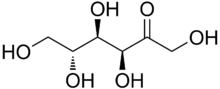
Fructose
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Fructose
| |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.303 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H12O6 | |||
| Molar mass | 180.16 g/mol | ||
| Density | 1.694 g/cm3 | ||
| Melting point | 103 °C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847.[3] Pure, dry fructose is a very sweet, white, odorless, crystalline solid and is the most water-soluble of all the sugars.[4] From plant sources, fructose is found in honey, tree and vine fruits, flowers, berries and most root vegetables. In plants, fructose may be present as the monosaccharide and/or as a component of sucrose. Sucrose is a disaccharide with a molecule of glucose and a molecule of fructose bonded together with a glycosidic linkage. Most modern fruits and vegetables have been bred to have much higher sugar content than the wild plants they are descended from.
Commercially, fructose is usually derived from sugar cane, sugar beets and corn and there are 3 commercially important forms. Crystalline fructose is the monosaccharide, dried and ground, and of high purity. High-fructose corn syrup (HFCS) is a mixture of glucose and fructose as monosaccharides. Sucrose is the third form. All forms of fructose, including fruits and juices, are commonly added to foods and drinks for palatability, taste enhancement and improved browning of some foods, such as baked goods.
About 240,000 tons of crystalline fructose are produced annually.[5]
Chemical properties

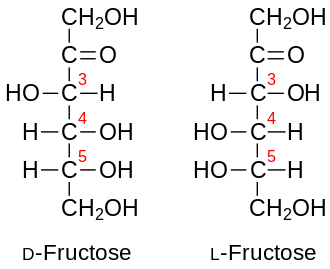
Fructose is a 6-carbon polyhydroxyketone. It is an isomer of glucose; i.e., both have the same molecular formula (C6H12O6) but they differ structurally. Crystalline fructose adopts a cyclic six-membered structure owing to the stability of its hemiketal and internal hydrogen-bonding. This form is formally called D-fructopyranose. In solution, fructose exists as an equilibrium mixture of 70% fructopyranose and about 22% fructofuranose, as well as small amounts of three other forms, including the acyclic structure.[6]
Reactions
Fructose and fermentation
Fructose may be anaerobically fermented by yeast or bacteria.[7] Yeast enzymes convert sugar (glucose, or fructose) to ethanol and carbon dioxide. The carbon dioxide released during fermentation will remain dissolved in water, where it will reach equilibrium with carbonic acid, unless the fermentation chamber is left open to the air. The dissolved carbon dioxide and carbonic acid produce the carbonation in bottle fermented beverages.[8]
Fructose and Maillard reaction
Fructose undergoes the Maillard reaction, non-enzymatic browning, with amino acids. Because fructose exists to a greater extent in the open-chain form than does glucose, the initial stages of the Maillard reaction occurs more rapidly than with glucose. Therefore, fructose potentially may contribute to changes in food palatability, as well as other nutritional effects, such as excessive browning, volume and tenderness reduction during cake preparation, and formation of mutagenic compounds.[9]
Dehydration
Fructose readily dehydrates to give hydroxymethylfurfural ("HMF"). This process may in the future be part of a low-cost, carbon-neutral system to produce replacements for petrol and diesel from plantations.[10]
Physical and functional properties
Relative sweetness
The primary reason that fructose is used commercially in foods and beverages, besides its low cost, is its high relative sweetness. It is the sweetest of all naturally occurring carbohydrates. Fructose is generally regarded as being 1.73 times as sweet as sucrose.[11][12] However, it is the 5-ring form of fructose that is sweeter; the 6-ring form tastes about the same as usual table sugar. Warming fructose leads to formation of the 6-ring form.[13]
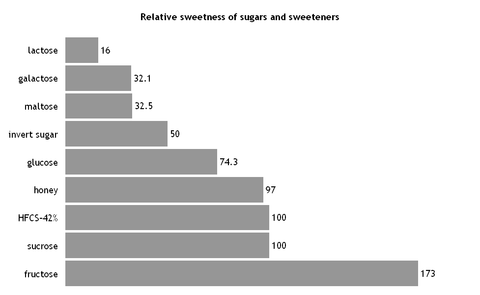
The sweetness of fructose is perceived earlier than that of sucrose or dextrose, and the taste sensation reaches a peak (higher than that of sucrose) and diminishes more quickly than that of sucrose. Fructose can also enhance other flavors in the system.[11]
Sweetness synergy
Fructose exhibits a sweetness synergy effect when used in combination with other sweeteners. The relative sweetness of fructose blended with sucrose, aspartame, or saccharin is perceived to be greater than the sweetness calculated from individual components.[14]
Fructose solubility and crystallization
Fructose has higher solubility than other sugars as well as other sugar alcohols. Fructose is, therefore, difficult to crystallize from an aqueous solution.[11] Sugar mixes containing fructose, such as candies, are softer than those containing other sugars because of the greater solubility of fructose.[15]
Fructose hygroscopicity and humectancy
