Chemical symbol
Chemical symbols are abbreviations used in chemistry for chemical elements, functional groups and chemical compounds. Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised.
Earlier symbols for chemical elements stem from classical Latin and Greek vocabulary. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (plumbum in Latin); Hg is the symbol for mercury (hydrargyrum in Greek); and He is the symbol for helium (a new Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (Wolfram in German) which was not known in Roman times.
A 3-letter temporary symbol may be assigned to a newly synthesized (or not yet synthesized) element. For example, "Uno" was the temporary symbol for hassium (element 108) which had the temporary name of unniloctium, based on its atomic number being 8 greater than 100. There are also some historical symbols that are no longer officially used.
In addition to the letters for the element itself, additional details may be added to the symbol as superscripts or subscripts a particular isotope, ionization, or oxidation state, or other atomic detail.[1] A few isotopes have their own specific symbols rather than just an isotopic detail added to their element symbol.
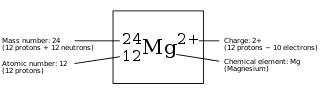
Attached subscripts or superscripts specifying a nuclide or molecule have the following meanings and positions:
- The nucleon number (mass number) is shown in the left superscript position (e.g., 14N). This number defines the specific isotope. Various letters, such as "m" and "f" may also be used here to indicate a nuclear isomer (e.g., 99mTc). Alternately, the number here can represent a specific spin state (e.g., 1O2). These details can be omitted if not relevant in a certain context.
- The proton number (atomic number) may be indicated in the left subscript position (e.g., 64Gd). The atomic number is redundant to the chemical element, but is sometimes used to emphasize the change of numbers of nucleons in a nuclear reaction.
- If necessary, a state of ionization or an excited state may be indicated in the right superscript position (e.g., state of ionization Ca2+).
- The number of atoms of an element in a molecule or chemical compound is shown in the right subscript position (e.g., N2 or Fe2O3). If this number is one, it is normally omitted - the number one is implicitly understood if unspecified.
- A radical is indicated by a dot on the right side (e.g., Cl• for a neutral chlorine atom). This is often omitted unless relevant to a certain context because it is already deducible from the charge and atomic number, as generally true for nonbonded valence electrons in skeletal structures.
In Chinese, each chemical element has a dedicated character, usually created for the purpose (see Chemical elements in East Asian languages). However, Latin symbols are also used, especially in formulas.
Many functional groups also have their own chemical symbol, e.g. Ph for the phenyl group, and Me for the methyl group.

A list of current, dated, as well as proposed and historical signs and symbols is included here with its signification. Also given is each element's atomic number, atomic weight, or the atomic mass of the most stable isotope, group and period numbers on the periodic table, and etymology of the symbol.
Hazard pictographs are another type of symbols used in chemistry.
Symbols for chemical elements
| List of chemical elements | ||||||
|---|---|---|---|---|---|---|
| Z[I] | Symbol | Element | Origin of name[2][3] | Group | Period | Atomic weight[4][5] (u (±)) |
| 1 | style="background:#f0ff8f" | H | Hydrogen | Greek elements hydro- and -gen, meaning 'water-forming' | 1 | 1 | 1.008[II][III][IV][V] |
| 2 | He | Helium | Greek hḗlios, 'sun' | 18 | 1 | 4.002602(2)[II][IV] |
| 3 | style="background:#ff9d9d" | Li | Lithium | Greek líthos, 'stone' | 1 | 2 | 6.94[II][III][IV][VI][V] |
| 4 | style="background:#ffdead" | Be | Beryllium | beryl, a mineral (ultimately from the name of Belur in southern India) | 2 | 2 | 9.0121831(5) |
| 5 | B | Boron | borax, a mineral (from Arabic bawraq) | 13 | 2 | 10.81[II][III][IV][V] |
| 6 | style="background:#f0ff8f" | C | Carbon | Latin carbo, 'coal' | 14 | 2 | 12.011[II][IV][V] |
| 7 | style="background:#f0ff8f" | N | Nitrogen | Greek nítron and -gen, meaning 'niter-forming' | 15 | 2 | 14.007[II][IV][V] |
| 8 | style="background:#f0ff8f" | O | Oxygen | Greek oxy- and -gen, meaning 'acid-forming' | 16 | 2 | 15.999[II][IV][V] |
| 9 | style="background:#f0ff8f" | F | Fluorine | Latin fluere, 'to flow' | 17 | 2 | 18.998403163(6) |
| 10 | Ne | Neon | Greek néon, 'new' | 18 | 2 | 20.1797(6)[II][III] |
| 11 | style="background:#ff9d9d" | Na | Sodium | English soda (the symbol Na is derived from New Latin natrium, coined from German Natron, 'natron') | 1 | 3 | 22.98976928(2) |
| 12 | style="background:#ffdead" | Mg | Magnesium | Magnesia, a district of Eastern Thessaly in Greece | 2 | 3 | 24.305[V] |
| 13 | style="background:#cccccc" | Al | Aluminium | alumina, from Latin alumen (gen. aluminis), 'bitter salt, alum' | 13 | 3 | 26.9815384(3) |
| 14 | Si | Silicon | Latin silex, 'flint' (originally silicium) | 14 | 3 | 28.085[IV][V] |
| 15 | style="background:#f0ff8f" | P | Phosphorus | Greek phōsphóros, 'light-bearing' | 15 | 3 | 30.973761998(5) |
| 16 | style="background:#f0ff8f" | S | Sulfur | Latin sulphur, 'brimstone' | 16 | 3 | 32.06[II][IV][V] |
| 17 | style="background:#f0ff8f" | Cl | Chlorine | Greek chlōrós, 'greenish yellow' | 17 | 3 | 35.45[II][III][IV][V] |
| 18 | Ar | Argon | Greek argós, 'idle' (because of its inertness) | 18 | 3 | 39.948[II][IV][V] |
