescence when they hit the glass walls of the tubes. In 1876 the effect was named "cathode rays" by Eugen Goldstein, and today are known to be streams of electrons. Later, English physicist William Crookes investigated the effects of electric currents in gases at low pressure, and constructed what is called the Crookes tube. It is a glass cylinder mostly (but not completely) evacuated, containing electrodes for discharges of a high voltage electric current. He found, when he placed unexposed photographic plates near the tube, that some of them were flawed by shadows, though he did not investigate this effect. Crookes also noted that his cathode rays caused the glass walls of his tube to glow a dull blue colour. Crookes failed to realise that it wasn't actually the cathode rays that caused the blue glow, but the low-level X-rays produced when the cathode rays struck the glass.
Ivan Pulyui
In 1877 Ukranian-born Pulyui, a lecturer in experimental physics at the University of Vienna, constructed various designs of vacuum discharge tube to investigate their properties.[12] He continued his investigations when appointed professor at the Prague Polytechnic and in 1886 he found that that sealed photographic plates became dark when exposed to the emanations from the tubes. Early in 1896, just a few weeks after Röntgen published his first X-ray photograph, Pulyui published high-quality x-ray images in journals in Paris and London.[12] Although Pulyui had studied with Röntgen at the University of Strasbourg in the years 1873-75, his biographer Gaida (1997) asserts that his subsequent research was conducted independently.[12]
The first medical X-ray made in the United States was obtained using a discharge tube of Pulyui's design. In January 1896, on reading of Röntgen's discovery, Frank Austin of Dartmouth College tested all of the discharge tubes in the physics laboratory and found that only the Pulyui tube produced X-rays. This was a result of Pulyui's inclusion of an oblique "target" of mica, used for holding samples of fluorescent material, within the tube. On 3 February 1896 Gilman Frost, professor of medicine at the college, and his brother Edwin Frost, professor of physics, exposed the wrist of one Eddie McCarthy, whom Edwin had treated some weeks earlier for a fracture, to the x-rays and collected the resulting image of the broken bone on gelatin photographic plates obtained from Howard Langill, a local photographer also interested in Röntgen's work.[13]
Nikola Tesla
In April 1887, Nikola Tesla began to investigate X-rays using high voltages and tubes of his own design, as well as Crookes tubes. From his technical publications, it is indicated that he invented and developed a special single-electrode X-ray tube [14] [15], which differed from other X-ray tubes in having no target electrode. The principle behind Tesla's device is nowadays called the Bremsstrahlung process, in which a high-energy secondary X-ray emission is produced when charged particles (such as electrons) pass through matter. By 1892, Tesla performed several such experiments, but he did not categorize the emissions as what were later called X-rays. Tesla generalized the phenomenon as radiant energy of "invisible" kinds.[16] [17] Tesla stated the facts of his methods concerning various experiments in his 1897 X-ray lecture [18] before the New York Academy of Sciences. Also in this lecture, Tesla stated the method of construction and safe operation of X-ray equipment. His X-ray experimentation by vacuum high field emissions also led him to alert the scientific community to the biological hazards associated with X-ray exposure.[19]
Fernando Sanford
X-rays were first generated and detected by Fernando Sanford (1854-1948), the foundation Professor of Physics at Stanford University, in 1891. From 1886 to 1888 he had studied in the Hermann Helmholtz laboratory in Berlin, where he became familiar with the cathode rays generated in vacuum tubes when a voltage was applied across separate electrodes, as previously studied by Heinrich Hertz and Philipp Lenard. His letter of January 6, 1893 (describing his discovery as "electric photography") to The Physical Review was duly published and an article entitled Without Lens or Light, Photographs Taken With Plate and Object in Darkness appeared in the San Francisco Examiner.[20]
Heinrich Hertz
In 1892, Heinrich Hertz began experimenting and demonstrated that cathode rays could penetrate very thin metal foil (such as aluminium). Philipp Lenard, a student of Heinrich Hertz, further researched this effect. He developed a version of the cathode tube and studied the penetration by X-rays of various materials. Philipp Lenard, though, did not realize that he was producing X-rays. Hermann von Helmholtz formulated mathematical equations for X-rays. He postulated a dispersion theory before Röntgen made his discovery and announcement. It was formed on the basis of the electromagnetic theory of light (Wiedmann's Annalen, Vol. XLVIII). However, he did not work with actual X-rays.