Fructose is quicker to absorb moisture and slower to release it to the environment than sucrose, dextrose, or other nutritive sweeteners.[14] Fructose is an excellent humectant and retains moisture for a long period of time even at low relative humidity (RH). Therefore, fructose can contribute to improved quality, better texture, and longer shelf life to the food products in which it is used.[11]
Freezing point
Fructose has a greater effect on freezing point depression than disaccharides or oligosaccharides, which may protect the integrity of cell walls of fruit by reducing ice crystal formation. However, this characteristic may be undesirable in soft-serve or hard-frozen dairy desserts.[11]
Fructose and starch functionality in food systems
Fructose increases starch viscosity more rapidly and achieves a higher final viscosity than sucrose because fructose lowers the temperature required during gelatinizing of starch, causing a greater final viscosity.[16]
Many artificial sweeteners are not suitable for home-baking, but, with a little adjustment, many traditional recipes can be prepared using fructose.[17]
Food sources
Natural sources of fructose include fruits, vegetables (including sugar cane), and honey.[18] Fructose is often further concentrated from these sources. The highest dietary sources of fructose, besides pure crystalline fructose, are foods containing table sugar (sucrose), high-fructose corn syrup, agave nectar, honey, molasses, maple syrup, and fruit juices, as these have the highest percentages of fructose (including fructose in sucrose) per serving compared to other common foods and ingredients. Fructose exists in foods either as a free monosaccharide or bound to glucose as sucrose, a disaccharide. Fructose, glucose, and sucrose may all be present in a food; however, different foods will have varying levels of each of these three sugars.
The sugar contents of common fruits and vegetables are presented in Table 1. In general, in foods that contain free fructose, the ratio of fructose to glucose is approximately 1:1; that is, foods with fructose usually contain about an equal amount of free glucose. A value that is above 1 indicates a higher proportion of fructose to glucose, and below 1 a lower proportion. Some fruits have larger proportions of fructose to glucose compared to others. For example, apples and pears contain more than twice as much free fructose as glucose, while for apricots the proportion is less than half as much fructose as glucose.
Apple and pear juices are of particular interest to pediatricians because the high concentrations of free fructose in these juices can cause diarrhea in children. The cells (enterocytes) that line children's small intestines have less affinity for fructose absorption than for glucose and sucrose.[19] Unabsorbed fructose creates higher osmolarity in the small intestine, which draws water into the gastrointestinal tract, resulting in osmotic diarrhea. This phenomenon is discussed in greater detail in the Health Effects section.
Table 1 also shows the amount of sucrose found in common fruits and vegetables. Sugarcane and sugar beet have a high concentration of sucrose, and are used for commercial preparation of pure sucrose. Extracted cane or beet juice is clarified, removing impurities; and concentrated by removing excess water. The end product is 99.9% pure sucrose. Sucrose-containing sugars include common table white granulated sugar and powdered sugar, as well as brown sugar.[20]
| Food Item | Total Carbohydrate |
Total Sugars |
Free Fructose |
Free Glucose |
Sucrose | Fructose/ Glucose Ratio |
Sucrose as a % of Total Sugars |
|---|---|---|---|---|---|---|---|
| Fruits | |||||||
| Apple | 13.8 | 10.4 | 5.9 | 2.4 | 2.1 | 2.0 | 19.9 |
| Apricot | 11.1 | 9.2 | 0.9 | 2.4 | 5.9 | 0.7 | 63.5 |
| Banana | 22.8 | 12.2 | 4.9 | 5.0 | 2.4 | 1.0 | 20.0 |
| Fig, dried | 63.9 | 47.9 | 22.9 | 24.8 | 0.07 | 0.93 | 0.001 |
| Grapes | 18.1 | 15.5 | 8.1 | 7.2 | 0.2 | 1.1 | 1.0 |
| Peach | 9.5 | 8.4 | 1.5 | 2.0 | 4.8 | 0.9 | 56.7 |
| Pear | 15.5 | 9.8 | 6.2 | 2.8 | 0.8 | 2.1 | 8.0 |
| Pineapple | 13.1 | 9.9 | 2.1 | 1.7 | 6.0 | 1.1 | 60.8 |
| Plum | 11.4 | 9.9 | 3.1 | 5.1 | 1.6 | 0.66 | 0.16 |
| Vegetables | |||||||
| Beet, Red | 9.6 | 6.8 | 0.1 | 0.1 | 6.5 | 1.0 | 96.2 |
| Carrot | 9.6 | 4.7 | 0.6 | 0.6 | 3.6 | 1.0 | 70.0 |
| Corn, Sweet | 19.0 | 6.2 | 1.9 | 3.4 | 0.9 | 0.61 | 15.0 |
| Red Pepper, Sweet | 6.0 | 4.2 | 2.3 | 1.9 | 0.0 | 1.2 | 0.0 |
| Onion, Sweet | 7.6 | 5.0 | 2.0 | 2.3 | 0.7 | 0.9 | 14.3 |
| Sweet Potato | 20.1 | 4.2 | 0.7 | 1.0 | 2.5 | 0.9 | 60.3 |
| Yam | 27.9 | 0.5 | tr | tr | tr | na | tr |
| Sugar Cane | 13 - 18 | 0.2 – 1.0 | 0.2 – 1.0 | 11 - 16 | 1.0 | 100 | |
| Sugar Beet | 17 - 18 | 0.1 – 0.5 | 0.1 – 0.5 | 16 - 17 | 1.0 | 100 |
All data with a unit of g (gram) are based on 100 g of a food item. The fructose / glucose ratio is calculated by dividing the sum of free fructose plus half sucrose by the sum of free glucose plus half sucrose.