| 19 | style="background:#ff9d9d" | K | Potassium | New Latin potassa, 'potash' (the symbol K is derived from Latin kalium) | 1 | 4 | 39.0983(1) |
| 20 | style="background:#ffdead" | Ca | Calcium | Latin calx, 'lime' | 2 | 4 | 40.078(4)[II] |
| 21 | style="background:#ffc0c0" | Sc | Scandium | Latin Scandia, 'Scandinavia' | 3 | 4 | 44.955908(5) |
| 22 | style="background:#ffc0c0" | Ti | Titanium | Titans, the sons of the Earth goddess of Greek mythology | 4 | 4 | 47.867(1) |
| 23 | style="background:#ffc0c0" | V | Vanadium | Vanadis, an Old Norse name for the Scandinavian goddess Freyja | 5 | 4 | 50.9415(1) |
| 24 | style="background:#ffc0c0" | Cr | Chromium | Greek chróma, 'colour' | 6 | 4 | 51.9961(6) |
| 25 | style="background:#ffc0c0" | Mn | Manganese | corrupted from magnesia negra; see Magnesium | 7 | 4 | 54.938043(2) |
| 26 | style="background:#ffc0c0" | Fe | Iron | English word (the symbol Fe is derived from Latin ferrum) | 8 | 4 | 55.845(2) |
| 27 | style="background:#ffc0c0" | Co | Cobalt | German Kobold, 'goblin' | 9 | 4 | 58.933194(3) |
| 28 | style="background:#ffc0c0" | Ni | Nickel | Nickel, a mischievous sprite of German miner mythology | 10 | 4 | 58.6934(4) |
| 29 | style="background:#ffc0c0" | Cu | Copper | English word, from Latin cuprum, from Ancient Greek Kýpros 'Cyprus' | 11 | 4 | 63.546(3)[IV] |
| 30 | style="background:#cccccc" | Zn | Zinc | Most likely from German Zinke, 'prong' or 'tooth', though some suggest Persian sang, 'stone' | 12 | 4 | 65.38(2) |
| 31 | style="background:#cccccc" | Ga | Gallium | Latin Gallia, 'France' | 13 | 4 | 69.723(1) |
| 32 | Ge | Germanium | Latin Germania, 'Germany' | 14 | 4 | 72.630(8) |
| 33 | As | Arsenic | French arsenic, from Greek arsenikón 'yellow arsenic' (influenced by arsenikós, 'masculine' or 'virile'), from a West Asian wanderword ultimately from Old Iranian *zarniya-ka, 'golden' | 15 | 4 | 74.921595(6) |
| 34 | style="background:#f0ff8f" | Se | Selenium | Greek selḗnē, 'moon' | 16 | 4 | 78.971(8)[IV] |
| 35 | style="background:#f0ff8f" | Br | Bromine | Greek brômos, 'stench' | 17 | 4 | 79.904[V] |
| 36 | Kr | Krypton | Greek kryptós, 'hidden' | 18 | 4 | 83.798(2)[II][III] |
| 37 | style="background:#ff9d9d" | Rb | Rubidium | Latin rubidus, 'deep red' | 1 | 5 | 85.4678(3)[II] |
| 38 | style="background:#ffdead" | Sr | Strontium | Strontian, a village in Scotland | 2 | 5 | 87.62(1)[II][IV] |
| 39 | style="background:#ffc0c0" | Y | Yttrium | Ytterby, a village in Sweden | 3 | 5 | 88.90584(1) |
| 40 | style="background:#ffc0c0" | Zr | Zirconium | zircon, a mineral | 4 | 5 | 91.224(2)[II] |
| 41 | style="background:#ffc0c0" | Nb | Niobium | Niobe, daughter of king Tantalus from Greek mythology | 5 | 5 | 92.90637(1) |
| 42 | style="background:#ffc0c0" | Mo | Molybdenum | Greek molýbdaina, 'piece of lead', from mólybdos, 'lead' | 6 | 5 | 95.95(1)[II] |
| 43 | style="background:#ffc0c0" | Tc | Technetium | Greek tekhnētós, 'artificial' | 7 | 5 | [98][VII] |
| 44 | style="background:#ffc0c0" | Ru | Ruthenium | New Latin Ruthenia, 'Russia' | 8 | 5 | 101.07(2)[II] |
| 45 | style="background:#ffc0c0" | Rh | Rhodium | Greek rhodóeis, 'rose-coloured', from rhódon, 'rose' | 9 | 5 | 102.90549(2) |
| 46 | style="background:#ffc0c0" | Pd | Palladium | the asteroid Pallas, considered a planet at the time | 10 | 5 | 106.42(1)[II] |
| 47 | style="background:#ffc0c0" | Ag | Silver | English word (The symbol derives from Latin argentum) | 11 | 5 | 107.8682(2)[II] |
| 48 | style="background:#cccccc" | Cd | Cadmium | New Latin cadmia, from King Kadmos | 12 | 5 | 112.414(4)[II] |
| 49 | style="background:#cccccc" | In | Indium | Latin indicum, 'indigo' (colour found in its spectrum) | 13 | 5 | 114.818(1) |
| 50 | style="background:#cccccc" | Sn | Tin | English word (The symbol derives from Latin stannum) | 14 | 5 | 118.710(7)[II] |
| 51 | Sb | Antimony | Latin antimonium, the origin of which is uncertain: folk etymologies suggest it is derived from Greek antí ('against') + mónos ('alone'), or Old French anti-moine, 'Monk's bane', but it could plausibly be from or related to Arabic ʾiṯmid, 'antimony', reformatted as a Latin word. (The symbol derives from Latin stibium 'stibnite'.) | 15 | 5 | 121.760(1)[II] |
| 52 | Te | Tellurium | Latin tellus, 'the ground, earth' | 16 | 5 | 127.60(3)[II] |
| 53 | style="background:#f0ff8f" | I | Iodine | French iode, from Greek ioeidḗs, 'violet' | 17 | 5 | 126.90447(3) |
| 54 | Xe | Xenon | Greek xénon, neuter form of xénos 'strange' | 18 | 5 | 131.293(6)[II][III] |
| 55 | style="background:#ff9d9d" | Cs | Caesium | Latin caesius, 'sky-blue' | 1 | 6 | 132.90545196(6) |
| 56 | style="background:#ffdead" | Ba | Barium | Greek barýs, 'heavy' | 2 | 6 | 137.327(7) |
| 57 | style="background:#ffbfff" | La | Lanthanum | Greek lanthánein, 'to lie hidden' | 3 | 6 | 138.90547(7)[II] |
| 58 | style="background:#ffbfff" | Ce | Cerium | the dwarf planet Ceres, considered a planet at the time | 6 | 140.116(1)[II] | |
| 59 | style="background:#ffbfff" | Pr | Praseodymium | Greek prásios dídymos, 'green twin' | 6 | 140.90766(1) | |
| 60 | style="background:#ffbfff" | Nd | Neodymium | Greek néos dídymos, 'new twin' | 6 | 144.242(3)[II] | |
| 61 | style="background:#ffbfff" | Pm | Promethium | Prometheus of Greek mythology | 6 | [145][VII] | |
| 62 | style="background:#ffbfff" | Sm | Samarium | samarskite, a mineral named after Colonel Vasili Samarsky-Bykhovets, Russian mine official | 6 | 150.36(2)[II] | |
| 63 | style="background:#ffbfff" | Eu | Europium | Europe | 6 | 151.964(1)[II] | |
