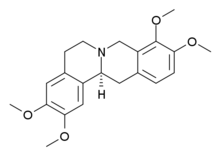
Tetrahydropalmatine
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.241.370 |
| Chemical and physical data | |
| Formula | C21H25NO4 |
| Molar mass | 355.428 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tetrahydropalmatine (THP) is an alkaloid found in several different plant species, mainly in the Corydalis genus (Yan Hu Suo),[1][2] but also in other plants such as Stephania rotunda.[3] These plants have traditional uses in Chinese herbal medicine. The pharmaceutical industry has synthetically produced the more potent enantiomer Levo-tetrahydropalmatine (Levo-THP), which has been marketed worldwide under different brand names as an alternative to anxiolytic and sedative drugs of the benzodiazepine group and analgesics such as opiates. It is also sold as a dietary supplement.
Effects
Tetrahydropalmatine has been demonstrated to possess analgesic effects and may be beneficial in the treatment of heart disease and liver damage.[4][5] It is a blocker of voltage-activated L-type calcium channel active potassium channels.[citation needed] It is a potent muscle relaxant.[citation needed] It has also shown potential in the treatment of drug addiction to both cocaine and opiates, and preliminary human studies have shown promising results.[6][7][8]
Animal experiments have shown that the sedative effect of THP results from blocking dopaminergic neurons in the brain. Dopamine is an important neurotransmitter in the central nervous system where it occurs in several important signaling systems that regulate muscular activity and attention, as well as feelings of joy, enthusiasm, and creativity. Therefore, THP causes no feelings of euphoria, and has been seen as an alternative to addictive drugs for people suffering from anxiety and pain, and as a possibility for relief for people not helped by existing drugs.[citation needed]
Cases of poisoning
Several cases of poisoning related to THP have been reported.[9] These cases involved negative effects on respiration, cardiac activity, and the nervous system. In addition, chronic hepatitis has been reported, caused by THP production in East Asia under conditions that were insufficiently sterile. Fatalities started to be reported in 1999 in cases where THP had been used in combination with other drugs having analgesic and anti-anxiety effects. All 1999 deaths could be tied to a single THP-based supplement, sold under the name "Jin Bu Huan Anodyne Tablets". This product was therefore blacklisted by US and European health authorities. In some other countries, such as Singapore, THP is treated as a controlled substance, and license is required to sell it.[citation needed]
See also
References
- ^ Sutin, EL; Jacobowitz, DM (1991). "Neurochemicals in the dorsal pontine tegmentum". Prog. Brain Res. 88: 3–14. doi:10.1016/S0079-6123(08)63796-6. PMID 1726029.
- ^ Ma, ZJ; Li, XD; Gu, XZ; Cheng, LP; Mao, SJ (March 2006). "[Effects of different types and standard of processing vinegaron inherent constituents in rhizoma of Corydalis yanhusuo]". Zhongguo Zhong Yao Za Zhi. 31 (6): 465–7. PMID 16722373.
- ^ Andersson, C; Bergarp, E; Hedman, G (January 1992). "[Sick-listed but active]". Lakartidningen. 89 (5): 281–3. PMID 1738250.
- ^ Wu, L; Ling, H; Li, L; Jiang, L; He, M (2007). "Beneficial effects of the extract from Corydalis yanhusuo in rats with heart failure following myocardial infarction". The Journal of pharmacy and pharmacology. 59 (5): 695–701. doi:10.1211/jpp.59.5.0010. PMID 17524235.
- ^ Min, Q; Bai, YT; Shu, SJ; Ren, P (2006). "Protective effect of dl-tetrahydropalmatine on liver injury induced by carbon tetrachloride in mice". Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. 31 (6): 483–4, 521. PMID 16722379.
- ^ Mantsch, JR; Li, SJ; Risinger, R; Awad, S; Katz, E; Baker, DA; Yang, Z (2007). "Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats". Psychopharmacology. 192 (4): 581–91. doi:10.1007/s00213-007-0754-7. PMID 17361394.
- ^ Chu, H; Jin, G; Friedman, E; Zhen, X (2008). "Recent development in studies of tetrahydroprotoberberines: Mechanism in antinociception and drug addiction". Cellular and molecular neurobiology. 28 (4): 491–9. doi:10.1007/s10571-007-9179-4. PMID 17710533.
- ^ Yang, Z; Shao, YC; Li, SJ; Qi, JL; Zhang, MJ; Hao, W; Jin, GZ (2008). "Medication of l-tetrahydropalmatine significantly ameliorates opiate craving and increases the abstinence rate in heroin users: A pilot study". Acta pharmacologica Sinica. 29 (7): 781–8. doi:10.1111/j.1745-7254.2008.00817.x. PMID 18565275.
- ^ Lai, Chi-Kong; Yan-Wo Chan, Albert (1999). "Tetrahydropalmatine Poisoning: Diagnoses of Nine Adult Overdoses Based on Toxicology Screens by HPLC with Diode-Array Detection and Gas Chromatography–Mass Spectrometry". Clinical Chemistry. 45 (2): 229–236. PMID 9931045.