Fructose is also found in the synthetically manufactured sweetener, high-fructose corn syrup (HFCS). Hydrolyzed corn starch is used as the raw material for production of HFCS. Through the enzymatic treatment, glucose molecules are converted into fructose.[20] There are three types of HFCS, each with a different proportion of fructose: HFCS-42, HFCS-55, and HFCS-90. The number for each HFCS corresponds to the percentage of synthesized fructose present in the syrup. HFCS-90 has the highest concentration of fructose, and is typically used to manufacture HFCS-55; HFCS-55 is used as sweetener in soft drinks, whereas HFCS-42 is used in many processed foods and baked goods.
Carbohydrate content of commercial sweeteners (percent)
| Sugar | Fructose | Glucose | Sucrose (Fructose-Glucose) | Other Sugars |
|---|---|---|---|---|
| Granulated Sugar | 0 | 0 | 100 | 0 |
| Brown Sugar | 1 | 1 | 97 | 1 |
| HFCS-42 | 42 | 53 | 0 | 5 |
| HFCS-55 | 55 | 41 | 0 | 4 |
| HFCS-90 | 90 | 5 | 0 | 5 |
| Honey | 50 | 44 | 1 | 5 |
| Maple Syrup | 1 | 4 | 95 | 0 |
| Molasses | 23 | 21 | 53 | 3 |
| Corn Syrup | 0 | 35 | 0 | 0 |
Data obtained from Kretchmer, N. & Hollenbeck, CB (1991). Sugars and Sweeteners, Boca Raton, FL: CRC Press, Inc.[20] for HFCS, and USDA for fruits and vegetables and the other refined sugars.[21]
Cane and beet sugars have been used as the major sweetener in food manufacturing for centuries. However, with the development of HFCS, a significant shift occurred in the type of sweetener consumption. As seen in Figure 3, this change happened in the 1970s. Contrary to the popular belief, however, with the increase of HFCS consumption, the total fructose intake has not dramatically changed. Granulated sugar is 99.9% pure sucrose, which means that it has equal ratio of fructose to glucose. The most commonly used forms of HFCS, HFCS-42 and HFCS-55, have a roughly equal ratio of fructose to glucose, with minor differences. HFCS has simply replaced sucrose as a sweetener. Therefore, despite the changes in the sweetener consumption, the ratio of glucose to fructose intake has remained relatively constant.[22]

Fructose digestion and absorption in humans
Fructose exists in foods either as a monosaccharide (free fructose) or as a unit of a disaccharide (sucrose). Free fructose is absorbed directly by the intestine; however, when fructose is consumed in the form of sucrose, digestion occurs entirely in the upper small intestine. As sucrose comes into contact with the membrane of the small intestine, the enzyme sucrase catalyzes the cleavage of sucrose to yield one glucose unit and one fructose unit. Fructose is absorbed in the small intestine, then enters the hepatic portal vein and is directed toward the liver.

The mechanism of fructose absorption in the small intestine is not completely understood. Some evidence suggests active transport, because fructose uptake has been shown to occur against a concentration gradient.[23] However, the majority of research supports the claim that fructose absorption occurs on the mucosal membrane via facilitated transport involving GLUT5 transport proteins. Since the concentration of fructose is higher in the lumen, fructose is able to flow down a concentration gradient into the enterocytes, assisted by transport proteins. Fructose may be transported out of the enterocyte across the basolateral membrane by either GLUT2 or GLUT5, although the GLUT2 transporter has a greater capacity for transporting fructose, and, therefore, the majority of fructose is transported out of the enterocyte through GLUT2.