| 64 | style="background:#ffbfff" | Gd | Gadolinium | gadolinite, a mineral named after Johan Gadolin, Finnish chemist, physicist and mineralogist | 6 | 157.25(3)[II] | |
| 65 | style="background:#ffbfff" | Tb | Terbium | Ytterby, a village in Sweden | 6 | 158.925354(8) | |
| 66 | style="background:#ffbfff" | Dy | Dysprosium | Greek dysprósitos, 'hard to get' | 6 | 162.500(1)[II] | |
| 67 | style="background:#ffbfff" | Ho | Holmium | New Latin Holmia, 'Stockholm' | 6 | 164.930328(7) | |
| 68 | style="background:#ffbfff" | Er | Erbium | Ytterby, a village in Sweden | 6 | 167.259(3)[II] | |
| 69 | style="background:#ffbfff" | Tm | Thulium | Thule, the ancient name for an unclear northern location | 6 | 168.934218(6) | |
| 70 | style="background:#ffbfff" | Yb | Ytterbium | Ytterby, a village in Sweden | 6 | 173.045(10)[II] | |
| 71 | style="background:#ffbfff" | Lu | Lutetium | Latin Lutetia, 'Paris' | 6 | 174.9668(1)[II] | |
| 72 | style="background:#ffc0c0" | Hf | Hafnium | New Latin Hafnia, 'Copenhagen' (from Danish havn) | 4 | 6 | 178.49(2) |
| 73 | style="background:#ffc0c0" | Ta | Tantalum | King Tantalus, father of Niobe from Greek mythology | 5 | 6 | 180.94788(2) |
| 74 | style="background:#ffc0c0" | W | Tungsten | Swedish tung sten, 'heavy stone' (The symbol is from wolfram, the old name of the tungsten mineral wolframite) | 6 | 6 | 183.84(1) |
| 75 | style="background:#ffc0c0" | Re | Rhenium | Latin Rhenus, 'the Rhine' | 7 | 6 | 186.207(1) |
| 76 | style="background:#ffc0c0" | Os | Osmium | Greek osmḗ, 'smell' | 8 | 6 | 190.23(3)[II] |
| 77 | style="background:#ffc0c0" | Ir | Iridium | Iris, the Greek goddess of the rainbow | 9 | 6 | 192.217(2) |
| 78 | style="background:#ffc0c0" | Pt | Platinum | Spanish platina, 'little silver', from plata 'silver' | 10 | 6 | 195.084(9) |
| 79 | style="background:#ffc0c0" | Au | Gold | English word (The symbol derives from Latin aurum) | 11 | 6 | 196.966570(4) |
| 80 | style="background:#cccccc" | Hg | Mercury | Mercury, Roman god of commerce, communication, and luck, known for his speed and mobility (The symbol is from the element's Latin name hydrargyrum, derived from Greek hydrárgyros, 'water-silver') | 12 | 6 | 200.592(3) |
| 81 | style="background:#cccccc" | Tl | Thallium | Greek thallós, 'green shoot or twig' | 13 | 6 | 204.38[V] |
| 82 | style="background:#cccccc" | Pb | Lead | English word (The symbol derives from Latin plumbum) | 14 | 6 | 207.2(1)[II][IV] |
| 83 | style="background:#cccccc" | Bi | Bismuth | German Wismut, from weiß Masse 'white mass', unless from Arabic | 15 | 6 | 208.98040(1)[VII] |
| 84 | style="background:#cccccc" | Po | Polonium | Latin Polonia, 'Poland' (the home country of Marie Curie) | 16 | 6 | [209][VII] |
| 85 | style="background:#f0ff8f" | At | Astatine | Greek ástatos, 'unstable' | 17 | 6 | [210][VII] |
| 86 | Rn | Radon | radium | 18 | 6 | [222][VII] |
| 87 | style="background:#ff9d9d" | Fr | Francium | France | 1 | 7 | [223][VII] |
| 88 | style="background:#ffdead" | Ra | Radium | French radium, from Latin radius, 'ray' | 2 | 7 | [226][VII] |
| 89 | style="background:#ff99cc" | Ac | Actinium | Greek aktís, 'ray' | 3 | 7 | [227][VII] |
| 90 | style="background:#ff99cc" | Th | Thorium | Thor, the Scandinavian god of thunder | 7 | 232.0377(4)[VII][II] | |
| 91 | style="background:#ff99cc" | Pa | Protactinium | proto- (from Greek prôtos, 'first, before') + actinium, which is produced through the radioactive decay of protactinium | 7 | 231.03588(1)[VII] | |
| 92 | style="background:#ff99cc" | U | Uranium | Uranus, the seventh planet in the Solar System | 7 | 238.02891(3)[VII] | |
| 93 | style="background:#ff99cc" | Np | Neptunium | Neptune, the eighth planet in the Solar System | 7 | [237][VII] | |
| 94 | style="background:#ff99cc" | Pu | Plutonium | the dwarf planet Pluto, considered the ninth planet in the Solar System at the time | 7 | [244][VII] | |
| 95 | style="background:#ff99cc" | Am | Americium | The Americas, as the element was first synthesised on the continent, by analogy with europium | 7 | [243][VII] | |
| 96 | style="background:#ff99cc" | Cm | Curium | Pierre Curie and Marie Curie, French physicists and chemists | 7 | [247][VII] | |
| 97 | style="background:#ff99cc" | Bk | Berkelium | Berkeley, California, where the element was first synthesised, by analogy with terbium | 7 | [247][VII] | |
| 98 | style="background:#ff99cc" | Cf | Californium | California, where the element was first synthesised | 7 | [251][VII] | |
| 99 | style="background:#ff99cc" | Es | Einsteinium | Albert Einstein, German physicist | 7 | [252][VII] | |
| 100 | style="background:#ff99cc" | Fm | Fermium | Enrico Fermi, Italian physicist | 7 | [257][VII] | |
| 101 | style="background:#ff99cc" | Md | Mendelevium | Dmitri Mendeleev, Russian chemist and inventor who proposed the periodic table | 7 | [258][VII] | |
| 102 | style="background:#ff99cc" | No | Nobelium | Alfred Nobel, Swedish chemist and engineer | 7 | [259][VII] | |
| 103 | style="background:#ff99cc" | Lr | Lawrencium | Ernest O. Lawrence, American physicist | 7 | [266][VII] | |
| 104 | style="background:#ffc0c0" | Rf | Rutherfordium | Ernest Rutherford, British chemist and physicist | 4 | 7 | [267][VII] |
| 105 | style="background:#ffc0c0" | Db | Dubnium | Dubna, Russia, where the Joint Institute for Nuclear Research is located | 5 | 7 | [268][VII] |
| 106 | style="background:#ffc0c0" | Sg | Seaborgium | Glenn T. Seaborg, American chemist | 6 | 7 | [269][VII] |
| 107 | style="background:#ffc0c0" | Bh | Bohrium | Niels Bohr, Danish physicist | 7 | 7 | [270][VII] |