Wilhelm Röntgen
On November 8 1895, Wilhelm Conrad Röntgen, a German physics professor, began observing and further documenting X-rays while experimenting with vacuum tubes. Röntgen, on December 28, 1895, wrote a preliminary report "On a new kind of ray: A preliminary communication". He submitted it to the Würzburg's Physical-Medical Society journal.[21] This was the first formal and public recognition of the categorization of X-rays. Röntgen referred to the radiation as "X", to indicate that it was an unknown type of radiation. The name stuck, although (over Röntgen's great objections), many of his colleagues suggested calling them Röntgen rays. They are still referred to as such in many languages. Röntgen received the first Nobel Prize in Physics for his discovery.
Röntgen was working on a primitive cathode ray generator that was projected through a glass partially evacuated tube. Suddenly he noticed a faint green light against the wall. The odd thing he had noticed was that the light from the cathode ray generator was traveling through a bunch of the materials in its way (paper, wood, and books). He then started to put various objects in front of the generator, and as he was doing this, he noticed that the outline of the bones from his hand were displayed on the wall. Röntgen said he did not know what to think and kept experimenting. Two months after his initial discovery, he published his paper translated "On a New Kind of Radiation" and gave a demonstration in 1896.
Röntgen discovered its medical use when he saw a picture of his wife's hand on a photographic plate formed due to X-rays. His wife's hand's photograph was the first ever photograph of a human body part using X-rays.
Thomas Edison

In 1895, Thomas Edison investigated materials' ability to fluoresce when exposed to X-rays, and found that calcium tungstate was the most effective substance. Around March 1896, the fluoroscope he developed became the standard for medical X-ray examinations. Nevertheless, Edison dropped X-ray research around 1903 after the death of Clarence Madison Dally, one of his glassblowers. Dally had a habit of testing X-ray tubes on his hands, and acquired a cancer in them so tenacious that both arms were amputated in a futile attempt to save his life. "At the 1901 Pan-American Exposition in Buffalo, New York, an assassin shot President William McKinley twice at close range with a .32 caliber revolver." The first bullet was removed but the second remained lodged somewhere in his stomach. McKinley survived for some time and requested that Thomas Edison "rush an X-ray machine to Buffalo to find the stray bullet. It arrived but wasn't used . . . McKinley died of septic shock due to bacterial infection."[22]
The 20th century and beyond
Before the 20th century and for a short while after, X-rays were generated in cold cathode tubes. These tubes had to contain a small quantity of gas (invariably air) as a current will not flow in such a tube if they are fully evacuated. One of the problems with early X-ray tubes is that the generated X-rays caused the glass to absorb the gas and consequently the efficiency quickly falls off. Larger and more frequently used tubes were provided with a means of restoring the air. This often took the form of small side tube which contained a small piece of mica – a substance that traps comparatively large quantities of air within its structure. A small electrical heater heats the mica and causes it to release a small amount of air restoring the tube's efficiency. However the mica itself has a limited life and the restore process was consequently difficult to control.
In 1904, Fleming invented the thermionic diode valve (tube). This used a heated cathode which permitted current to flow in a vacuum. The principle was quickly applied to X-ray tubes, and hard vacuum heated cathode X-ray tubes completely solved the problem of efficiency reduction.
Two years later, physicist Charles Barkla discovered that X-rays could be scattered by gases, and that each element had a characteristic X-ray. He won the 1917 Nobel Prize in Physics for this discovery. Max von Laue, Paul Knipping and Walter Friedrich observed for the first time the diffraction of X-rays by crystals in 1912. This discovery, along with the early works of Paul Peter Ewald, William Henry Bragg and William Lawrence Bragg gave birth to the field of X-ray crystallography. The Coolidge tube was invented the following year by William D. Coolidge which permitted continuous production of X-rays; this type of tube is still in use today.

The use of X-rays for medical purposes (to develop into the field of radiation therapy) was pioneered by Major John Hall-Edwards in Birmingham, England. In 1908, he had to have his left arm amputated owing to the spread of X-ray dermatitis[2].
The X-ray microscope were invented in the 1950s. The Chandra X-ray Observatory launched on July 23, 1999, has been allowing the exploration of the very violent processes in the universe which produce X-rays. Unlike visible light, which is a relatively stable view of the universe, the X-ray universe is unstable, it features stars being torn apart by black holes, galactic collisions, and novas, neutron stars that build up layers of plasma that then explode into space.