Capacity and rate of absorption
The absorption capacity for fructose in monosaccharide form ranges from less than 5 g to 50 g and adapts with changes in dietary fructose intake. Studies show the greatest absorption rate occurs when glucose and fructose are administered in equal quantities.[24] When fructose is ingested as part of the disaccharide sucrose, absorption capacity is much higher because fructose exists in a 1:1 ratio with glucose. It appears that the GLUT5 transfer rate may be saturated at low levels, and absorption is increased through joint absorption with glucose.[25] One proposed mechanism for this phenomenon is a glucose-dependent cotransport of fructose. In addition, fructose transfer activity increases with dietary fructose intake. The presence of fructose in the lumen causes increased mRNA transcription of GLUT5, leading to increased transport proteins. High-fructose diets[vague] have been shown to increase abundance of transport proteins within 3 days of intake.[26]
Malabsorption
Several studies have measured the intestinal absorption of fructose using hydrogen breath test.[27][28][29][30] These studies indicate that fructose is not completely absorbed in the small intestine. When fructose is not absorbed in the small intestine, it is transported into the large intestine, where it is fermented by the colonic flora. Hydrogen is produced during the fermentation process and dissolves into the blood of the portal vein. This hydrogen is transported to the lungs, where it is exchanged across the lungs and is measurable by the hydrogen breath test. The colonic flora also produces carbon dioxide, short-chain fatty acids, organic acids, and trace gases in the presence of unabsorbed fructose.[31] The presence of gases and organic acids in the large intestine causes gastrointestinal symptoms such as bloating, diarrhea, flatulence, and gastrointestial pain[27] Exercise immediately after consumption can exacerbate these symptoms by decreasing transit time in the small intestine, resulting in a greater amount of fructose emptied into the large intestine.[32]
Fructose metabolism
All three dietary monosaccharides are transported into the liver by the GLUT2 transporter.[33] Fructose and galactose are phosphorylated in the liver by fructokinase (Km= 0.5 mM) and galactokinase (Km = 0.8 mM). By contrast, glucose tends to pass through the liver (Km of hepatic glucokinase = 10 mM) and can be metabolised anywhere in the body. Uptake of fructose by the liver is not regulated by insulin. However, insulin is capable of increasing the abundance and functional activity of GLUT5 in skeletal muscle cells.[34]
Fructolysis
Main article: fructolysis
The initial catabolism of fructose is sometimes referred to as fructolysis, in analogy with glycolysis, the catabolism of glucose. In fructolysis, the enzyme fructokinase initially produces fructose 1-phosphate, which is split by aldolase B to produce the trioses dihydroxyacetone phosphate and glyceraldehyde [2]. Unlike glycolysis, in fructolysis the triose glyceraldehyde lacks a phosphate group. A third enzyme, triokinase, is therefore required to phosphorylate glyceraldehyde, producing glyceraldehyde-3-phosphate. The resulting trioses are identical to those obtained in glycolysis and can enter the gluconeogenic pathway for glucose or glycogen synthesis, or be further catabolized through the lower glycolytic pathway to pyruvate.
Metabolism of fructose to DHAP and glyceraldehyde
The first step in the metabolism of fructose is the phosphorylation of fructose to fructose 1-phosphate by fructokinase, thus trapping fructose for metabolism in the liver. Fructose 1-phosphate then undergoes hydrolysis by aldolase B to form DHAP and glyceraldehydes; DHAP can either be isomerized to glyceraldehyde 3-phosphate by triosephosphate isomerase or undergo reduction to glycerol 3-phosphate by glycerol 3-phosphate dehydrogenase. The glyceraldehyde produced may also be converted to glyceraldehyde 3-phosphate by glyceraldehyde kinase or converted to glycerol 3-phosphate by glyceraldehyde 3-phosphate dehydrogenase. The metabolism of fructose at this point yields intermediates in the gluconeogenic and fructolytic pathways leading to glycogen synthesis as well as fatty acid and triglyceride synthesis.
Synthesis of glycogen from DHAP and glyceraldehyde 3 phosphate
The resultant glyceraldehyde formed by aldolase B then undergoes phosphorylation to glyceraldehyde 3-phosphate. Increased concentrations of DHAP and glyceraldehyde 3-phosphate in the liver drive the gluconeogenic pathway toward glucose and subsequent glycogen synthesis.[35] It appears that fructose is a better substrate for glycogen synthesis than glucose and that glycogen replenishment takes precedence over triglyceride formation.[citation needed] Once liver glycogen is replenished, the intermediates of fructose metabolism are primarily directed toward triglyceride synthesis.[citation needed]

Synthesis of triglyceride from DHAP and glyceraldehyde 3 phosphate
Carbons from dietary fructose are found in both the free fatty acid and glycerol moieties of plasma triglycerides. High fructose consumption can lead to excess pyruvate production, causing a buildup of Krebs cycle intermediates.[36] Accumulated citrate can be transported from the mitochondria into the cytosol of hepatocytes, converted to acetyl CoA by citrate lyase and directed toward fatty acid synthesis.[36][37] Additionally, DHAP can be converted to glycerol 3-phosphate as previously mentioned, providing the glycerol backbone for the triglyceride molecule.[37] Triglycerides are incorporated into very low density lipoproteins (VLDL), which are released from the liver destined toward peripheral tissues for storage in both fat and muscle cells.

