Management of HIV/AIDS
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs in an attempt to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple drugs that act on different viral targets is known as highly active antiretroviral therapy (HAART). HAART decreases the patient's total burden of HIV, maintains function of the immune system, and prevents opportunistic infections that often lead to death.[1]
Treatment has been so successful that in many parts of the world, HIV has become a chronic condition in which progression to AIDS is increasingly rare. Anthony Fauci, head of the United States National Institute of Allergy and Infectious Diseases, has written, "With collective and resolute action now and a steadfast commitment for years to come, an AIDS-free generation is indeed within reach." In the same paper, he noted that an estimated 700,000 lives were saved in 2010 alone by antiretroviral therapy.[2] As another commentary in The Lancet noted, "Rather than dealing with acute and potentially life-threatening complications, clinicians are now confronted with managing a chronic disease that in the absence of a cure will persist for many decades."[3]
The United States Department of Health and Human Services and the World Health Organization[4] recommend offering antiretroviral treatment to all patients with HIV.[5] Because of the complexity of selecting and following a regimen, the potential for side effects, and the importance of taking medications regularly to prevent viral resistance, such organizations emphasize the importance of involving patients in therapy choices and recommend analyzing the risks and the potential benefits.[5]
History
The first effective therapy against HIV was the nucleoside reverse transcriptase inhibitor (NRTI) zidovudine (AZT). It was approved by the US FDA in 1987.[6] Subsequently several more NRTIs were developed but even in combination were unable to suppress the virus for long periods of time and patients still inevitably died.[7] To distinguish from this early anti-retroviral therapy (ART), the term highly active anti-retroviral therapy (HAART) was introduced. In 1996 by sequential publications in The New England Journal of Medicine by Hammer and colleagues[8] and Gulick and coinvestigators[9] illustrating the substantial benefit of combining 2 NRTIs with a new class of anti-retrovirals, protease inhibitors, namely indinavir. This concept of 3-drug therapy was quickly incorporated into clinical practice and rapidly showed impressive benefit with a 60% to 80% decline in rates of AIDS, death, and hospitalization.[1]
Classes of drugs
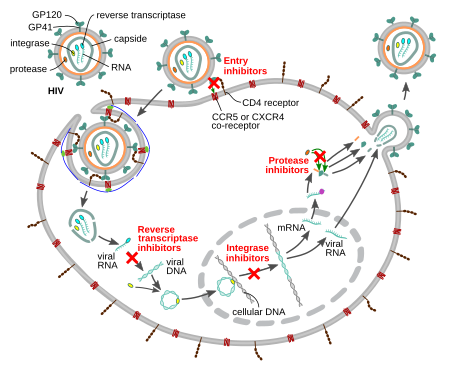
There are five classes of drugs, which are usually used in combination, to treat HIV infection. Use of these drugs in combination can be termed anti-retroviral therapy (ART), combination anti-retroviral therapy (cART) or highly active anti-retroviral therapy (HAART). Anti-retroviral (ARV) drugs are broadly classified by the phase of the retrovirus life-cycle that the drug inhibits. Typical combinations include 2 NRTIs as a "backbone" along with 1 NNRTI, PI or INSTI as a "base."[5]
- Entry inhibitors (or fusion inhibitors) interfere with binding, fusion and entry of HIV-1 to the host cell by blocking one of several targets. Maraviroc and enfuvirtide are the two currently available agents in this class. Maraviroc works by targeting CCR5, a co-receptor located on human helper T-cells. Caution should be used when administering this drug however due to a possible shift in tropism which allows HIV to target an alternative co-receptor such as CXCR4. In rare cases, individuals may have a mutation in the CCR5 delta gene which results in a nonfunctional CCR5 co-receptor and in turn, a means of resistance or slow progression of the disease. However, as mentioned previously, this can be overcome if an HIV variant that targets CXCR4 becomes dominant.[10] To prevent fusion of the virus with the host membrane, enfuvirtide can be used. Enfuvirtide is a peptide drug that must be injected and acts by interacting with the N-terminal heptad repeat of gp41 of HIV to form an inactive hetero six-helix bundle, therefore preventing infection of host cells.[11]
- Nucleoside reverse transcriptase inhibitors (NRTI) and nucleotide reverse transcriptase inhibitors (NtRTI) are nucleoside and nucleotide analogues which inhibit reverse transcription. HIV is an RNA virus and hence unable to become integrated into the DNA in the nucleus of the human cell; it must be "reverse" transcribed into DNA. Since the conversion of RNA to DNA is not done in the mammalian cell it is performed by a viral protein which makes it a selective target for inhibition. NRTIs are chain terminators such that once incorporated, work by preventing other nucleosides from also being incorporated into the DNA chain because of the absence of a 3' OH group. Both act as competitive substrate inhibitors. Examples of currently used NRTIs include zidovudine, abacavir, lamivudine, emtricitabine, and tenofovir.[12]
- Non-Nucleoside reverse transcriptase inhibitors (NNRTI) inhibit reverse transcriptase by binding to an allosteric site of the enzyme; NNRTIs act as non-competitive inhibitors of reverse transcriptase. NNRTIs affect the handling of substrate (nucleotides) by reverse transcriptase by binding near the active site. NNRTIs can be further classified into 1st generation and 2nd generation NNRTIs. 1st generation NNRTIs include nevirapine and efavirenz. 2nd generation NNRTIs are etravirine and rilpivirine.[12] HIV-2 is naturally resistant to NNRTIs.[13]
- Integrase inhibitors (also known as integrase nuclear strand transfer inhibitors or INSTIs) inhibit the viral enzyme integrase, which is responsible for integration of viral DNA into the DNA of the infected cell. There are several integrase inhibitors currently under clinical trial, and raltegravir became the first to receive FDA approval in October 2007. Raltegravir has two metal binding groups that compete for substrate with two Mg2+ ions at the metal binding site of integrase. As of early 2014, two other clinically approved integrase inhibitors are elvitegravir and dolutegravir.[14]
- Protease inhibitors block the viral protease enzyme necessary to produce mature virions upon budding from the host membrane. Particularly, these drugs prevent the cleavage of gag and gag/pol precursor proteins.[15] Virus particles produced in the presence of protease inhibitors are defective and mostly non-infectious. Examples of HIV protease inhibitors are lopinavir, indinavir, nelfinavir, amprenavir and ritonavir. Darunavir and atazanavir are currently recommended as first line therapy choices.[5] Maturation inhibitors have a similar effect by binding to gag, but development of two experimental drugs in this class, bevirimat and vivecon, was halted in 2010.[16] Resistance to some protease inhibitors is high. Second generation drugs have been developed that are effective against otherwise resistant HIV variants.[15]
Combination therapy
The life cycle of HIV can be as short as about 1.5 days from viral entry into a cell, through replication, assembly, and release of additional viruses, to infection of other cells.[17] HIV lacks proofreading enzymes to correct errors made when it converts its RNA into DNA via reverse transcription. Its short life-cycle and high error rate cause the virus to mutate very rapidly, resulting in a high genetic variability of HIV. Most of the mutations either are inferior to the parent virus (often lacking the ability to reproduce at all) or convey no advantage, but some of them have a natural selection superiority to their parent and can enable them to slip past defenses such as the human immune system and antiretroviral drugs. The more active copies of the virus, the greater the possibility that one resistant to antiretroviral drugs will be made.[18]
When antiretroviral drugs are used improperly, multi-drug resistant strains can become the dominant genotypes very rapidly. In the era before multiple drug classes were available (pre-1997), the reverse transcriptase inhibitors zidovudine, didanosine, zalcitabine, stavudine, and lamivudine were used serially or in combination leading to the development of multi-drug resistant mutations.[19]
Antiretroviral combination therapy defends against resistance by suppressing HIV replication as much as possible, thus reducing the potential pool of spontaneous resistance mutations.[18]
Combinations of antiretrovirals create multiple obstacles to HIV replication to keep the number of offspring low and reduce the possibility of a superior mutation. If a mutation that conveys resistance to one of the drugs being taken arises, the other drugs continue to suppress reproduction of that mutation. With rare exceptions, no individual antiretroviral drug has been demonstrated to suppress an HIV infection for long; these agents must be taken in combinations in order to have a lasting effect. As a result, the standard of care is to use combinations of antiretroviral drugs.[5] Combinations usually consist of three drugs from at least two different classes.[5] This three drug combination is commonly known as a triple cocktail.[20] Combinations of antiretrovirals are subject to positive and negative synergies, which limits the number of useful combinations.