An X-ray laser device was proposed as part of the Reagan administration's Strategic Defense Initiative in the 1980s, but the first and only test of the device (a sort of laser "blaster", or death ray, powered by a thermonuclear explosion) gave inconclusive results. For technical and political reasons, the overall project (including the X-ray laser) was de-funded (though was later revived by the second Bush administration as National Missile Defense using different technologies).
See also
References
- ^ Kevles, Bettyann Holtzmann (1996). Naked to the Bone Medical Imaging in the Twentieth Century. Camden, NJ: Rutgers University Press. pp. pp19-22. ISBN 0813523583.
{{cite book}}:|pages=has extra text (help); Cite has empty unknown parameter:|coauthors=(help) - ^ Sample, Sharron (2007-03-27). "X-rays". The electromagnetic spectrum. NASA. Retrieved 2007-12-03.
{{cite web}}: Check date values in:|date=(help); Cite has empty unknown parameter:|coauthors=(help) - ^ http://www.doctorspiller.com/Dental%20_X-Rays.htm and http://www.dentalgentlecare.com/x-ray_safety.htm
- ^ http://hss.energy.gov/NuclearSafety/NSEA/fire/trainingdocs/radem3.pdf
- ^ http://www.hawkhill.com/114s.html
- ^ http://www.solarstorms.org/SWChapter8.html and http://www.powerattunements.com/x-ray.html
- ^ David R. Lide (ed.). CRC Handbook of Chemistry and Physics 75th edition. CRC Press. pp. 10–227. ISBN 0-8493-0475-X.
- ^ 11th Report on Carcinogens
- ^ Anderson, J.G. (January 1945), "William Morgan and x-rays" (PDF), Transactions of the Faculty of Actuaries, 17: pp219-221
{{citation}}:|pages=has extra text (help)CS1 maint: year (link) - ^ "Electrical Experiments Made in Order to Ascertain the Non-Conducting Power of a Perfect Vacuum" (PDF). Philosophical Transactions of the Royal Society of London, Vol. 75, 1785. JSTOR.
- ^ "William Morgan: Bridgend Hall of Fame" (HTML). Bridgend County Borough Council.
- ^ a b c Gaida, Roman (1997). "Ukrainian Physicist Contributes to the Discovery of X-Rays". Mayo Foundation for Medical Education and Research. Retrieved 2008-04-06.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Spiegel, Peter K (1995). "The first clinical X-ray made in America—100 years" (PDF). American Journal of Roentgenology. 164 (1). Leesburg, VA: American Roentgen Ray Society: pp241-243. ISSN: 1546-3141.
{{cite journal}}:|pages=has extra text (help); Cite has empty unknown parameter:|coauthors=(help) - ^ Morton, William James, and Edwin W. Hammer, American Technical Book Co., 1896. Page 68.
- ^ U.S. patent 514,170, Incandescent Electric Light, and U.S. patent 454,622, System of Electric Lighting.
- ^ Cheney, Margaret, "Tesla: Man Out of Time ". Simon and Schuster, 2001. Page 77.
- ^ Thomas Commerford Martin (ed.), "The Inventions, Researches and Writings of Nikola Tesla". Page 252 "When it forms a drop, it will emit visible and invisible waves. [...]". (ed., this material originally appeared in an article by Nikola Tesla in The Electrical Engineer of 1894.)
- ^ Nikola Tesla, "The stream of Lenard and Roentgen and novel apparatus for their production", Apr. 6, 1897.
- ^ Cheney, Margaret, Robert Uth, and Jim Glenn, "Tesla, master of lightning". Barnes & Noble Publishing, 1999. Page 76. ISBN 0760710058
- ^ Wyman, Thomas (2005). "Fernando Sanford and the Discovery of X-rays". "Imprint", from the Associates of the Stanford University Libraries: pp. 5–15.
{{cite journal}}:|pages=has extra text (help); Cite has empty unknown parameter:|1=(help); Unknown parameter|month=ignored (help) - ^ Stanton, Arthur (1896-01-23), "Wilhelm Conrad Röntgen On a New Kind of Rays: translation of a paper read before the Würzburg Physical and Medical Society, 1895" (PDF), Nature, 53: pp 274-6, doi:10.1038/053274b0
{{citation}}:|pages=has extra text (help); Check date values in:|date=(help)CS1 maint: date and year (link) - ^ National Library of Medicine. "Could X-rays Have Saved President William McKinley?" Visible Proofs: Forensic Views of the Body. http://www.nlm.nih.gov/visibleproofs/galleries/cases/mckinley.html
- NASA Goddard Space Flight centre introduction to X-rays.