Acesulfame potassium
| |

| |
| Names | |
|---|---|
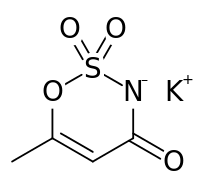
| IUPAC name
potassium 6-methyl-2,2-dioxo-2H-1,2λ6,3-oxathiazin-4-olate
| |
| Other names
Acesulfame K
Ace K
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.054.269 |
| EC Number |
|
| E number | E950 (glazing agents, ...) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H4KNO4S | |
| Molar mass | 201.242 |
| Appearance | white crystalline powder |
| Density | 1.81 g/cm3 |
| Melting point | 225 °C (437 °F; 498 K) |
| 270 g/L at 20 °C | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Acesulfame potassium (ace-SUHL-faym), also known as acesulfame K (K is the symbol for potassium) or Ace K, is a calorie-free sugar substitute (artificial sweetener), and marketed under the trade names Sunett and Sweet One. In the European Union, it is known under the E number (additive code) E950.[1] It was discovered accidentally in 1967 by German chemist Karl Clauss at Hoechst AG (now Nutrinova).[2] In chemical structure, acesulfame potassium is the potassium salt of 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide. It is a white crystalline powder with molecular formula C4H4KNO4S and a molecular weight of 201.24 g/mol.[3]
Properties
Acesulfame K is 200 times sweeter than sucrose (common sugar), as sweet as aspartame, about 2/3 as sweet as saccharin, and 1/3 as sweet as sucralose. Like saccharin, it has a slightly bitter aftertaste, especially at high concentrations. Kraft Foods patented the use of sodium ferulate to mask acesulfame's aftertaste.[4] Acesulfame K is often blended with other sweeteners (usually sucralose or aspartame). These blends are reputed[by whom?] to give a more sucrose-like taste whereby each sweetener masks the other's aftertaste, or exhibits a synergistic effect by which the blend is sweeter than its components. Acesulfame potassium has a smaller particle size than sucrose, allowing for its mixtures with other sweeteners to be more uniform.[5]
Unlike aspartame, acesulfame K is stable under heat, even under moderately acidic or basic conditions, allowing it to be used as a food additive in baking, or in products that require a long shelf life. Although acesulfame potassium has a stable shelf life, it can eventually degrade to acetoacetamide, which is toxic in high doses.[6] In carbonated drinks, it is almost always used in conjunction with another sweetener, such as aspartame or sucralose. It is also used as a sweetener in protein shakes and pharmaceutical products,[7] especially chewable and liquid medications, where it can make the active ingredients more palatable. The acceptable daily intake of acesulfame potassium is listed as 15 mg/kg/day.[8]
Discovery
Acesulfame potassium was developed after the accidental discovery of a similar compound (5,6-dimethyl-1,2,3-oxathiazin-4(3H)-one 2,2-dioxide) in 1967 by Karl Clauss and Harald Jensen at Hoechst AG.[9][10] After accidentally dipping his fingers into the chemicals with which he was working, Clauss licked them to pick up a piece of paper.[11] Clauss is the inventor listed on a United States patent issued in 1975 to the assignee Hoechst Aktiengesellschaft for one process of manufacturing acesulfame potassium.[12] Subsequent research showed a number of compounds with the same basic ring structure had varying levels of sweetness. 6-methyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide had particularly favourable taste characteristics and was relatively easy to synthesize, so it was singled out for further research, and received its generic name (acesulfame-K) from the World Health Organization in 1978.[9] Acesulfame potassium first received approval for table top use in the United States in 1988.[8]
Safety
As with other artificial sweeteners, concern exists over the safety of acesulfame potassium. However, the United States Food and Drug Administration (FDA) has approved its general use. Critics say acesulfame potassium has not been studied adequately and may be carcinogenic,[13] although these claims have been dismissed by the FDA[14] and equivalent authorities in the European Union.[15]
As for potential negative effects, when injected directly in very large doses (the equivalent of 10 g for an average sized human male), acesulfame K has been shown to stimulate dose-dependent insulin secretion in rats, though no hypoglycemia was observed.[16]
One rodent study showed no increased incidence of tumors in response to administration of acesulfame K.[17] In this study, conducted by the National Toxicology Program, 60 rats were given acesulfame K for 40 weeks, making up as much as 3% of their total diet (which would be equivalent to a human consuming 1,343 12 oz cans of artificially sweetened soft drinks every day). No sign indicated these (or lower) levels of acesulfame K increased the rats' risk of cancer or other neoplasms. However, a similar study conducted with p53 haploinsufficient mice showed signs of carcinogenicity in males but not females.[17] Further food safety research has been recommended.[13][18] Acesulfame K did not show any DNA-damaging properties.[19]
Research suggests acesulfame K may affect prenatal development. One study appeared to show acesulfame K is ingested by mice through their mothers' amniotic fluid or breast milk, and this influences the adult mouse's sweet preference.[20]
Additional research on the effects of acesulfame K on mice revealed chronic use over a period of 40 weeks resulted in a moderate but limited effect on neurometabolic function. These results suggest chronic usage of acesulfame K may alter neurological function.[21]
Environment Canada tested the water from the Grand River at 23 sites between its headwaters and where it dumps into Lake Erie. The results suggest the artificial sweetener acesulfame is the best at evading wastewater treatment, and it appears in far higher concentrations than saccharin or sucralose at the various test sites.[22]
Compendial status
References
- ^ "Current EU approved additives and their E Numbers". UK: Food Standards Agency. 2012-03-14.