In recent years, drug companies have worked together to combine these complex regimens into simpler formulas, termed fixed-dose combinations.[21] For instance, there are now several options that combine 3 drugs into one pill taken once daily.[22][23][24] This greatly increases the ease with which they can be taken, which in turn increases the consistency with which medication is taken (adherence),[25] and thus their effectiveness over the long-term. Not taking anti-retrovirals regularly is a cause of resistance development in people who have started taking them previously.[26] Patients who take medications regularly can stay on one regimen without developing resistance.[26] This greatly increases life expectancy and leaves more drugs available to the individual should the need arise.
Fixed-dose combinations
Fixed dose combinations are multiple antiretroviral drugs combined into a single pill.
Treatment guidelines
Initiation of antiretroviral therapy
Antiretroviral drug treatment guidelines have changed over time. Before 1987, no antiretroviral drugs were available and treatment consisted of treating complications from opportunistic infections and malignancies. After antiretroviral medications were introduced, most clinicians agreed that HIV positive patients with low CD4 counts should be treated, but no consensus formed as to whether to treat patients with high CD4 counts.[28]
In April 1995, Merck and the National Institute of Allergy and Infectious Diseases began recruiting patients for a trial examining the effects of a three drug combination of the protease inhibitor indinavir and two nucleoside analogs.[9] illustrating the substantial benefit of combining 2 NRTIs with a new class of anti-retrovirals, protease inhibitors, namely indinavir. Later that year David Ho became an advocate of this "hit hard, hit early" approach with aggressive treatment with multiple antiretrovirals early in the course of the infection.[29] Later reviews in the late 90s and early 2000s noted that this approach of "hit hard, hit early" ran significant risks of increasing side effects and development of multidrug resistance, and this approach was largely abandoned. The only consensus was on treating patients with advanced immunosuppression (CD4 counts less than 350/μL).[30] Treatment with antiretrovirals was expensive at the time, ranging from $10,000 to $15,000 a year.[31]
The timing of when to start therapy has continued to be a core controversy within the medical community, though recent studies have led to more clarity. The NA-ACCORD[32] study observed patients who started antiretroviral therapy either at a CD4 count of less than 500 versus less than 350 and showed that patients who started ART at lower CD4 counts had a 69% increase in the risk of death.[33] In 2015 the START[34] and TEMPRANO[35] studies both showed that patients lived longer if they started antiretrovirals at the time of their diagnosis, rather than waiting for their CD4 counts to drop to a specified level.
Other arguments for starting therapy earlier are that people who start therapy later have been shown to have less recovery of their immune systems,[36] and higher CD4 counts are associated with less cancer.[37]
Treatment as prevention
A separate argument for starting antiretroviral therapy that has gained more prominence is its effect on HIV transmission. ART reduces the amount of virus in the blood and genital secretions.[38][39] This has been shown to lead to dramatically reduced transmission of HIV when one partner with a suppressed viral load (<50 copies/ml) has sex with a partner who is HIV negative. In clinical trial HPTN 052, 1763 serodiscordant heterosexual couples in 9 countries were planned to be followed for at least 10 years, with both groups receiving education on preventing HIV transmission and condoms, but only one group getting ART. The study was stopped early for ethical reasons when it became clear that antiviral treatment provided significant protection. Of the 28 couples where cross-infection had occurred, all but one had taken place in the control group consistent with a 96% reduction in risk of transmission while on ART.[40] The term "treatment as prevention" has been used for the concept of treating patients with HIV to help prevent the spread of HIV.[41] In 2011, the journal Science gave the Breakthrough of the Year award to treatment as prevention.[42]
In summary, as the WHO HIV treatment guidelines state, "The ARV regimens now available, even in the poorest countries, are safer, simpler, more efficacious and more affordable than ever before."[43]
There is a consensus among experts that, once initiated, antiretroviral therapy should never be stopped. This is because the selection pressure of incomplete suppression of viral replication in the presence of drug therapy causes the more drug sensitive strains to be selectively inhibited. This allows the drug resistant strains to become dominant. This in turn makes it harder to treat the infected individual as well as anyone else they infect.[5] One trial where ART therapy was periodically stopped had higher rates of opportunistic infections, cancers, heart attacks and death in patients who interrupted their ART.[44][45]
Guideline Sources
There are several treatment guidelines for HIV-1 infected adults in the developed world (that is, those countries with access to all or most therapies and laboratory tests). In the United States there are both the International AIDS Society-USA (IAS-USA) (a 501(c)(3) not-for-profit organization in the USA)[46] as well as the US government's Department of Health and Human Services guidelines.[5] In Europe there are the European AIDS Clinical Society guidelines.[47]
For resource limited countries, most national guidelines closely follow the World Health Organization guidelines.[4]
Current guidelines
The current guidelines use new criteria to consider starting HAART, as described below. However, there remain a range of views on this subject and the decision of whether to commence treatment ultimately rests with the patient and his or her doctor.
Current US DHHS guidelines (published April 8, 2015) state:
- Antiretroviral therapy (ART) is recommended for all HIV-infected individuals to reduce the risk of disease progression.
- ART also is recommended for HIV-infected individuals for the prevention of transmission of HIV.
- Patients starting ART should be willing and able to commit to treatment and understand the benefits and risks of therapy and the importance of adherence. Patients may choose to postpone therapy, and providers, on a case-by-case basis, may elect to defer therapy on the basis of clinical and/or psychosocial factors.
The newest World Health Organization guidelines (dated September 30, 2015) now agree and state:[4]
- Antiretroviral therapy (ART) should be initiated in everyone living with HIV at any CD4 cell count
Baseline resistance
Baseline resistance is the presence of resistance mutations in patients who have never been treated before for HIV. In countries with a high rate of baseline resistance, resistance testing is recommended before starting treatment; or, if the initiation of treatment is urgent, then a "best guess" treatment regimen should be started, which is then modified on the basis of resistance testing.[13] In the UK, there is 11.8% medium to high-level resistance at baseline to the combination of efavirenz + zidovudine + lamivudine, and 6.4% medium to high level resistance to stavudine + lamivudine + nevirapine.[48] In the US, 10.8% of one cohort of patients who had never been on ART before had at least one resistance mutation in 2005.[49] Various surveys in different parts of the world have shown increasing or stable rates of baseline resistance as the era of effective HIV therapy continues.[50][51][52][53] With baseline resistance testing, a combination of antiretrovirals that are likely to be effective can be customized for each patient.