Health effects
Digestive problems
Fructose absorption occurs in the small intestine via the GLUT-5[38] (fructose only) transporter, and the GLUT2 transporter, for which it competes with glucose and galactose. Over consumption of fructose, inhibition of GLUT2 by other phytochemicals, such as flavonoids,[39] or other issues, may result in unabsorbed fructose's being carried into the large intestine, where, like any sugar, it may provide nutrients for the existing gut flora that produce gas. It may also cause water retention in the intestine. These effects may lead to bloating, excessive flatulence, loose stools, and even diarrhea depending on the amounts eaten and other factors. For people with celiac disease, irritable bowel syndrome, or other fructose absorption issues, fructose malabsorption may be a major health concern. {{citation}}: Empty citation (help)
Metabolic syndrome
Excess fructose consumption has been hypothesized to be a cause of insulin resistance, obesity,[40] elevated LDL cholesterol and triglycerides, leading to metabolic syndrome.[41] Fructose consumption has been shown to be correlated with obesity,[42][43] especially central obesity, which is thought to be the most dangerous kind of obesity. A study in mice showed that a high fructose intake increases adiposity.[44]
Although all simple sugars have nearly identical chemical formulae, each has distinct chemical properties. This can be illustrated with pure fructose. A journal article reports that, "...fructose given alone increased the blood glucose almost as much as a similar amount of glucose (78% of the glucose-alone area)".[45][46][47][47][48]
One study concluded that fructose "produced significantly higher fasting plasma triacylglycerol values than did the glucose diet in men" and "...if plasma triacylglycerols are a risk factor for cardiovascular disease, then diets high in fructose may be undesirable".[49] Bantle et al. "noted the same effects in a study of 14 healthy volunteers who sequentially ate a high-fructose diet and one almost devoid of the sugar."[50]
Fructose is a reducing sugar, as are all monosaccharides. The spontaneous chemical reaction of simple sugar molecules binding to proteins, known as glycation, is thought to be a significant cause of damage in diabetics. Fructose appears to be equivalent to glucose in this regard and so does not seem to be a better answer for diabetes for this reason alone, save for the smaller quantities required to achieve equivalent sweetness in some foods.[51] This may be an important contribution to senescence and many age-related chronic diseases.[52]
Compared to sucrose
Studies that have compared high-fructose corn syrup (an ingredient in nearly all soft drinks sold in the US) to sucrose (common table sugar) find that most measured physiological effects are equivalent.[dubious – discuss] For instance, Melanson et al. (2006), studied the effects of HFCS and sucrose-sweetened drinks on blood glucose, insulin, leptin, and ghrelin levels. They found no significant differences in any of these parameters.[53] This is not surprising, since sucrose is a disaccharide that digests to 50% fructose and 50% glucose, whereas the high-fructose corn syrup most commonly used on soft drinks is 55% fructose and 45% glucose. The difference between the two lies in the fact that HFCS contains little sucrose, the fructose and glucose being independent moieties.
Fructose is often recommended for diabetics because it does not trigger the production of insulin by pancreatic β cells, probably because β cells have low levels of GLUT5.[54][55][56] Fructose has a very low glycemic index of 19 ± 2, compared with 100 for glucose and 68 ± 5 for sucrose.[57] Fructose is also seventy-three percent sweeter than sucrose (see relative sweetness) at room temperature, so diabetics can use less of it. Studies show that fructose consumed before a meal may even lessen the glycemic response of the meal.[58] Its sweetness changes at higher temperatures, so its effects in recipes are not equivalent to those of sucrose (i.e., table sugar).[citation needed]
Liver disease
"The medical profession thinks fructose is better for diabetics than sugar," says Meira Field, PhD, a research chemist at United States Department of Agriculture, "but every cell in the body can metabolize glucose. However, all fructose must be metabolized in the liver. The livers of the rats on the high-fructose diet looked like the livers of alcoholics, plugged with fat and cirrhotic."[59] While a few other tissues (e.g., sperm cells[60] and some intestinal cells) do use fructose directly, fructose is almost entirely metabolized in the liver.[59]
"When fructose reaches the liver," says Dr. William J. Whelan, a biochemist at the University of Miami School of Medicine, "the liver goes bananas and stops everything else to metabolize the fructose." Eating fructose instead of glucose results in lower circulating insulin and leptin levels, and higher ghrelin levels after the meal.[61] Since leptin and insulin decrease appetite and ghrelin increases appetite, some researchers suspect that eating large amounts of fructose increases the likelihood of weight gain.[62]
Excessive fructose consumption is also believed to contribute to the development of non-alcoholic fatty liver disease.[63]
Gout
A 2008 British Medical Journal (BMJ) study linked the level of fructose consumption to incidence of gout. (The authors noted, "We found that the risk of incident gout associated with fructose or fructose rich foods was substantial.")[64] Cases of gout have risen in recent years, despite commonly being thought of as a Victorian disease, and it is suspected that the fructose found in soft drinks (e.g., carbonated beverages) and other sweetened drinks is the reason for this.[65][66]
In order for the liver to process fructose, it must be phosphorylated by removal of phosphates from adenosine triphosphate (ATP). The ATP gets converted to adenosine monophosphate (AMP), then to inositol monophosphate (IMP), and finally to uric acid,[67] the agent in gout.