- ^ Clauss, K.; Jensen, H. (1973). "Oxathiazinone Dioxides - A New Group of Sweetening Agents". Angewandte Chemie International Edition. 12 (11): 869–876. doi:10.1002/anie.197308691.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ager, D. J.; Pantaleone, D. P.; Henderson, S. A.; Katritzky, A. R.; Prakash, I.; Walters, D. E. (1998). "Commercial, Synthetic Nonnutritive Sweeteners" (PDF). Angewandte Chemie International Edition. 37 (13–14): 1802–1817. doi:10.1002/(SICI)1521-3773(19980803)37:13/14<1802::AID-ANIE1802>3.0.CO;2-9.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ United States Patent 5,336,513 (expired in 2006)
- ^ Mullarney, M.; Hancock, B.; Carlson, G.; Ladipo, D.; Langdon, B. The powder flow and compact mechanical properties of sucrose and three high-intensity sweeteners used in chewable tablets. Int. J. Pharm. 2003, 257, 227–236.
- ^ Findikli, Z.; Zeynep, F.; Sifa, T. Determination of the effects of some artificial sweeteners on human peripheral lymphocytes using the comet assay. Journal of toxicology and environmental health sciences 2014, 6, 147–153.
- ^ http://www.who.int/prequal/trainingresources/pq_pres/TrainingZA-April07/Excipients.ppt
- ^ a b Whitehouse, C.; Boullata, J.; McCauley, L. The potential toxicity of artificial sweeteners. AAOHN J. 2008, 56, 251-9 quiz 260.
- ^ a b O'Brien-Nabors, L. (2001). Alternative Sweeteners. New York, NY: Marcel Dekker. p. 13. ISBN 0-8247-0437-1.
- ^ Williams, R. J.; Goldberg, I. (1991). Biotechnology and Food Ingredients. New York: Van Nostrand Reinhold. ISBN 0-442-00272-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Newton, D. E. (2007). Food Chemistry (New Chemistry). New York: Infobase Publishing. p. 69. ISBN 0-8160-5277-8.
- ^ Clauss, K. Process for the manufacture of 6-methyl-3,4-dihydro-1,2,3-oxathiazine-4-one-2,2-dioxide. US Patent 3917589, 1975.
- ^ a b Karstadt, M. L. (2006). "Testing Needed for Acesulfame Potassium, an Artificial Sweetener" (PDF). Environmental Health Perspectives. 114 (9): A516. doi:10.1289/ehp.114-a516a. PMC 1570055. PMID 16966071.
- ^ Kroger, M.; Meister, K.; Kava, R. (2006). "Low-Calorie Sweeteners and Other Sugar Substitutes: A Review of the Safety Issues". Comprehensive Reviews in Food Science and Food Safety. 5 (2): 35–47. doi:10.1111/j.1541-4337.2006.tb00081.x.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Scientific Committee on Food (2000). "Opinion - Re-evaluation of acesulfame K with reference to the previous SCF opinion of 1991" (PDF). SCF/CS/ADD/EDUL/194 final. EU Commission.
- ^ Liang, Y.; Steinbach, G.; Maier, V.; Pfeiffer, E. F. (1987). "The Effect of Artificial Sweetener on Insulin Secretion. 1. The Effect of Acesulfame K on Insulin Secretion in the Rat (Studies in Vivo)". Hormone and Metabolic Research. 19 (6): 233–238. doi:10.1055/s-2007-1011788. PMID 2887500.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b National Toxicology Program (2005). "Toxicity Studies of Acesulfame Potassium (CAS No. 55589-62-3) in FVB/N-TgN(v-Ha-ras)Led (Tg.AC) Hemizygous Mice and Carcinogenicity Studies of Acesulfame Potassium in B6.129-Trp53tm1Brd (N5) Haploinsufficient Mice (Feed Studies)" (PDF). Genetically Modified Model Report. 2005 (NTP GMM-2). National Institutes of Health: 1–113. PMID 18784762. NIH Publication No. 06-4460.
- ^ Soffritti, M. (2006). "Acesulfame Potassium: Soffritti Responds" (PDF). Environmental Health Perspectives. 114 (9): A516–A517. doi:10.1289/ehp.114-a516b. PMC 1570058.
- ^ "Artificial sweeteners—do they bear a carcinogenic risk?". Ann Oncol. 15 (10): 1460–5. 2004. PMID 15367404.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Zhang, G. H.; Chen, M. L.; Liu, S. S.; Zhan, Y. H.; Quan, Y.; Qin, Y. M.; Deng, S. P. (2011). "Effects of Mother's Dietary Exposure to Acesulfame-K in Pregnancy or Lactation on the Adult Offspring's Sweet Preference". Chemical Senses. 36 (9): 763–770. doi:10.1093/chemse/bjr050. PMID 21653241.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cong W-n, Wang R, Cai H, Daimon CM, Scheibye-Knudsen M; et al. (2013). "Long-Term Artificial Sweetener Acesulfame Potassium Treatment Alters Neurometabolic Functions in C57BL/6J Mice". PLOS ONE. 8 (8): e70257. doi:10.1371/journal.pone.0070257.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ "Major Canadian river contains artificial sweeteners". Waterloo News. University of Waterloo. December 13, 2013.
- ^ British Pharmacopoeia Commission Secretariat (2009). "Index, BP 2009" (PDF).
External links
This article's use of external links may not follow Wikipedia's policies or guidelines. (December 2015) |
- Joint FAO/WHO Expert Committee on Food Additives evaluation monograph of Acesulfame Potassium
- FDA approval of Acesulfame Potassium
- FDA approval of Acesulfame Potassium as a General Purpose Sweetener in Food
- Elmhurst College, Illinois Virtual ChemBook Acesulfame K
- Hazardous substances databank entry at the national library of medicine (outdated source)
- Discovery News Sweeteners Linger in Groundwater