Regimens
Most current HAART regimens consist of three drugs: 2 NRTIs ("backbone")+ a PI/NNRTI/INSTI ("base"). Initial regimens use "first-line" drugs with a high efficacy and low side-effect profile.
The US DHHS preferred initial regimens for adults and adolescents in the United States, as of April 2015, are:[5]
- tenofovir/emtricitabine and raltegravir (an integrase inhibitor)
- tenofovir/emtricitabine and dolutegravir (an integrase inhibitor)
- abacavir/lamivudine (two NRTIs) and dolutegravir for patients who have been tested negative for the HLA-B*5701 gene allele
- tenofovir/emtricitabine, elvitegravir (an integrase inhibitor) and cobicistat (inhibiting metabolism of the former) in patients with good kidney function (gfr > 70)
- tenofovir/emtricitabine, ritonavir, and darunavir (both latter are protease inhibitors)
In the case of the protease inhibitor based regimens, ritonavir is used at low doses to inhibit cytochrome p450 enzymes and "boost" the levels of other protease inhibitors, rather than for its direct antiviral effect. This boosting effect allows them to be taken less frequently throughout the day.[54] Cobicistat is used with elvitegravir for a similar effect but does not have any direct antiviral effect itself.[55]
The WHO preferred initial regimen for adults and adolescents as of June 30, 2013 is:[43]
- tenofovir + lamivudine (or emtricitabine) + efavirenz
Special populations
Acute infection
In the first 6 months after infection HIV viral loads tend to be elevated and people are more often symptomatic than in later latent phases of HIV disease. There may be special benefits to starting antiretroviral therapy early during this acute phase, including lowering the viral "set-point" or baseline viral load, reduce the mutation rate of the virus, and reduce the size of the viral reservoir (See section below on viral reservoirs).[5] The SPARTAC trial compared 48 weeks of ART vs 12 weeks vs no treatment in acute HIV infection and found that 48 weeks of treatment delayed the time to decline in CD4 count below 350 cells per ml by 65 weeks and kept viral loads significantly lower even after treatment was stopped.[56] Since viral loads are usually very high during acute infection, this period carries an estimated 26 times higher risk of transmission.[57] By treating acutely infected patients, it is presumed that it could have a significant impact on decreasing overall HIV transmission rates since lower viral loads are associated with lower risk of transmission (See section on treatment as prevention). However an overall benefit has not been proven and has to be balanced with the risks of HIV treatment. Therapy during acute infection carries a grade BII recommendation from the US DHHS.[5]
Children
HIV can be especially harmful to infants and children, with one study in Africa showing that 52% of untreated children born with HIV had died by age 2.[58] By five years old, the risk of disease and death from HIV starts to approach that of young adults. The WHO recommends treating all children less than 5 years old, and starting all children older than 5 with stage 3 or 4 disease or CD4 <500 cells/ml.[43] DHHS guidelines are more complicated but recommend starting all children less than 12 months old and children of any age who have symptoms.[59]
As for which antiretrovirals to use, this is complicated by the fact that many children who are born to mothers with HIV are given a single dose of nevirapine (an NNRTI) at the time of birth to prevent transmission. If this fails it can lead to NNRTI resistance.[60] Also, a large study in Africa and India found that a PI based regimen was superior to an NNRTI based regimen in children less than 3 years who had never been exposed to NNRTIs in the past.[61] Thus the WHO recommends PI based regimens for children less than 3.
The WHO recommends for children less than 3 years:[43]
- abacavir (or zidovudine) + lamivudine + lopinivir + ritonivir
and for children 3 years to less than 10 years and adolescents <35 kilograms:
- abacavir + lamivudine + efavirenz
US DHHS guidelines are similar but include PI based options for children > 3 years old.[59]
A systematic review assessed the effects and safety of abacavir-containing regimens as first-line therapy for children between 1 month and 18 years of age when compared to regimens with other NRTIs.[62] This review included two trials and two observational studies with almost eleven thousand HIV infected children and adolescents. They measured virologic suppression, death and adverse events. The authors found that there is no meaningful difference between abacavir-containing regimens and other NRTI-containing regimens. The evidence if of low to moderate quality and therefore it is likely that future research may change these findings.
Pregnant women
The goals of treatment for pregnant women include the same benefits to the mother as in other infected adults as well as prevention of transmission to her child. The risk of transmission from mother to child is proportional to the plasma viral load of the mother. Untreated mothers with a viral load >100,000 copies/ml have a transmission risk of over 50%.[63] The risk when viral loads are < 1000 copies/ml are less than 1%.[64] ART for mothers both before and during delivery and to mothers and infants after delivery are recommended to substantially reduce the risk of transmission.[65] The mode of delivery is also important, with a planned Caesarian section having a lower risk than vaginal delivery or emergency Caesarian section.[66] HIV can also be detected in breast milk of infected mothers and transmitted through breast feeding.[67] The WHO balances the low risk of transmission through breast feeding from women who are on ART with the benefits of breastfeeding against diarrhea, prneumonia and malnutrition. It also strongly recommends that breastfeeding infants receive prophylactic ART.[43] In the US, the DHHS recommends against women with HIV breastfeeding.[65]
Older adults
With improvements in HIV therapy, several studies now estimate that patients on treatment in high-income countries can expect a normal life expectancy.[68][69] This means that a higher proportion of people living with HIV are now older and research is ongoing into the unique aspects of HIV infection in the older adult. There is data that older people with HIV have a blunted CD4 response to therapy but are more likely to achieve undetectable viral levels.[70] However, not all studies have seen a difference in response to therapy.[71] Current guidelines do not have separate treatment recommendations for older adults, but it is important to take into account that older patients are more likely to be on multiple non-HIV medications and consider drug interactions with any potential HIV medications.[72] There are also increased rates of HIV associated non-AIDS conditions (HANA) such as heart disease, liver disease and dementia that are multifactorial complications from HIV, associated behaviors, coinfections like hepatitis B, hepatitis C, and human papilloma virus (HPV) as well as HIV treatment.[72]
Concerns
There are several concerns about antiretroviral regimens that should be addressed before initiating:
- Intolerance: The drugs can have serious side-effects which can lead to harm as well as keep patients from taking their medications regularly.
- Resistance: Not taking medication consistently can lead to low blood levels that foster drug resistance.[73]
- Cost: The WHO maintains a database of world ART costs[74] which have dropped dramatically in recent years as more first line drugs have gone off-patent.[75] A one pill, once a day combination therapy has been introduced in South Africa for as little as $10 per patient per month.[76] One recent study estimated an overall cost savings to ART therapy in South Africa given reduced transmission.[77] In the United States, new on-patent regimens can cost up to $28,500 per patient, per year.[78][79]
- Public health: Individuals who fail to use antiretrovirals as directed can develop multi-drug resistant strains which can be passed onto others.[80]
Response to therapy
Virologic response
Suppressing the viral load to undetectable levels (<50 copies per ml) is the primary goal of ART.[54] This should happen by 24 weeks after starting combination therapy.[81] Viral load monitoring is the most important predictor of response to treatment with ART.[82] Levels higher than 200 copies per ml is considered virologic failure, and should prompt further testing for potential viral resistance.[5] Lack of viral load suppression on ART is termed virologic failure.
