Cobalt(II,III) oxide

| |
| |
| Names | |
|---|---|
| IUPAC name
cobalt(II) dicobalt(III) oxide
| |
| Other names
cobalt oxide, cobalt(II,III) oxide, cobaltosic oxide, tricobalt tetroxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.780 |
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Co3O4 CoO.Co2O3 | |
| Molar mass | 240.80 g/mol |
| Appearance | black solid |
| Density | 6.11 g/cm3 |
| Melting point | 895 °C (1,643 °F; 1,168 K) |
| Boiling point | 900 °C (1,650 °F; 1,170 K) (decomposes) |
| Insoluble | |
| Solubility | soluble in acids and alkalis |
| Structure | |
| cubic | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
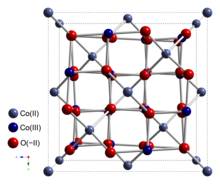
Cobalt(II,III) oxide is inorganic compound with the formula Co3O4. It is one of two well characterized cobalt oxides. It is a black antiferromagnetic solid. As a mixed valence compound, its formula is sometimes written as CoIICoIII2O4 and sometimes as CoO•Co2O3.[2]
Structure
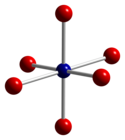
Co3O4 adopts the normal spinel structure, with Co2+ ions in tetrahedral interstices and Co3+ ions in the octahedral interstices of the cubic close-packed lattice of oxide anions.[2]
 |
 |
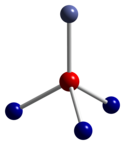 |
| tetrahedral coordination geometry of Co(II) | distorted octahedral coordination geometry of Co(III) | distorted tetrahedral coordination geometry of O |
Synthesis
Cobalt(II) oxide, CoO, converts to Co3O4 if heated to around 600-700 °C in air.[3] Above 900 °C, CoO is stable.[3][4] These reaction are described by the following equilibrium:
- 2 Co3O4 ⇌ 6 CoO + O2
Research
This inorganic compound is currently utilized in the process of artificial photosynthesis.[citation needed]
Safety
Cobalt compounds are potentially poisonous in large amounts.[5]
See also
References
- ^ Sigma-Aldrich product page
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1118. ISBN 978-0-08-037941-8.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1118. ISBN 978-0-08-037941-8.
- ^ Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. p. 1520.
- ^ MSDS