| 108 | style="background:#ffc0c0" | Hs | Hassium | New Latin Hassia, 'Hesse' (a state in Germany) | 8 | 7 | [270][VII] |
| 109 | Mt | Meitnerium | Lise Meitner, Austrian physicist | 9 | 7 | [278][VII] |
| 110 | Ds | Darmstadtium | Darmstadt, Germany, where the element was first synthesised | 10 | 7 | [281][VII] |
| 111 | Rg | Roentgenium | Wilhelm Conrad Röntgen, German physicist | 11 | 7 | [282][VII] |
| 112 | style="background:#cccccc" | Cn | Copernicium | Nicolaus Copernicus, Polish astronomer | 12 | 7 | [285][VII] |
| 113 | Nh | Nihonium | Japanese Nihon, 'Japan' (where the element was first synthesised) | 13 | 7 | [286][VII] |
| 114 | Fl | Flerovium | Flerov Laboratory of Nuclear Reactions, part of JINR, where the element was synthesised; itself named after Georgy Flyorov, Russian physicist | 14 | 7 | [289][VII] |
| 115 | Mc | Moscovium | Moscow Oblast, Russia, where the element was first synthesised | 15 | 7 | [290][VII] |
| 116 | Lv | Livermorium | Lawrence Livermore National Laboratory in Livermore, California, which collaborated with JINR on its synthesis | 16 | 7 | [293][VII] |
| 117 | Ts | Tennessine | Tennessee, United States | 17 | 7 | [294][VII] |
| 118 | Og | Oganesson | Yuri Oganessian, Russian physicist | 18 | 7 | [294][VII] |
Notes
| ||||||
Antimatter atoms are denoted by a bar above the symbol for their matter counterpart, so e.g. H is the symbol for antihydrogen.
Symbols and names not currently used
This section needs additional citations for verification. (May 2018) |
The following is a list of symbols and names formerly used or suggested for elements, including symbols for placeholder names and names given by discredited claimants for discovery.
| Chemical symbol | Name | Atomic number | Origin of symbol | Why not used | Refs |
|---|---|---|---|---|---|
| A | Argon | 18 | A used for Argon until 1957. Current symbol is Ar. | [nb 1] | [6] |
| Ab | Alabamine | 85 | Discredited claim to discovery of astatine. | [nb 2] | [7][8] |
| Ad | Aldebaranium | 70 | Former name for ytterbium. | [nb 2] | |
| Am | Alabamine | 85 | Discredited claim to discovery of astatine. The symbol is now used for americium. | [nb 2] | [7][8] |
| An | Athenium | 99 | Proposed name for einsteinium. | [nb 3] | |
| Ao | Ausonium | 93 | Discredited claim to discovery of neptunium. | [nb 2] | [7] |
| Az | Azote | 7 | Former name for nitrogen. | [nb 1] | |
| Bo | Boron | 5 | Current symbol is B. | [nb 1] | |
| Bv | Brevium | 91 | Former name for protactinium. | [nb 1] | |
| Bz | Berzelium | 90 | Baskerville wrongly believed berzelium to be a new element. Was actually thorium. | [nb 3] | [9] |
| Cb | Columbium | 41 | Former name for niobium. | [nb 1] | [7][9] |
| Ch | Chromium | 24 | Current symbol is Cr. | [nb 1] | |
| Cl | Columbium | 41 | Former name for niobium. The symbol is now used for chlorine. | [nb 1] | |
| Cn | Carolinium | 90 | Baskerville wrongly believed carolinium to be a new element. Was actually thorium. The symbol is now used for copernicium. | [9] | |
| Cp | Cassiopeium | 71 | Former name for lutetium. | [nb 1] | |
| Cp | Copernicium | 112 | Current symbol is Cn. | [nb 1] | |
| Ct | Celtium | 72 | Discredited claim to discovery of hafnium. | [nb 2] | |
| Ct | Centurium | 100 | Proposed name for fermium. | [nb 3] | |
| D | Didymium | 59/60 | Mixture of the elements praseodymium and neodymium. Mosander wrongly believed didymium to be an element. | [10] | |
| Da | Davyum | 43 | Discredited claim to discovery of technetium. | [nb 2] | [7] |
| Db | Dubnium | 104 | Proposed name for rutherfordium. The symbol and name were instead used for element 105. | [nb 1][nb 3] | [7] |
| Di | Didymium | 59/60 | Mixture of the elements praseodymium and neodymium. Mosander wrongly believed didymium to be an element. | [10] | |
| Ds | Dysprosium | 66 | Current symbol is Dy. The symbol is now used for darmstadtium. | [nb 1] | |
| E | Einsteinium | 99 | Current symbol is Es. | [nb 1] | |
| E | Erbium | 68 | Current symbol is Er. | [nb 1] | |
| Ea | Ekaaluminium | 31 | Name given by Mendeleev to an as of then undiscovered element. When discovered, gallium closely matched the prediction. | [nb 3][nb 4] | |
| Eb | Ekaboron | 21 | Name given by Mendeleev to an as of then undiscovered element. When discovered, scandium closely matched the prediction. | [nb 3][nb 4] | [7] |
| El | Ekaaluminium | 31 | Name given by Mendeleev to an as of then undiscovered element. When discovered, gallium closely matched the prediction. | [nb 3][nb 4] | [7] |
| Em | Ekamanganese | 43 | Name given by Mendeleev to an as of then undiscovered element. When discovered, technetium closely matched the prediction. | [nb 3][nb 4] | [7] |
| Em | Emanation | 86 | Also called "radium emanation", the name was originally given by Friedrich Ernst Dorn in 1900. In 1923, this element officially became radon (the name given at one time to 222Rn, an isotope identified in the decay chain of radium). | [nb 1] | [7] |
| Em | Emanium | 89 | Alternate name formerly proposed for actinium. | [nb 3] | |
| Es | Ekasilicon | 32 | Name given by Mendeleev to an as of then undiscovered element. When discovered, germanium closely matched the prediction. The symbol is now used for einsteinium. | [nb 3][nb 4] | [7] |
| Es | Esperium | 94 | Discredited claim to discovery of plutonium. The symbol is now used for einsteinium. | [nb 2] | [7] |
| Fa | Francium | 87 | Current symbol is Fr. | [nb 1] | |
| Fl | Florentium | 61 | Discredited claim to discovery of promethium. The symbol is now used for flerovium. | [nb 2] | |