Immunologic response
CD4 cell counts are another key measure of immune status and ART effectiveness.[81] CD4 counts should rise 50 to 100 cells per ml in the first year of therapy.[54] There can be substantial fluctuation in CD4 counts of up to 25% based on the time of day or concominant infections.[83] In one long term study, the majority of increase in CD4 cell counts was in the first two years after starting ART with little increase afterwards. This study also found that patients who began ART at lower CD4 counts continued to have lower CD4 counts than those who started at higher CD4 counts.[84] When viral suppression on ART is achieved but without a corresponding increase in CD4 counts it can be termed immunologic nonresponse or immunologic failure. While this is predictive of worse outcomes, there is no consensus on how to adjust therapy to immunologic failure and whether switching therapy is beneficial. DHHS guidelines do not recommend switching an otherwise suppressive regimen.[5][85]
Salvage therapy
In patients who have persistently detectable viral loads while taking ART, tests can be done to investigate whether there is drug resistance. Most commonly a genotype is sequenced which can be compared with databases of other HIV viral genotypes and resistance profiles to predict response to therapy.[86] If there is extensive resistance a phenotypic test of a patient's virus against a range of drug concentrations can be performed, but is expensive and can take several weeks, so genotypes are generally preferred.[5] Using information from a genotype or phenotype, a regimen of 3 drugs from at least 2 classes is constructed that will have the highest probability of suppressing the virus. If a regimen cannot be constructed from recommended first line agents it is termed salvage therapy, and when 6 or more drugs are needed it is termed mega-HAART.[87]
Structured treatment interruptions
Drug holidays (or "structured treatment interruptions") are intentional discontinuations of antiretroviral drug treatment. As mentioned above, randomized controlled studies of structured treatment interruptions have shown higher rates of opportunistic infections, cancers, heart attacks and death in patients who took drug holidays.[44][45] With the exception of post exposure prophylaxis, current treatment guidelines do not call for the interruption of drug therapy once it has been initiated.[5][43][81]
Adverse effects
Each class and individual antiretroviral carries unique risks of adverse side effects.
NRTIs
The NRTIs can interfere with mitochondrial DNA synthesis and lead to high levels of lactate and lactic acidosis, liver steatosis, peripheral neuropathy, myopathy and lipoatrophy.[54] Current first line NRTIs such as lamivudine/emtrictabine, tenofovir, and abacavir are less likely to cause mitochondrial dysfunction.[88][89]
NNRTIs
NNRTIs are generally safe and well tolerated. The main reason for discontinuation of efavirenz is neuro-psychiatric effects including suicidal ideation. Nevirapine can cause severe hepatotoxicity, especially in women with high CD4 counts.[90]
Protease inhibitors
Protease inhibitors (PIs) are often given with ritonavir, a strong inhibitor of cytochrome P450 enzymes, leading to numerous drug-drug interactions. They are also associated with lipodystrophy, elevated triglycerides and elevated risk of heart attack.[91]
Integrase inhibitors
Integrase inhibitors (INSTIs) are among the best tolerated of the antiretrovirals with excellent short and medium term outcomes. Given their relatively new development there is less long term safety data. They are associated with an increase in creatinine kinase levels and rarely myopathy.[92]
HIV postexposure prophylaxis (PEP)
When people are exposed to HIV-positive infectious bodily fluids either through skin puncture, contact with mucous membranes or contact with damaged skin, they are at risk for acquiring HIV. Pooled estimates give a risk of transmission with puncture exposures of 0.3%[93] and mucous membrane exposures 0.63%.[94] United States guidelines state that "feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are not considered potentially infectious unless they are visibly bloody."[95] Given the rare nature of these events, rigorous study of the protective abilities of antiretrovirals are limited but do suggest that taking antiretrovirals afterwards can prevent transmission.[96] It is unknown if three medications are better than two. The sooner after exposure that ART is started the better, but after what period they become ineffective is unknown, with the US Public Health Service Guidelines recommending starting prophylaxis up to a week after exposure.[95] They also recommend treating for a duration of four weeks based on animal studies. Their recommended regimen is emtricitabine + tenofovir + raltegravir (an INSTI). The rationale for this regimen is that it is "tolerable, potent, and conveniently administered, and it has been associated with minimal drug interactions."[95] People who are exposed to HIV should have follow up HIV testing at six, 12, and 24 weeks.
Pregnancy planning
Women with HIV have been shown to have decreased fertility which can affect available reproductive options.[97] In cases where the woman is HIV negative and the man is HIV positive, the primary assisted reproductive method used to prevent HIV transmission is sperm washing followed by intrauterine insemination (IUI) or in vitro fertilization (IVF). Preferably this is done after the man has achieved an undetectable plasma viral load.[98] In the past there have been cases of HIV transmission to an HIV-negative partner through processed artificial insemination,[99] but a large modern series in which followed 741 couples where the man had a stable viral load and semen samples were tested for HIV-1, there were no cases of HIV transmission.[100]
For cases where the woman is HIV positive and the man is HIV negative, the usual method is artificial insemination.[98] With appropriate treatment the risk of mother-to-child infection can be reduced to below 1%.[101]
Towards a cure
People living with HIV can currently expect to live a normal life span if able to achieve durable viral suppression on combination antiretroviral therapy. However this requires lifelong medication and will still suffer from higher rates of cardiovascular, renal, liver and neurologic disease.[102] This has prompted further research towards a cure for HIV.
"Berlin patient"
So far only one adult (the so-called "Berlin patient") has been potentially cured and has been off of treatment since 2006 with no detectable virus.[103] This was achieved through two bone marrow transplants that replaced his immune system with a donor's that did not have the CCR5 cell surface receptor, which is needed for some variants of HIV to enter a cell.[104] Bone marrow transplants carry their own significant risks including potential death and was only attempted because it was necessary to treat a blood cancer he had. Attempts to replicate this have not been successful and given the risks, expense and rarity of CCR5 negative donors, bone marrow transplant is not seen as a mainstream option.[102] It has inspired research into other methods to try to block CCR5 expression through gene therapy. A zinc-finger nuclease has been used in a Phase I trial of 12 humans and led to an increase in CD4 count and decrease in their viral load while off antiretroviral treatment.[105]
Viral reservoirs
The main obstacle to conventional antiretroviral therapy eliminating HIV infection is that HIV is able to integrate itself into the DNA of host cells and rest in a latent state, while antiretrovirals only attack actively replicating HIV. The cells in which HIV lays dormant are called the viral reservoir, and one of the main sources is thought to be central memory and transitional memory CD4+ T cells.[106] Recent reports of the cure of HIV in two infants[107] are presumably due to the fact that treatment was initiated within hours of infection, preventing HIV from establishing a deep reservoir.[108] Currently there is work being done to try to activate reservoir cells into replication so that the virus is forced out of latency and can be attacked by antiretrovirals and the host immune system. Targets include histone deacetylase (HDAC) which represses transcription and if inhibited can lead to increased cell activation. The HDAC inhibitors valproic acid and vorinostat have been used in human trials with only preliminary results so far.[109][110]
Immune activation
Even with all latent virus deactivated, it is thought that a vigorous immune response will need to be induced to clear all the remaining infected cells.[102] Current strategies include using cytokines to restore CD4+ cell counts as well as therapeutic vaccines to prime immune responses.[111] One such candidate vaccine is Tat Oyi, developed by Biosantech.[112] This vaccine is based on the HIV protein tat. A brief report of their phase I/II clinical trial reported it was safe and well tolerated in 48 HIV-positive patients.[113] Animal models have shown the generation of neutralizing antibodies and lower levels of HIV viremia.[114]
Drug advertisements
Direct-to-consumer and other advertisements for HIV drugs in the past were criticized for their use of healthy, glamorous models rather than typical HIV+ patients and by featuring patients in unrealistically strenuous activities, such as mountain climbing.[115] The US FDA reprimanded multiple pharmaceutical manufacturers for publishing such ads in 2001 because the misleading advertisements harmed consumers by implying unproven benefits and failing to disclose important information about the drug.[116]
See also
- Antiviral drug
- Discovery and Development of HIV Protease Inhibitors
- Discovery and Development of Non-Nucleoside Reverse Transcriptase Inhibitors
- Discovery and development of nucleoside and nucleotide reverse transcriptase inhibitors
References
- ^ a b "Natural history of HIV infection in the era of combination antiretroviral therapy". AIDS. 13 (14): 1933–42. October 1, 1999. doi:10.1097/00002030-199910010-00017. PMID 10513653.
