Lorvotuzumab mertansine
Appearance
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized |
| Target | CD56 |
| Clinical data | |
| ATC code |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| | |
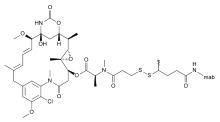
Lorvotuzumab mertansine (IMGN901) is an antibody-drug conjugate. It comprises the CD56-binding antibody, lorvotuzumab (huN901), with a maytansinoid cell-killing agent, DM1, attached using a disulfide linker, SPP. (When DM1 is attached to an antibody with the SPP linker, it is mertansine; when it is attached with the thioether linker, SMCC, it is emtansine.)
Lorvotuzumab mertansine is an experimental agent created for the treatment of CD56 positive cancers (e.g. small-cell lung cancer, ovarian cancer).[1][2]
It has been granted Orphan drug status for Merkel cell carcinoma.[3]
It has reported encouraging Phase II results for small-cell lung cancer.[4]
References
- ^ Dimond PF (9 March 2010). "Antibody-Drug Conjugates Stage a Comeback". Genetic Engineering & Biotechnology News.
- ^ ImmunoGen reports encouraging clinical data of IMGN901. News-medical.net. Retrieved on 2010-11-20.
- ^ "ImmunoGen receives FDA orphan drug designation for IMGN901 compound in treatment of MCC". News-Medical.net. 8 March 2010.
- ^ "ImmunoGen Announces Encouraging New Clinical Data With The Company's IMGN901 Compound In The Treatment Of Small-Cell Lung Cancer". 2009.[permanent dead link]