| Fl | Fluorine | 9 | Current symbol is F. The symbol is now used for flerovium. | [nb 1] | |
| Fr | Florentium | 61 | Discredited claim to discovery of promethium. The symbol is now used for francium. | [nb 2] | [7] |
| G | Glucinium | 4 | Former name for beryllium. | [nb 1] | |
| Gl | Glucinium | 4 | Former name for beryllium. | [nb 1] | [7] |
| Ha | Hahnium | 105 | Proposed name for dubnium. | [nb 3] | |
| Hn | Hahnium | 108 | Proposed name for hassium. | [nb 3] | [7] |
| Hv | Helvetium | 85 | Discredited claim to discovery of astatine. | [nb 2] | [9] |
| Hy | Mercury | 80 | Hy from the Greek hydrargyrum for "liquid silver". Current symbol is Hg. | [nb 1] | [6] |
| I | Iridium | 77 | Current symbol is Ir. The symbol is now used for iodine. | [nb 1] | |
| Il | Illinium | 61 | Discredited claim to discovery of promethium. | [nb 2] | [7] |
| J | Jodium | 53 | Former name for iodine. | [nb 1] | |
| Jg | Jargonium | 72 | Discredited claim to discovery of hafnium. | [nb 2] | [7] |
| Jl | Joliotium | 105 | Proposed name for dubnium. | [nb 3] | [7] |
| Ka | Potassium | 19 | Current symbol is K. | [nb 1] | |
| Ku | Kurchatovium | 104 | Proposed name for rutherfordium. | [nb 3] | [7] |
| L | Lithium | 3 | Current symbol is Li. | [nb 1] | |
| Lw | Lawrencium | 103 | Current symbol is Lr. | [nb 1] | |
| M | Muriaticum | 17 | Former name for chlorine. | [nb 1] | |
| Ma | Manganese | 25 | Current symbol is Mn. | [nb 1] | |
| Ma | Masurium | 43 | Disputed claim to discovery of technetium. | [nb 2] | [7] |
| Md | Mendelevium | 97 | Proposed name for berkelium. The symbol and name were later used for element 101. | [nb 1][nb 3] | |
| Ml | Moldavium | 87 | Discredited claim to discovery of francium. | [nb 2] | [9] |
| Ms | Magnesium | 12 | Current symbol is Mg. | [nb 1] | |
| Ms | Masurium | 43 | Disputed claim to discovery of technetium. | [nb 2] | |
| Mv | Mendelevium | 101 | Current symbol is Md. | [nb 1] | |
| Ng | Norwegium | 72 | Discredited claim to discovery of hafnium. | [nb 2] | |
| No | Norium | 72 | Discredited claim to discovery of hafnium. The symbol is now used for nobelium. | [nb 2] | |
| Np | Nipponium | 43 | Discredited claim to discovery of technetium. The symbol is now used for neptunium. | [nb 2] | [7] |
| Ns | Nielsbohrium | 105 | Proposed name for dubnium. | [nb 3] | [7] |
| Ns | Nielsbohrium | 107 | Proposed name for bohrium. | [nb 3] | [7] |
| Nt | Niton | 86 | Former name for radon. | [nb 1] | [7] |
| Ny | Neoytterbium | 70 | Former name for ytterbium. | [nb 1] | |
| P | Lead | 82 | Current symbol is Pb. The symbol is now used for phosphorus. | [nb 1] | |
| Pa | Palladium | 46 | Current symbol is Pd. The symbol is now used for protactinium. | [nb 1] | |
| Pe | Pelopium | 41 | Former name for niobium. | [nb 1] | |
| Pl | Palladium | 46 | Current symbol is Pd. | [nb 1] | |
| Po | Potassium | 19 | Current symbol is K. The symbol is now used for polonium. | [nb 1] | |
| R | Rhodium | 45 | Current symbol is Rh. (The symbol is now sometimes used for an alkyl group.) | [nb 1] | |
| Rd | Radium | 88 | Current symbol is Ra. | [nb 1] | |
| Rf | Rutherfordium | 106 | Proposed name for seaborgium. The symbol and name were instead used for element 104. | [nb 1][nb 3] | [7] |
| Ro | Rhodium | 45 | Current symbol is Rh. | [nb 1] | |
| Sa | Samarium | 62 | Current symbol is Sm. | [nb 1] | [7] |
| So | Sodium | 11 | Current symbol is Na. | [nb 1] | |
| St | Antimony | 51 | Current symbol is Sb. | [nb 1] | |
| St | Tin | 50 | Current symbol is Sn. | [nb 1] | |
| Tn | Tungsten | 74 | Current symbol is W. | [nb 1] | |
| Tr | Terbium | 65 | Current symbol is Tb. | [nb 1] | |
| Tu | Thulium | 69 | Current symbol is Tm. | [nb 1] | |
| Tu | Tungsten | 74 | Current symbol is W. | [nb 1] | |
| Unb | Unnilbium | 102 | Temporary name given to nobelium until it was permanently named by IUPAC. | [nb 4] | |
| Une | Unnilennium | 109 | Temporary name given to meitnerium until it was permanently named by IUPAC. | [nb 4] | |
| Unh | Unnilhexium | 106 | Temporary name given to seaborgium until it was permanently named by IUPAC. | [nb 4] | |
| Uno | Unniloctium | 108 | Temporary name given to hassium until it was permanently named by IUPAC. | [nb 4] | |
| Unp | Unnilpentium | 105 | Temporary name given to dubnium until it was permanently named by IUPAC. | [nb 4] | |
| Unq | Unnilquadium | 104 | Temporary name given to rutherfordium until it was permanently named by IUPAC. | [nb 4] | |
| Uns | Unnilseptium | 107 | Temporary name given to bohrium until it was permanently named by IUPAC. | [nb 4] | |
| Unt | Unniltrium | 103 | Temporary name given to lawrencium until it was permanently named by IUPAC. | [nb 4] | |
| Unu | Unnilunium | 101 | Temporary name given to mendelevium until it was permanently named by IUPAC. | [nb 4] | |
| Uub | Ununbium | 112 | Temporary name given to copernicium until it was permanently named by IUPAC. | [nb 4] | |
| Uuh | Ununhexium | 116 | Temporary name given to livermorium until it was permanently named by IUPAC. | [nb 4] | |
| Uun | Ununnilium | 110 | Temporary name given to darmstadtium until it was permanently named by IUPAC. | [nb 4] | |
| Uuo | Ununoctium | 118 | Temporary name given to oganesson until it was permanently named by IUPAC. | [nb 4] | |
| Uup | Ununpentium | 115 | Temporary name given to moscovium until it was permanently named by IUPAC. | [nb 4] | |
| Uuq | Ununquadium | 114 | Temporary name given to flerovium until it was permanently named by IUPAC. | [nb 4] | |
| Uus | Ununseptium | 117 | Temporary name given to tennessine until it was permanently named by IUPAC. | [nb 4] | |