- ^ Fauci, AS (25 July 2012). "Toward an AIDS-free generation". JAMA. 308 (4): 343. doi:10.1001/jama.2012.8142.
- ^ Deeks, Steven G (8 November 2013). "The end of AIDS: HIV infection as a chronic disease". The Lancet. 382 (9903): 1525–1533. doi:10.1016/S0140-6736(13)61809-7.
- ^ a b c "Guidelines: HIV". World Health Organization. Retrieved 2015-10-27.
- ^ a b c d e f g h i j k l m n o "Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents" (PDF). US Department of Health and Human Services. 2015-04-08.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "U.S Approves Drug to Prolong Lives of AIDS Patients". New York Times. 1987-03-21.
- ^ "Natural History of Opportunistic Disease in an HIV-Infected Urban Clinical Cohort". Annals of Internal Medicine. 124 (7): 633. doi:10.7326/0003-4819-124-7-199604010-00003.
- ^ "A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less". N. Engl. J. Med. 337 (11): 725–33. Sep 1997. doi:10.1056/NEJM199709113371101. PMID 9287227.
- ^ a b "Treatment with Indinavir, Zidovudine, and Lamivudine in Adults with Human Immunodeficiency Virus Infection and Prior Antiretroviral Therapy". New England Journal of Medicine. 337 (11): 734–739. 1997. doi:10.1056/NEJM199709113371102.
- ^ Lieberman-Blum, SS; Fung, HB; Bandres, JC (2008). "Maraviroc: A CCR5-receptor antagonist for the treatment of HIV-1 infection". Clinical Therapeutics. 30 (7): 1228–50. doi:10.1016/S0149-2918(08)80048-3. PMID 18691983.
- ^ Bai, Y; Xue, H; Wang, K; Cai, L; Qiu, J; Bi, S; Lai, L; Cheng, M; Liu, S; Liu, K (2013). "Covalent fusion inhibitors targeting HIV-1 gp41 deep pocket". Amino Acids. 44 (2): 701–13. doi:10.1007/s00726-012-1394-8. PMID 22961335.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b Das, K; Arnold, E (2013). "HIV-1 reverse transcriptase and antiviral drug resistance. Part 1". Current Opinion in Virology. 3 (2): 111–8. doi:10.1016/j.coviro.2013.03.012. PMID 23602471.
- ^ a b Geretti, ed. (2006). "9". Antiretroviral Resistance in Clinical Practice. Mediscript. ISBN 978-0-955-16690-7.
- ^ "HIV Integrase Inhibitors: 20-Year Landmark and Challenges". Advances in Pharmacology: 75–105. 2013. doi:10.1016/B978-0-12-405880-4.00003-2.
- ^ a b Wensing, AM; van Maarseveen, NM; Nijhuis, M (2010). "Fifteen years of HIV protease inhibitors: Raising the barrier to resistance". Antiviral Research. 85 (1): 59–74. doi:10.1016/j.antiviral.2009.10.003. PMID 19853627.
- ^ "Myriad Genetics suspends its HIV maturation inhibitor program". AIDSmeds. 8 June 2012. Retrieved 27 June 2012.
- ^ Perelson, AS; Neumann, AU; Markowitz, M; Leonard, JM; Ho, DD (1996). "HIV-1 dynamics in vivo: Virion clearance rate, infected cell life-span, and viral generation time". Science. 271 (5255): 1582–6. doi:10.1126/science.271.5255.1582. PMID 8599114.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b "The origin of genetic diversity in HIV-1". Virus Research. 169 (2): 415–429. November 2012. doi:10.1016/j.virusres.2012.06.015.
- ^ Schmit, JC; Cogniaux, J; Hermans, P; Van Vaeck, C; Sprecher, S; Van Remoortel, B; Witvrouw, M; Balzarini, J; Desmyter, J; De Clercq, E; Vandamme, AM (1996). "Multiple drug resistance to nucleoside analogues and nonnucleoside reverse transcriptase inhibitors in an efficiently replicating human immunodeficiency virus type 1 patient strain" (PDF). The Journal of Infectious Diseases. 174 (5): 962–8. doi:10.1093/infdis/174.5.962. PMID 8896496.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Henkel, J (July–August 1999). "Attacking AIDS with a 'cocktail' therapy". FDA Consumer. Food and Drug Administration, US Dept. of Health and Human Services. Archived from the original on 2009-01-14.
- ^ "Fixed-dose combinations". aidsmap. March 2011. Retrieved 2014-04-09.
- ^ "Atripla Prescribing Information" (PDF).
- ^ "Stribild Prescribing Information". Retrieved 2014-04-09.
- ^ "Complera Prescribing Information". Retrieved 2014-04-09.
- ^ "Fixed-dose combinations improve medication compliance: a meta-analysis". Am. J. Med. 120 (8): 713–9. August 2007. doi:10.1016/j.amjmed.2006.08.033. PMID 17679131.
- ^ a b "Adherence-resistance relationships to combination HIV antiretroviral therapy". Curr HIV/AIDS Rep. 4 (2): 65–72. May 2007. doi:10.1007/s11904-007-0010-0. PMID 17547827.
- ^ "Descovy Prescribing Information" (PDF). Retrieved 5 April 2016.
- ^ Darbyshire, J (1995). "Perspectives in drug therapy of HIV infection". Drugs. 49 Suppl 1 (Supplement 1): 1–3, discussion 38–40. doi:10.2165/00003495-199500491-00003. PMID 7614897.
- ^ Ho, DD (1995). "Time to hit HIV, early and hard". The New England Journal of Medicine. 333 (7): 450–1. doi:10.1056/NEJM199508173330710. PMID 7616996.
- ^ Harrington, M; Carpenter, CC (2000). "Hit HIV-1 hard, but only when necessary". The Lancet. 355 (9221): 2147–52. doi:10.1016/S0140-6736(00)02388-6. PMID 10902643.
- ^ Sonenklar, C (2011). "Chapter 6: Treatment for HIV and AIDS". AIDS. USA Today Health Reports: Diseases and Disorders. Minneapolis, MN: Twenty-First Century Books. pp. 90–101. ISBN 9780822585817.
- ^ Kitahata, Mari M.; Gange, Stephen J.; Abraham, Alison G.; Merriman, Barry; Saag, Michael S.; Justice, Amy C.; Hogg, Robert S.; Deeks, Steven G.; Eron, Joseph J. (2009-04-30). "Effect of early versus deferred antiretroviral therapy for HIV on survival". The New England Journal of Medicine. 360 (18): 1815–1826. doi:10.1056/NEJMoa0807252. ISSN 1533-4406. PMC 2854555. PMID 19339714.