Glycemic index
Fructose has the lowest glycemic index (GI = 19) of all the natural sugars and used to be recommended for use by diabetics due to its small effect on blood glucose levels. In comparison, ordinary table sugar (sucrose, which is half fructose) has a GI of 65 and honey (usually about 40% fructose content) has a GI of 55. Due to the potential that excessive consumption of fructose may be a factor in some diseases, including metabolic syndrome and insulin resistance,[68] products containing high contents of fructose may be discouraged.
See also
- Tagatose
- Inverted sugar
- DMF (potential fructose-based biofuel)
- Fructose intolerance
- Fructose malabsorption
- Fructan (fructose polymer)
- Galactose
- Glucose
- Glycation
- High fructose corn syrup
- Hyperuricemia
- Seliwanoff's test
- Sucrose
- Sugars in wine
- Agave nectar
References
- ^ Fructose - Merriam Webster dictionary
- ^ Levulose comes from the Latin word laevus, levo, "left side", levulose is the old word for the most occurring isomer of fructose. D-fructose rotate plane-polarised light to the left, hence the name.[1].
- ^ Fruton, J.S. Molecules of Life 1972, Wiley-Interscience
- ^ Hyvonen, L., & Koivistoinen, P (1982). "Fructose in Food Systems". In Birch, G.G. & Parker, K.J (ed.). Nutritive Sweeteners. London & New Jersey: Applied Science Publishers. pp. 133–144. ISBN 0-85334-997-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Wolfgang Wach "Fructose" in Ullmann's Encyclopedia of Industrial Chemistry 2004, Wiley-VCH, Weinheim.doi:10.1002/14356007.a12_047.pub2
- ^ "Institute of Organic Chemistry".
- ^ McWilliams, Margaret. Foods: Experimental Perspectives, 4th Edition. ISBN 0130212822.
- ^ Keusch, P. "Yeast and Sugar- the Chemistry must be right".
- ^ Dills, WL (1993). "Protein fructosylation: Fructose and the Maillard reaction". Journal of Clinical Nutrition. 58: 779–787.
- ^ Huber, G. W.; Iborra, S.; Corma, A. Chem. Rev. 2006, 106, 4044 - 4098. doi:10.1021/cr068360d
- ^ a b c d e Hanover, LM (1993). "Manufacturing, composition, and application of fructose". Journal of Clinical Nutrition. 58: 724s–732.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Oregon State University. "Sugar Sweetness". Last accessed May 5, 2008. http://food.oregonstate.edu/sugar/sweet.html Template:Wayback
- ^ Fructose in our diet: http://www.medbio.info/Horn/Time%201-2/carbohydrate_metabolism.htm last visited 2008-12-28
- ^ a b Nabors, LO (2001). "American Sweeteners": 374–375.
{{cite journal}}: Cite journal requires|journal=(help) - ^ McWilliams, Margaret (2001). Foods: Experimental Perspectives, 4th Edition. Upper Saddle River, NJ : Prentice Hall. ISBN 0130212822.
- ^ White, DC (1990). "Predicting gelatinization temperature of starch/sweetener system for cake formulation by differential scanning calorimetry I. Development of a model". Cereal Foods Wold. 35: 728–731.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Recipe Information for home baking at
- ^ Park, KY (1993). "Intakes and food sources of fructose in the United States". American Journal of Clinical Nutrition. 58 (5 Suppl): 737S–747S. PMID 8213605.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Riby, JE (1993). "Fructose absorption". American Journal of Clinical Nutrition. 58 (5 Suppl): 748S–753S. PMID 8213606.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Kretchmer, N (1991). "Sugars and Sweeteners". CRC Press, Inc.