| Uut | Ununtrium | 113 | Temporary name given to nihonium until it was permanently named by IUPAC. | [nb 4] | |
| Uuu | Unununium | 111 | Temporary name given to roentgenium until it was permanently named by IUPAC. | [nb 4] | |
| Ur | Uranium | 92 | Current symbol is U. | [nb 1] | |
| Vi | Virginium | 87 | Discredited claim to discovery of francium. | [nb 2] | [7] |
| Vm | Virginium | 87 | Discredited claim to discovery of francium. | [nb 2] | [7] |
| Va | Vanadium | 23 | Current symbol is V. | [nb 1] | |
| Wo | Tungsten | 74 | Current symbol is W. | [nb 1] | |
| X | Xenon | 54 | Current symbol is Xe. The symbol is now used for halogens. | [nb 1] | |
| Yt | Yttrium | 39 | Current symbol is Y. | [nb 1] | [7] |
Pictographic symbols
The following is a list of pictographic symbols employed to symbolize elements known since ancient times (for example to the alchemists). Not included in this list are symbolic representations of substances previously called elements (such as certain rare-earth mineral blends and the classical elements fire and water of ancient philosophy) which are known today to be multi-atomic. Also not included are symbolic representations currently used for elements in other languages such as the Chinese characters for elements. Modern alphabetic notation was introduced in 1814 by Jöns Jakob Berzelius.[11]
| Chemical symbol | Original name | Modern name | Atomic number | Origin of symbol |
|---|---|---|---|---|
| ☉ | Hydrogen | Hydrogen | 1 | Daltonian symbol circa 1808. |
| ⬤ | Carbon | Carbon | 6 | Daltonian symbol circa 1808. |
| ⦶ | Azote | Nitrogen | 7 | Daltonian symbol circa 1808. |
| ◯ | Oxygen | Oxygen | 8 | Daltonian symbol circa 1808. |
| ⦷ | Soda | Sodium | 11 | Daltonian symbol circa 1808. |
| ⊛ | Magnesium | Magnesium | 12 | Alchemical symbol. Also adopted as the symbol for Magnesium by Dalton in 1808. |
| 🟕 | Silicon | Silicon | 14 | Daltonian symbol circa 1808. |
| Sulfur | Sulfur | 16 | Alchemical symbol. | |
| Pallas | Sulfur | 16 | Alchemical symbol. | |
| 🜍 | Sulfur | Sulfur | 16 | Alchemical symbol. |
| ⴲ | Sulfur | Sulfur | 16 | Daltonian symbol circa 1808. |
| ⦾ | Calcium | Calcium | 20 | Daltonian symbol circa 1808. |
| ♂ | Mars | Iron | 26 | Alchemical symbol. |
| Ⓘ | Iron | Iron | 26 | Daltonian symbol circa 1808. |
| Ⓝ | Nickel | Nickel | 28 | Daltonian symbol circa 1808. |
| Stellae Fixae | Copper | 29 | Pre–16th-century alchemical symbol. | |
| ♀ | Venus | Copper | 29 | Alchemical symbol. |
| Copper | Copper | 29 | Alchemical symbol. | |
| Ⓒ | Copper | Copper | 29 | Daltonian symbol circa 1808. |
| Ⓩ | Zinc | Zinc | 30 | Daltonian symbol circa 1808. |
| Arsenic | Arsenic | 33 | Alchemical symbol. | |
| 🜺 | Arsenic | Arsenic | 33 | Alchemical symbol. |
| ⊕ | Yttrium | Yttrium | 39 | Daltonian symbol circa 1808. |
| ☽ | Luna | Silver | 47 | Alchemical symbol. |
| 🜛 | Silver | Silver | 47 | Alchemical symbol. |
| Ⓢ | Silver | Silver | 47 | Daltonian symbol circa 1808. |
| ♃ | Iupiter | Tin | 50 | Alchemical symbol. |
| Ⓣ | Tin | Tin | 50 | Daltonian symbol circa 1808. |
| ♁ | Antimony | Antimony | 51 | Alchemical symbol. |
| ☉☾ | Platinum | Platinum | 78 | Alchemical symbol. |
| ⛢ | Uranus | Platinum | 78 | Alchemical symbol. |
| Ⓟ | Platinum | Platinum | 78 | Daltonian symbol circa 1808. |
| ☼ | Sol | Gold | 79 | Alchemical symbol from the 16th century. |
| ☉ | Sol | Gold | 79 | Alchemical symbol from 1700 through 1783. |
| 🜚 | Gold | Gold | 79 | Alchemical symbol. |
| Ⓖ | Gold | Gold | 79 | Daltonian symbol circa 1808. |
| ♓︎ | Pisces | Mercury | 80 | Pre–16th-century alchemical symbol. |
| ♆ | Neptunus | Mercury | 80 | Alchemical symbol from the 17th century. |
| ☿ | Mercurius | Mercury | 80 | Alchemical symbol from 1700 through 1783. |
| ♄ | Saturnus | Lead | 82 | Alchemical symbol circa 1783. |
| Ⓛ | Lead | Lead | 82 | Daltonian symbol circa 1808. |
| ♉︎ | Taurus | Bismuth | 83 | Alchemical symbol. |
| Ⓑ | Bismuth | Bismuth | 83 | Daltonian symbol circa 1808. |
| Ⓤ | Uranium | Uranium | 92 | Daltonian symbol circa 1808. |
Symbols for named isotopes
The following is a list of isotopes of elements given in the previous tables which have been designated unique symbols. By this it is meant that a comprehensive list of current systematic symbols (in the uAtom form) are not included in the list and can instead be found in the Isotope index chart. The symbols for the named isotopes of hydrogen, deuterium (D), and tritium (T) are still in use today, as is thoron (Tn) for radon-220 (though not actinon; An is usually used instead for a generic actinide). Heavy water and other deuterated solvents are commonly used in chemistry, and it is convenient to use a single character rather than a symbol with a subscript in these cases. The practice also continues with tritium compounds. When the name of the solvent is given, a lowercase d is sometimes used. For example, d6-benzene and C6D6 can be used instead of [2H6]C6H6.[12]
The symbols for isotopes of elements other than hydrogen and radon are no longer in use within the scientific community. Many of these symbols were designated during the early years of radiochemistry, and several isotopes (namely those in the decay chains of actinium, radium, and thorium) bear placeholder names using the early naming system devised by Ernest Rutherford.[13]
| Chemical symbol | Name | Atomic number | Origin of symbol |
|---|---|---|---|
| Ac | Actinium | 89 | From the Greek aktinos. Name restricted at one time to 227Ac, an isotope of actinium. This named isotope later became the official name for element 89. |