- ^ "Effect of early versus deferred antiretroviral therapy for HIV on survival". New England Journal of Medicine. 360 (18): 1815–1826. April 30, 2009. doi:10.1056/NEJMoa0807252. PMC 2854555. PMID 19339714.
- ^ "Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection". New England Journal of Medicine. 373 (9): 795–807. 2015-08-27. doi:10.1056/NEJMoa1506816. ISSN 0028-4793. PMC 4569751. PMID 26192873.
- ^ Danel, Christine; Moh, Raoul; Gabillard, Delphine; Badje, Anani; Le Carrou, Jérôme; Ouassa, Timothée; Ouattara, Eric; Anzian, Amani (2015-08-27). "A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa". The New England Journal of Medicine. 373 (9): 808–822. doi:10.1056/NEJMoa1507198. ISSN 1533-4406. PMID 26193126.
{{cite journal}}: Missing|author1=(help) - ^ "Incomplete Peripheral CD4+ Cell Count Restoration in HIV-Infected Patients Receiving Long-Term Antiretroviral Treatment". Clinical Infectious Diseases. 48 (6): 787–794. 2009-03-15. doi:10.1086/597093.
- ^ "HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies". AIDS. 22 (16): 2143–2153. Oct 18, 2008. doi:10.1097/QAD.0b013e3283112b77.
- ^ "Initiation of antiretroviral therapy leads to a rapid decline in cervical and vaginal HIV-1 shedding". AIDS. 21 (4): 501–507. Feb 19, 2007. doi:10.1097/QAD.0b013e32801424bd.
- ^ "Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study". AIDS. 14 (2): 117–21. Jan 28, 2000. doi:10.1097/00002030-200001280-00006. PMID 10708281.
- ^ "Prevention of HIV-1 infection with early antiretroviral therapy". New England Journal of Medicine. 365 (6): 493–505. 2011. doi:10.1056/NEJMoa1105243. PMC 3200068. PMID 21767103.
- ^ "Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: where do we go from here?". Lancet. 382 (9903): 1515–1524. Nov 8, 2013. doi:10.1016/S0140-6736(13)61998-4.
- ^ Cohen, J (2011). "Breakthrough of the year. HIV treatment as prevention". Science. 334 (6063): 1628. doi:10.1126/science.334.6063.1628. PMID 22194547.
- ^ a b c d e f "WHO | Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection". June 30, 2013. p. 38. ISBN 978 92 4 150572 7.
- ^ a b "CD4+ Count–Guided Interruption of Antiretroviral Treatment". New England Journal of Medicine. 355 (22): 2283–2296. 2006. doi:10.1056/NEJMoa062360.
- ^ a b "Risk of cancers during interrupted antiretroviral therapy in the SMART study". AIDS. 21 (14): 1957–1963. 2007. doi:10.1097/QAD.0b013e3282ed6338.
- ^ "Antiretroviral treatment of adult hiv infection: 2014 recommendations of the international antiviral society–usa panel". JAMA. 312 (4): 410–425. 2014-07-23. doi:10.1001/jama.2014.8722. ISSN 0098-7484.
- ^ "EACS Guidelines 8.0". www.eacsociety.org. Retrieved 2016-01-14.
- ^ UK Group on Transmitted HIV Drug Resistance; Cane, P; Chrystie, I; Dunn, D; Evans, B; Geretti, AM; Green, H; Phillips, A; Pillay, D; Porter, K; Pozniak, A; Sabin, C; Smit, E; Weber, J; Zuckerman, M (2005). "Time trends in primary resistance to HIV drugs in the United Kingdom: Multicentre observational study". BMJ. 331 (7529): 1368–71. doi:10.1136/bmj.38665.534595.55. PMC 1309643. PMID 16299012.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ "Prevalence of antiretroviral drug resistance mutations in chronically HIV-infected, treatment-naive patients: implications for routine resistance screening before initiation of antiretroviral therapy". Clinical Infectious Diseases. 40 (3): 468–74. Feb 1, 2005. doi:10.1086/427212. PMID 15668873.
- ^ "National sentinel surveillance of transmitted drug resistance in antiretroviral-naive chronically HIV-infected patients in France over a decade: 2001-2011". J Antimicrob Chemother. 68 (11): 2626–31. Nov 2013. doi:10.1093/jac/dkt238. PMID 23798669.
- ^ "Surveillance of transmitted HIV drug resistance in antiretroviral-naive patients aged less than 25 years, in Bangkok, Thailand". J Int Assoc Provid AIDS Care. 13 (1): 12–4. Jan–Feb 2014. doi:10.1177/2325957413488200. PMID 23708678.
- ^ "A multicenter study of initiation of antiretroviral therapy and transmitted drug resistance in antiretroviral-naive adolescents and young adults with HIV in New York City". PLoS ONE. 58 (6): 865–72. March 2014. doi:10.1093/cid/ciu003. PMID 24429431.
- ^ "Antiretroviral drug resistance in HIV-1 therapy-naive patients in Cuba". Infect Genet Evol. 16: 144–50. June 2013. doi:10.1016/j.meegid.2013.02.002. PMID 23416260.
- ^ a b c d "Antiretroviral Therapy for Human Immunodeficiency Virus Infection". Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 7th Edition. Churchill Livingstone. 2009. ISBN 978-0-443-06839-3.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ "Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro". Antimicrob Agents Chemother. 56 (10): 5409–13. October 2012. doi:10.1128/AAC.01089-12. PMC 3457391. PMID 22850510. Retrieved 2014-06-06.
- ^ "Short-Course Antiretroviral Therapy in Primary HIV Infection". NEJM. 368 (3): 207–217. 2013-1-17. doi:10.1056/NEJMoa1110039.
{{cite journal}}: Check date values in:|date=(help) - ^ "HIV-1 Transmission, by Stage of Infection". J Infect Dis. 198 (5): 687–693. 9/1/2008. doi:10.1086/590501.
{{cite journal}}: Check date values in:|date=(help) - ^ "Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis". The Lancet. 364 (9441): 1236–1243. October 8, 2004. doi:10.1016/S0140-6736(04)17140-7.
- ^ a b "Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection" (PDF). US Department of Health and Human Services. February 12, 2014. pp. 50–61. Retrieved April 11, 2014.
- ^ "Response to Nonnucleoside Reverse Transcriptase Inhibitor-Based Therapy in HIV-Infected Children with Perinatal Exposure to Single-Dose Nevirapine". AIDS Research and Human Retroviruses. 25 (10): 989–996. October 2009. doi:10.1089/aid.2009.0054.
- ^ "Nevirapine versus Ritonavir-Boosted Lopinavir for HIV-Infected Children". New England Journal of Medicine. 366 (25): 2380–2389. 2012. doi:10.1056/NEJMoa1113249.
- ^ Adetokunboh, Olatunji O.; Schoonees, Anel; Balogun, Tolulope A.; Wiysonge, Charles S. (26 October 2015). "Efficacy and safety of abacavir-containing combination antiretroviral therapy as first-line treatment of HIV infected children and adolescents: a systematic review and meta-analysis". BMC Infectious Diseases. 15 (1). doi:10.1186/s12879-015-1183-6.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Maternal Levels of Plasma Human Immunodeficiency Virus Type 1 RNA and the Risk of Perinatal Transmission". NEJM. 341 (6): 394–402. 1999. doi:10.1056/NEJM199908053410602.