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ a b USDA National Nutrient Database
- ^ Guthrie, FJ (2000). "Food sources of added sweeteners in the diets of Americans". Journals of American Dietetic Association. 100: 43–51. doi:10.1016/S0002-8223(00)00018-3.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Stipanuk, Marsha H (2006). "Biochemical, Physiological, and Molecular Aspects of Human Nutrition, 2nd Edition". W.B. Saunders, Philadelphia, PA.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Fujisawa, T (1991). "Intestinal absorption of fructose in the rat". Gastroenterology. 101: 360–367. PMID 206591.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ushijima, K (1991). "Absorption of fructose by isolated small intestine of rats is via a specific saturable carrier in the absence of glucose and by the disaccharidase-related transport system in the presence of glucose". Journal of Nurtition. 125 (8): 2156–2164. PMID 7643250.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ferraris, R (2001). "Dietary and developmental regulation of intestinal sugar transport". Journal of Biochemistry. 360 (Pt 2): 265–276. doi:10.1042/0264-6021:3600265. PMC 1222226. PMID 11716754.
- ^ a b Beyer, PL (2005). "Fructose intake at current levels in the United States may cause gastrointestinal distress in normal adults". J. Am. Diet. Assoc. 105 (10): 1559–1566. doi:10.1016/j.jada.2005.07.002. PMID 16183355.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ravich, WJ (1983). "Fructose: incomplete intestinal absorption in humans". Gastroenterology. 84 (1): 26–29. PMID 6847852.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Riby, JE (1993). "Fructose absorption". American Journal of Clinical Nutrition. 58 (5 Suppl): 748S–753S. PMID 8213606.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Rumessen, JJ (1986). "Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides". Gut. 27 (10): 1161–1168. doi:10.1136/gut.27.10.1161. PMC 1433856. PMID 3781328.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Skoog, SM (2004). "Dietary fructose and gastrointestinal symptoms: a review". Am. J. Gastroenterol. 99 (10): 2046–50. doi:10.1111/j.1572-0241.2004.40266.x. PMID 15447771.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Fujisawa, T, T (1993). "The effect of exercise on fructose absorption". Am. J. Clin. Nutr. 58 (1): 75–9. PMID 8317393.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Quezada-Calvillo, R (2006). Carbohydrate Digestion and Absorption. Missouri: Saunders, Elsevier. pp. 182–185. ISBN 141600209X.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hajduch, E (2003). "Insulin regulates the expression of the GLUT5 transporter in L6 skeletal muscle cells". FEBS letters. 549 (1–3): 77–82. PMID 12914929.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Parniak, MA; Kalant, N (1988). "Enhancement of glycogen concentrations in primary cultures of rat hepatocytes exposed to glucose and fructose". Biochemical Journal. 251 (3): 795–802. PMC 1149073. PMID 3415647.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b McGrane, MM (2006). Carbohydrate metabolism: Synthesis and oxidation. Missouri: Saunders, Elsevier. pp. 258–277. ISBN 141600209X.
- ^ a b Sul, HS (2006). Metabolism of Fatty Acids, Acylglycerols, and Sphingolipids. Missouri: Saunders, Elsevier. pp. 450–467. ISBN 141600209X.
- ^ Buchs, AE (1998). "Characterization of GLUT5 domains responsible for fructose transport". Endocrinology. 139 (3): 827–31. doi:10.1210/en.139.3.827. PMID 9492009.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kwon, Oran (22). "Inhibition of the intestinal glucose transporter GLUT2 by flavonoids" (PDF). FASEB Journal. 21 (2): 366–377. doi:10.1096/fj.06-6620com. PMID 17172639.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ (2002). "Fructose, weight gain, and the insulin resistance syndrome". Am. J. Clin. Nutr. 76 (5): 911–22. PMID 12399260.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Basciano H, Federico L, Adeli K (2005). "Fructose, insulin resistance, and metabolic dyslipidemia". Nutrition & Metabolism. 2 (5): 5. doi:10.1186/1743-7075-2-5. PMC 552336. PMID 15723702.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Lustig RH (2006). "Childhood obesity: behavioral aberration or biochemical drive? Reinterpreting the First Law of Thermodynamics". Nature clinical practice. Endocrinology & metabolism. 2 (8): 447–58. doi:10.1038/ncpendmet0220. PMID 16932334.
- ^ Isganaitis E; Lustig RH (2005). "Fast food, central nervous system insulin resistance, and obesity". Arterioscler. Thromb. Vasc. Biol. 25 (12): 2451–62. doi:10.1161/01.ATV.0000186208.06964.91. PMID 16166564.
- ^ Jürgens H, Haass W, Castañeda TR; et al. (2005). "Consuming fructose-sweetened beverages increases body adiposity in mice". Obes. Res. 13 (7): 1146–56. doi:10.1038/oby.2005.136. PMID 16076983.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Hughes TA, Atchison J, Hazelrig JB, Boshell BR (1989). "Glycemic responses in insulin-dependent diabetic patients: effect of food composition". Am. J. Clin. Nutr. 49 (4): 658–66. PMID 2929488.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wylie-Rosett, Judith (2004). "Carbohydrates and Increases in Obesity: Does the Type of Carbohydrate Make a Difference?". Obesity Res. 12: 124S–129S. doi:10.1038/oby.2004.277. PMID 15601960.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Havel PJ (2001). "Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis". Exp. Biol. Med. (Maywood). 226 (11): 963–77. PMID 11743131.