| AcA | Actinium A | 84 | From actinium and A. Placeholder name given at one time to 215Po, an isotope of polonium identified in the decay chain of actinium. |
| AcB | Actinium B | 82 | From actinium and B. Placeholder name given at one time to 211Pb, an isotope of lead identified in the decay chain of actinium. |
| AcC | Actinium C | 83 | From actinium and C. Placeholder name given at one time to 211Bi, an isotope of bismuth identified in the decay chain of actinium. |
| AcC' | Actinium C' | 84 | From actinium and C'. Placeholder name given at one time to 211Po, an isotope of polonium identified in the decay chain of actinium. |
| AcC" | Actinium C" | 81 | From actinium and C". Placeholder name given at one time to 207Tl, an isotope of thallium identified in the decay chain of actinium. |
| AcK | Actinium K | 87 | Name given at one time to 223Fr, an isotope of francium identified in the decay chain of actinium. |
| AcU | Actino-uranium | 92 | Name given at one time to 235U, an isotope of uranium. |
| AcX | Actinium X | 88 | Name given at one time to 223Ra, an isotope of radium identified in the decay chain of actinium. |
| An | Actinon | 86 | From actinium and emanation. Name given at one time to 219Rn, an isotope of radon identified in the decay chain of actinium. |
| D | Deuterium | 1 | From the Greek deuteros. Name given to 2H. |
| Io | Ionium | 90 | Name given to 230Th, an isotope of thorium identified in the decay chain of uranium. |
| MsTh1 | Mesothorium 1 | 88 | Name given at one time to 228Ra, an isotope of radium. |
| MsTh2 | Mesothorium 2 | 89 | Name given at one time to 228Ac, an isotope of actinium. |
| Pa | Protactinium | 91 | From the Greek protos and actinium. Name restricted at one time to 231Pa, an isotope of protactinium. This named isotope later became the official name for element 91. |
| Ra | Radium | 88 | From the Latin radius. Name restricted at one time to 226Ra, an isotope of radium. This named isotope later became the official name for element 88. |
| RaA | Radium A | 84 | From radium and A. Placeholder name given at one time to 218Po, an isotope of polonium identified in the decay chain of radium. |
| RaB | Radium B | 82 | From radium and B. Placeholder name given at one time to 214Pb, an isotope of lead identified in the decay chain of radium. |
| RaC | Radium C | 83 | From radium and C. Placeholder name given at one time to 214Bi, an isotope of bismuth identified in the decay chain of radium. |
| RaC' | Radium C' | 84 | From radium and C'. Placeholder name given at one time to 214Po, an isotope of polonium identified in the decay chain of radium. |
| RaC" | Radium C" | 81 | From radium and C". Placeholder name given at one time to 210Tl, an isotope of thallium identified in the decay chain of radium. |
| RaD | Radium D | 82 | From radium and D. Placeholder name given at one time to 210Pb, an isotope of lead identified in the decay chain of radium. |
| RaE | Radium E | 83 | From radium and E. Placeholder name given at one time to 210Bi, an isotope of bismuth identified in the decay chain of radium. |
| RaE" | Radium E" | 81 | From radium and E". Placeholder name given at one time to 206Tl, an isotope of thallium identified in the decay chain of radium. |
| RaF | Radium F | 84 | From radium and F. Placeholder name given at one time to 210Po, an isotope of polonium identified in the decay chain of radium. |
| RdAc | Radioactinium | 90 | Name given at one time to 227Th, an isotope of thorium. |
| RdTh | Radiothorium | 90 | Name given at one time to 228Th, an isotope of thorium. |
| Rn | Radon | 86 | From radium and emanation. Name restricted at one time to 222Rn, an isotope of radon identified in the decay chain of radium. This named isotope later became the official name for element 86 in 1923. |
| T | Tritium | 1 | From the Greek tritos. Name given to 3H. |
| Th | Thorium | 90 | After Thor. Name restricted at one time to 232Th, an isotope of thorium. This named isotope later became the official name for element 90. |
| ThA | Thorium A | 84 | From thorium and A. Placeholder name given at one time to 216Po, an isotope of polonium identified in the decay chain of thorium. |
| ThB | Thorium B | 82 | From thorium and B. Placeholder name given at one time to 212Pb, an isotope of lead identified in the decay chain of thorium. |
| ThC | Thorium C | 83 | From thorium and C. Placeholder name given at one time to 212Bi, an isotope of bismuth identified in the decay chain of thorium. |
| ThC' | Thorium C' | 84 | From thorium and C'. Placeholder name given at one time to 212Po, an isotope of polonium identified in the decay chain of thorium. |
| ThC" | Thorium C" | 81 | From thorium and C". Placeholder name given at one time to 208Tl, an isotope of thallium identified in the decay chain of thorium. |
| ThX | Thorium X | 88 | Name given at one time to 224Ra, an isotope of radium identified in the decay chain of thorium. |
| Tn | Thoron | 86 | From thorium and emanation. Name given at one time to 220Rn, an isotope of radon identified in the decay chain of thorium. |
| UI | Uranium I | 92 | Name given at one time to 238U, an isotope of uranium. |
| UII | Uranium II | 92 | Name given at one time to 234U, an isotope of uranium. |
| UX1 | Uranium X1 | 90 | Name given at one time to 234Th, an isotope of thorium identified in the decay chain of uranium. |
| UX2 | Uranium X2 | 91 | Name given at one time to 234mPa, an isotope of protactinium identified in the decay chain of uranium. |
| UY | Uranium Y | 90 | Name given at one time to 231Th, an isotope of thorium identified in the decay chain of uranium. |