- ^ "Mother-to-Child Transmission of HIV Infection in the Era of Highly Active Antiretroviral Therapy". Clin Infect Dis. 40 (3): 458–465. 02/01/2005. doi:10.1086/427287.
{{cite journal}}: Check date values in:|date=(help) - ^ a b "Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States" (PDF). US DHHS. March 28, 2014. Retrieved 2014-04-11.
- ^ "Mother-to-Child Transmission of HIV Infection in the Era of Highly Active Antiretroviral Therapy". Clin Infect Dis. 40 (3): 458–465. 02/01/2005. doi:10.1086/427287.
{{cite journal}}: Check date values in:|date=(help) - ^ "Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease". The Journal of Infectious Diseases. 187 (5): 741–747. Mar 1, 2003. doi:10.1086/374273.
- ^ "Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy: UK cohort study". AIDS: 1. Feb 19, 2014. doi:10.1097/QAD.0000000000000243.
- ^ "Life expectancy living with HIV: recent estimates and future implications". Current Opinion in Infectious Diseases. 26 (1): 17–25. 2013. doi:10.1097/QCO.0b013e32835ba6b1.
- ^ "Older age and the response to and tolerability of antiretroviral therapy". Arch Intern Med. 167 (7): 684. April 9, 2007. doi:10.1001/archinte.167.7.684.
- ^ "Virologic and immunologic response to HAART, by age and regimen class". AIDS. 24 (16): 2469–2479. October 2010. doi:10.1097/QAD.0b013e32833e6d14.
- ^ a b "Management of human immunodeficiency virus infection in advanced age". JAMA. 309 (13): 1397. April 3, 2013. doi:10.1001/jama.2013.2963.
- ^ "Antiretroviral medication adherence and the development of class-specific antiretroviral resistance". AIDS. 23 (9): 1035–1046. Jun 1, 2009. doi:10.1097/QAD.0b013e32832ba8ec.
- ^ "Global Price Reporting Mechanism for HIV, tuberculosis and malaria". World Health Organization. Retrieved 2014-04-11.
- ^ "Antiretroviral Drug Prices". Avert. Retrieved 2014-04-12.
- ^ "New one-pill, $10-per-month anti-retroviral AIDS treatment debuts in South Africa". The Raw Story. Agence France-Presse. 4/8/2013. Retrieved 2014-04-14.
{{cite web}}: Check date values in:|date=(help) - ^ "Cost-Effectiveness of HIV Treatment as Prevention in Serodiscordant Couples". NEJM. 369 (18): 1715–1725. 2013. doi:10.1056/NEJMsa1214720.
- ^ Horn, Tim (August 28, 2012). "Activists Protest Stribild's $28,500 Price Tag". AIDSMeds. Retrieved 2014-04-11.
- ^ "Stribild". GoodRx. Retrieved 2014-04-11.
- ^ Beardsley, T (1998). "Coping with HIV's ethical dilemmas". Scientific American. 279 (1): 106–7. doi:10.1038/scientificamerican0798-106. PMID 9648307.
- ^ a b c "Antiretroviral treatment of adult hiv infection: 2012 recommendations of the international antiviral society–usa panel". JAMA. 308 (4): 387–402. July 25, 2012. doi:10.1001/jama.2012.7961. PMID 22820792.
- ^ "The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs". AIDS. 13 (7): 797–804. May 7, 1999. doi:10.1097/00002030-199905070-00008. PMID 10357378.
- ^ "Within-Subject Variation in CD4 Lymphocyte Count in Asymptomatic Human Immunodeficiency Virus Infection: Implications for Patient Monitoring". J Infect Dis. 169 (1): 28–36. 1994-01-01. doi:10.1093/infdis/169.1.28.
- ^ "Long-term increase in CD4+ T-cell counts during combination antiretroviral therapy for HIV-1 infection". AIDS. 24 (12): 1867–1876. Jul 31, 2010. doi:10.1097/QAD.0b013e32833adbcf.
- ^ "The Absence of CD4+ T Cell Count Recovery Despite Receipt of Virologically Suppressive Highly Active Antiretroviral Therapy: Clinical Risk, Immunological Gaps, and Therapeutic Options". Clin Infect Dis. 48 (3): 328–337. 02/01/2009. doi:10.1086/695852.
{{cite journal}}: Check date values in:|date=(help) - ^ "Stanford University HIV Drug Resistance Database". Retrieved 2014-04-13.
- ^ "HIV drug susceptibility and treatment response to mega-HAART regimen in patients from the Frankfurt HIV cohort". Antivir Ther. 5 (1): 49–55. 2000. PMID 10846593.
- ^ "Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase". J Biol Chem. 276 (44): 40847–57. November 2001. doi:10.1074/jbc.M106743200. PMID 11526116.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors". Antimicrob Agents Chemother. 46 (3): 716–23. March 2002. doi:10.1128/aac.46.3.716-723.2002. PMC 127499. PMID 11850253.
- ^ "Non-nucleoside reverse transcriptase inhibitors: a review on pharmacokinetics, pharmacodynamics, safety and tolerability". J Int AIDS Soc. 16 (1): 1–14. 2013. doi:10.7448/ias.16.1.18567. PMC 3764307. PMID 24008177.
- ^ "Protease inhibitor-based regimens for HIV therapy: safety and efficacy". J Acquir Immune Defic Syndr. 45 Suppl 1 (Supplement 1): S5–13, quiz S28–31. June 2007. doi:10.1097/QAI.0b013e3180600709. PMID 17525691.
- ^ "Tolerability of HIV integrase inhibitors". Curr Opin HIV AIDS. 7 (5): 422–8. September 2012. doi:10.1097/COH.0b013e328356682a. PMID 22886031.
- ^ Bell, D M (May 19, 1997). "Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview". Am. J. Med. 102 (5B): 9–15. doi:10.1016/s0002-9343(97)89441-7. PMID 9845490.
- ^ Ippolito, Giuseppe; Puro, V; De Carli, G (June 28, 1993). "The risk of occupational human immunodeficiency virus infection in health care workers: Italian multicenter study". Arch Intern Med. 153 (12): 1451–8. doi:10.1001/archinte.1993.00410120035005. PMID 8512436.
- ^ a b c Kuhar, D. T.; Henderson, D. K.; Struble, K. A.; Heneine, W; Thomas, V; Cheever, L. W.; Gomaa, A; Panlilio, A. L.; US Public Health Service Working Group (September 1, 2013). "Updated US Public Health Service Guidelines for the Management of Occupational Exposures to Human Immunodeficiency Virus and Recommendations for Postexposure Prophylaxis". Infection Control and Hospital Epidemiology. 34 (9): 875–92. doi:10.1086/672271. JSTOR 672271. PMID 23917901.
- ^ Cardo, D. M.; Culver, D. H.; Ciesielski, C. A.; Srivastava, P. U.; Marcus, R; Abiteboul, D; Heptonstall, J; Ippolito, G; Lot, F; McKibben, P. S.; Bell, D. M. (1997). "A Case–Control Study of HIV Seroconversion in Health Care Workers after Percutaneous Exposure". NEJM. 337 (21): 1485–90. doi:10.1056/NEJM199711203372101. PMID 9366579.
- ^ Glynn, J. R.; Buvé, A; Caraël, M; Kahindo, M; MacAuley, I. B.; Musonda, R. M.; Jungmann, E; Tembo, F; Zekeng, L (Dec 1, 2000). "Decreased fertility among HIV-1-infected women attending antenatal clinics in three African cities". J. Acquir. Immune Defic. Syndr. 25 (4): 345–52. doi:10.1097/00126334-200012010-00008. PMID 11114835.