- ^ Dennison BA, Rockwell HL, Baker SL (1997). "Excess fruit juice consumption by preschool-aged children is associated with short stature and obesity". Pediatrics. 99 (1): 15–22. PMID 8989331.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bantle JP, Raatz SK, Thomas W, Georgopoulos A (2000). "Effects of dietary fructose on plasma lipids in healthy subjects". Am. J. Clin. Nutr. 72 (5): 1128–34. PMID 11063439.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Enerex.ca | Whey Protein and Fructose, an Unhealthy Combination
- ^ McPherson, JD (1988). "Role of fructose in glycation and cross-linking of proteins. PMID 3132203". Biochemistry. 27 (5): 1901–7. doi:10.1021/bi00406a016. PMID 3132203.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Levi, B (1998). Fulltext "Long-term fructose consumption accelerates glycation and several age-related variables in male rats. PMID 9732303". J Nutr. 128 (9): 1442–9. PMID 9732303.
{{cite journal}}: Check|url=value (help); Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Melanson, K. (2006). "Eating Rate and Satiation". Obesity Society (NAASO) 2006 Annual Meeting, October 20–24,Hynes Convention Center, Boston, Massachusetts.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ A. M. Grant, M. R. Christie, S. J. Ashcroft (1980). "Insulin Release from Human Pancreatic Islets in Vitro". Diabetologia. 19 (2): 114–117. doi:10.1007/BF00421856. PMID 6998814.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ D. L. Curry (1989). "Effects of Mannose and Fructose on the Synthesis and Secretion of Insulin". Pancreas. 4 (1): 2–9. doi:10.1097/00006676-198902000-00002. PMID 2654926.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - ^ Y. Sato, T. Ito, N. Udaka; et al. (1996). "Immunohistochemical Localization of Facilitated-Diffusion Glucose Transporters in Rat Pancreatic Islets". Tissue Cell. 28 (6): 637–643. doi:10.1016/S0040-8166(96)80067-X. PMID 9004533.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kaye Foster-Powell, Susanna H. A. Holt, and Janette C. Brand-Miller. July 2002. International Table of Glycemic Index and Glycemic Load Values: 2002. American Journal of Clinical Nutrition 76(1):5-56
- ^ Patricia M. Heacock, Steven R. Hertzler and Bryan W. Wolf. September 2002. Fructose Prefeeding Reduces the Glycemic Response to a High-Glycemic Index, Starchy Food in Humans. The Journal of Nutrition 132(9):2601-2604
- ^ a b Forristal, Linda (2001). "The Murky World of High-Fructose Corn Syrup". Weston A. Price Foundation.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Mann, T (1954). "The Biochemistry of Semen" (Document). London: Methuen & Co; New York: John Wiley & Sons.
{{cite document}}: Unknown parameter|url=ignored (help) - ^ Teff, KL (2004). "Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women". J Clin Endocrinol Metab. 89 (6): 2963–72. doi:10.1210/jc.2003-031855. PMID 15181085.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Swan, Norman; Lustig, Robert H. "ABC Radio National, The Health Report, The Obesity Epidemic". Retrieved 2007-07-15.
- ^ Ouyang X, Cirillo P, Sautin Y; et al. (2008). "Fructose Consumption as a Risk Factor for Non-alcoholic Fatty Liver Disease". J. Hepatol. 48 (6): 993–9. doi:10.1016/j.jhep.2008.02.011. PMC 2423467. PMID 18395287.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Choi, Hyon K. and Curhan, Gary. "Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study". BMJ. Retrieved 5 December 2011.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ "Gout surge blamed on sweet drinks". BBC News. 2008-02-01.
- ^ Johnson, Richard Joseph (2008). The Sugar Fix: The High-Fructose Fallout That is Making You Fat and Sick. US: Rodale. p. 304. ISBN 10 1-59486-665-1.
{{cite book}}: Check|isbn=value: length (help); External link in|publisher=|coauthors=ignored (|author=suggested) (help) - ^ Sugar, the Bitter Truth. University of California. 2009-07-02.
- ^ Lim, Jung (2010). "the role of fructose in the pathogenesis of naFlD and the metabolic syndrome". Nature reviews | gastroenterology & hepatology. 7 (5): 251–264. doi:10.1038/nrgastro.2010.41.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)
External links
- [3] Hereditary Fructose Intolerance
- [4]
- How Fructose Impairs the Memory
- Cancer cells metabolize fructose to increase proliferation