| UZ | Uranium Z | 91 | Name given at one time to 234Pa, an isotope of protactinium identified in the decay chain of uranium. |
Other symbols
General:
- A: A deprotonated acid or an anion
- An: any actinide
- B: A base, often in the context of Lewis acid–base theory or Brønsted–Lowry acid–base theory
- E: any element or electrophile
- L: any ligand
- Ln: any lanthanide
- M: any metal
- Mm: mischmetall (occasionally used)[14]
- Ng: any noble gas (Rg is sometimes used, but that is also used for the element roentgenium: see above)
- Nu: any nucleophile
- R: any unspecified radical (moiety) not important to the discussion
- St: steel (occasionally used)
- X: any halogen (or sometimes pseudohalogen)
From organic chemistry:
- Ac: acetyl – (also used for the element actinium: see above)
- Ad: 1-adamantyl
- All: allyl
- Am: amyl (pentyl) – (also used for the element americium: see above)
- Ar: aryl – (also used for the element argon: see above)
- Bn: benzyl
- Bs: brosyl or (outdated) benzenesulfonyl
- Bu: butyl (i-, s-, or t- prefixes may be used to denote iso-, sec-, or tert- isomers, respectively)
- Bz: benzoyl
- Cp: cyclopentadienyl
- Cp*: pentamethylcyclopentadienyl
- Cy: cyclohexyl
- Cyp: cyclopentyl
- Et: ethyl
- Me: methyl
- Mes: mesityl (2,4,6-trimethylphenyl)
- Ms: mesyl (methylsulfonyl)
- Np: neopentyl – (also used for the element neptunium: see above)
- Ns: nosyl
- Pent: pentyl
- Ph, Φ: phenyl
- Pr: propyl – (i- prefix may be used to denote isopropyl. Also used for the element praseodymium: see above)
- R: In organic chemistry contexts, an unspecified "R" is often understood to be an alkyl group
- Tf: triflyl (trifluoromethanesulfonyl)
- Tr, Trt: trityl (triphenylmethyl)
- Ts, Tos: tosyl (para-toluenesulfonyl) – (Ts also used for the element tennessine: see above)
- Vi: vinyl
Exotic atoms:
- Mu: muonium
- Pn: protonium
- Ps: positronium
See also
Notes
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba Name changed due to a standardization of, modernization of, or update to older formerly-used symbol.
- ^ a b c d e f g h i j k l m n o p q r s t Name designated by discredited/disputed claimant.
- ^ a b c d e f g h i j k l m n o p q r Name proposed prior to discovery/creation of element or prior to official re-naming of a placeholder name.
- ^ a b c d e f g h i j k l m n o p q r s t u v w Temporary placeholder name.
References
- ^ IUPAC Provisional Recommendations: IR-3: Elements and Groups of Elements (PDF) (Report). IUPAC. March 2004.
- ^ "Periodic Table – Royal Society of Chemistry". www.rsc.org.
- ^ "Online Etymology Dictionary". etymonline.com.
- ^ Wieser, Michael E.; Holden, Norman (2013). Atomic weights of the elements 2011 (IUPAC Technical Report). Pure Appl. Chem. (Report). Vol. 85. pp. 1047–1078. doi:10.1351/PAC-REP-13-03-02.
{{cite report}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) (for standard atomic weights of elements) - ^ Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brookhaven National Laboratory. Retrieved 2008-06-06. (for atomic weights of elements with atomic numbers 103–118)
- ^ a b Holden, N. E. (12 March 2004). "History of the Origin of the Chemical Elements and Their Discoverers". National Nuclear Data Center.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac Leal, João P. (2013). "The Forgotten Names of Chemical Elements". Foundations of Science. 19 (2): 175–183. doi:10.1007/s10699-013-9326-y.
- ^ a b "Fred Allison". Encyclopedia of Alabama.
- ^ a b c d e Fontani, Marco; Costa, Mariagrazia; Orna, Mary Virginia (2014). The Lost Elements: The Periodic Table's Shadow Side. Oxford University Press. ISBN 9780199383344.
- ^ a b Praseodymium on was.chemistryexplained.com.
- ^ Berzelius, Jöns Jakob. "Essay on the Cause of Chemical Proportions, and on Some Circumstances Relating to Them: Together with a Short and Easy Method of Expressing Them." Annals of Philosophy 2, Pp.443–454 (1813); 3, Pp.51–52, 93–106, 244–255, 353–364 (1814); (Subsequently republished in "A Source Book in Chemistry, 1400-1900", eds. Leicester, Henry M. & Herbert S. Klickstein. 1952.)
- ^ IUPAC. "Isotopically Modified Compounds". IUPAC. Retrieved 31 March 2015.
- ^ Morgan, G. T., ed. (1905). "Annual Reports on the Progress of Chemistry for 1904". Journal of the Chemical Society. 1. Gurney & Jackson: 268.
In view of the extraordinarily complex nature of the later changes occurring in Radium, Rutherford has proposed a new and convenient system of nomenclature. The first product of the change of the radium emanation is named radium A, the next radium B, and so on.
- ^ Jurczyk, M.; Rajewski, W.; Majchrzycki, W.; Wójcik, G. (1999-08-30). "Mechanically alloyed MmNi5-type materials for metal hydride electrodes". Journal of Alloys and Compounds. 290 (1–2): 262–266. doi:10.1016/S0925-8388(99)00202-9.
- Elementymology & Elements Multidict, element name etymologies. Retrieved July 15, 2005.
- Atomic Weights of the Elements 2001, Pure Appl. Chem. 75(8), 1107–1122, 2003. Retrieved June 30, 2005. Atomic weights of elements with atomic numbers from 1–109 taken from this source.
- IUPAC Standard Atomic Weights Revised (2005).
- WebElements Periodic Table. Retrieved June 30, 2005. Atomic weights of elements with atomic numbers 110–116 taken from this source.
- Lapp, Ralph E. Matter. Life Science Library. New York: Time, Inc. 1963.
- Leighton, Robert B. Principles of Modern Physics. New York: McGraw-Hill. 1959.
- Scerri, E.R. "The Periodic Table, Its Story and Its Significance". New York, Oxford University Press. 2007.