- ^ a b Savasi, V; Mandia, L; Laoreti, A; Cetin, I (2012). "Reproductive assistance in HIV serodiscordant couples". Human Reproduction Update. 19 (2): 136–50. doi:10.1093/humupd/dms046. PMID 23146867.
- ^ Centers for Disease Control (CDC) (Apr 20, 1990). "HIV-1 infection and artificial insemination with processed semen". MMWR. 39 (15): 249, 255–6. PMID 2109169.
- ^ Savasi, V.; Ferrazzi, E.; Lanzani, C.; Oneta, M.; Parrilla, B.; Persico, T. (03/01/2007). "Safety of sperm washing and ART outcome in 741 HIV-1-serodiscordant couples". Hum. Reprod. 22 (3): 772–777. doi:10.1093/humrep/del422. PMID 17107974.
{{cite journal}}: Check date values in:|date=(help) - ^ Coutsoudis, A; Kwaan, L; Thomson, M (2010). "Prevention of vertical transmission of HIV-1 in resource-limited settings". Expert Review of Anti-infective Therapy. 8 (10): 1163–75. doi:10.1586/eri.10.94. PMID 20954881.
- ^ a b c Passaes, Caroline P.; Sáez-Cirión, Asier (April 2014). "HIV cure research: Advances and prospects". Virology. 454–455: 340–352. doi:10.1016/j.virol.2014.02.021. PMID 24636252.
- ^ Rosenberg, Tina (May 29, 2011). "The Man Who Had HIV and Now Does Not". New York Magazine. Retrieved 2014-04-12.
- ^ Hütter, G; Nowak, D; Mossner, M; Ganepola, S; Müssig, A; Allers, K; Schneider, T; Hofmann, J; Kücherer, C; Blau, O; Blau, I. W.; Hofmann, W. K.; Thiel, E (2009). "Long-Term Control of HIV by CCR5 Delta32/Delta32 Stem-Cell Transplantation". NEJM. 360 (7): 692–8. doi:10.1056/NEJMoa0802905. PMID 19213682.
- ^ Tebas, P; Stein, D; Tang, W. W.; Frank, I; Wang, S. Q.; Lee, G; Spratt, S. K.; Surosky, R. T.; Giedlin, M. A.; Nichol, G; Holmes, M. C.; Gregory, P. D.; Ando, D. G.; Kalos, M; Collman, R. G.; Binder-Scholl, G; Plesa, G; Hwang, W. T.; Levine, B. L.; June, C. H. (2014). "Gene Editing of CCR5 in Autologous CD4 T Cells of Persons Infected with HIV". NEJM. 370 (10): 901–10. doi:10.1056/NEJMoa1300662. PMC 4084652. PMID 24597865.
- ^ Chomont, Nicolas; El-Far, Mohamed; Ancuta, Petronela; Trautmann, Lydie; Procopio, Francesco A; Yassine-Diab, Bader; Boucher, Geneviève; Boulassel, Mohamed-Rachid; Ghattas, Georges; Brenchley, Jason M; Schacker, Timothy W; Hill, Brenna J; Douek, Daniel C; Routy, Jean-Pierre; Haddad, Elias K; Sékaly, Rafick-Pierre (August 2009). "HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation". Nat Med. 15 (8): 893–900. doi:10.1038/nm.1972. PMID 19543283.
- ^ McNeil, Donald (3/5/2014). "Early Treatment Is Found to Clear H.I.V. in a 2nd Baby". New York Times. Retrieved 2014-04-14.
{{cite web}}: Check date values in:|date=(help) - ^ Persaud, Deborah; Gay, Hannah; Ziemniak, Carrie; Chen, Ya Hui; Piatak, Michael; Chun, Tae-Wook; Strain, Matthew; Richman, Douglas; Luzuriaga, Katherine (11/7/2013). "Absence of Detectable HIV-1 Viremia after Treatment Cessation in an Infant". NEJM. 369 (19): 1828–1835. doi:10.1056/NEJMoa1302976. PMID 24152233.
{{cite journal}}: Check date values in:|date=(help) - ^ Archin, N. M.; Cheema, M; Parker, D; Wiegand, A; Bosch, R. J.; Coffin, J. M.; Eron, J; Cohen, M; Margolis, D. M. (2010-2-23). "Antiretroviral Intensification and Valproic Acid Lack Sustained Effect on Residual HIV-1 Viremia or Resting CD4+ Cell Infection". PLoS ONE. 5 (2): e9390. doi:10.1371/journal.pone.0009390. PMC 2826423. PMID 20186346.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: unflagged free DOI (link) - ^ Archin, N. M.; Liberty, A. L.; Kashuba, A. D.; Choudhary, S. K.; Kuruc, J. D.; Crooks, A. M.; Parker, D. C.; Anderson, E. M.; Kearney, M. F.; Strain, M. C.; Richman, D. D.; Hudgens, M. G.; Bosch, R. J.; Coffin, J. M.; Eron, J. J.; Hazuda, D. J.; Margolis, D. M. (2012-07-26). "Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy". Nature. 487 (7408): 482–485. doi:10.1038/nature11286. PMID 22837004.
- ^ Carcelain, Guislaine; Autran, Brigitte (7/1/2013). "Immune interventions in HIV infection". Immunol Rev. 254 (1): 355–371. doi:10.1111/imr.12083. PMID 23772631.
{{cite journal}}: Check date values in:|date=(help) - ^ "Programs TAT – Vaccin VIH | BIOSANTECH SA ®". www.biosantech.org. Retrieved 2015-10-27.
- ^ "Tat Oyi-based candidate therapeutic vaccine: a successful phase 1 clinical trial in HIV-1 infected patients | Retroviruses-2015 | OMICS International". retrovirus.conferenceseries.com. Retrieved 2015-10-27.
- ^ Watkins, Jennifer D.; Lancelot, Sophie; Campbell, Grant R.; Esquieu, Didier; de Mareuil, Jean; Opi, Sandrine; Annappa, Sylvie; Salles, Jean-Pierre; Loret, Erwann P. (2006-01-01). "Reservoir cells no longer detectable after a heterologous SHIV challenge with the synthetic HIV-1 Tat Oyi vaccine". Retrovirology. 3: 8. doi:10.1186/1742-4690-3-8. ISSN 1742-4690. PMC 1434768. PMID 16441880.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kallen, Alexander; Woloshin, Steven; Shu, Jennifer; Juhl, Ellen; Schwartz, Lisa (2007-10-01). "Direct-to-consumer advertisements for HIV antiretroviral medications: a progress report". Health Affairs (Project Hope). 26 (5): 1392–1398. doi:10.1377/hlthaff.26.5.1392. ISSN 1544-5208. PMID 17848450.
- ^ Josefson, Deborah (2001-05-12). "FDA warning to manufacturers of AIDS drugs". BMJ: British Medical Journal. 322 (7295): 1143. ISSN 0959-8138. PMC 1120280. PMID 11348904.
External links
- AIDSinfo - Comprehensive resource for HIV/AIDS treatment and clinical trial information from the U. S. Department of Health and Human Services
- ASHM - Australian Commentary on HHS Guidelines for the use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents
- Origins of antiretroviral combination therapy
- Viral Load research papers, including effectiveness of HAART on reducing viral load
- Current status of gene therapy strategies to treat HIV/AIDS
