Origin recognition complex
| Origin recognition complex subunit 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | ORC2 | ||||||||
| Pfam | PF04084 | ||||||||
| InterPro | IPR007220 | ||||||||
| |||||||||
| Origin recognition complex (ORC) subunit 3 N-terminus | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | ORC3_N | ||||||||
| Pfam | PF07034 | ||||||||
| InterPro | IPR010748 | ||||||||
| |||||||||
| Origin recognition complex subunit 6 (ORC6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | ORC6 | ||||||||
| Pfam | PF05460 | ||||||||
| InterPro | IPR008721 | ||||||||
| |||||||||
In molecular biology, origin recognition complex (ORC) is a multi-subunit DNA binding complex (6 subunits) that binds in all eukaryotes and archaea in an ATP-dependent manner to origins of replication. The subunits of this complex are encoded by the ORC1, ORC2, ORC3, ORC4, ORC5 and ORC6 genes.[1][2][3] ORC is a central component for eukaryotic DNA replication, and remains bound to chromatin at replication origins throughout the cell cycle.[4]
ORC directs DNA replication throughout the genome and is required for its initiation.[5][6][7] ORC and Noc3p bound at replication origins serve as the foundation for assembly of the pre-replication complex (pre-RC), which includes Cdc6, Tah11 (a.k.a. Cdt1), and the Mcm2-Mcm7 complex.[8][9][10][11] Pre-RC assembly during G1 is required for replication licensing of chromosomes prior to DNA synthesis during S phase.[12][13][14] Cell cycle-regulated phosphorylation of Orc2, Orc6, Cdc6, and MCM by the cyclin-dependent protein kinase Cdc28 regulates initiation of DNA replication, including blocking reinitiation in G2/M phase.[4][15][16][17]
The ORC is present throughout the cell cycle bound to replication origins, but is only active in late mitosis and early G1.
In yeast, ORC also plays a role in the establishment of silencing at the mating-type loci Hidden MAT Left (HML) and Hidden MAT Right (HMR).[5][6][7] ORC participates in the assembly of transcriptionally silent chromatin at HML and HMR by recruiting the Sir1 silencing protein to the HML and HMR silencers.[7][18][19]
Both Orc1 and Orc5 bind ATP, though only Orc1 has ATPase activity.[20] The binding of ATP by Orc1 is required for ORC binding to DNA and is essential for cell viability.[11] The ATPase activity of Orc1 is involved in formation of the pre-RC.[21][22][23] ATP binding by Orc5 is crucial for the stability of ORC as a whole. Only the Orc1-5 subunits are required for origin binding; Orc6 is essential for maintenance of pre-RCs once formed.[24] Interactions within ORC suggest that Orc2-3-6 may form a core complex.[4] A 2020 report suggests that budding yeast ORC dimerizes in a cell cycle dependent manner to control licensing.[25][26]
Proteins
[edit]The following proteins are present in the ORC:
| S. cerevisiae | S. pombe | D. melanogaster | Vertebrates |
|---|---|---|---|
| ORC 1-6 | ORC 1-6 | ORC 1-6 | ORC 1-6 |
| Cdc6 | Cdc18 | Cdc6 | Cdc6 |
| Cdt1/Tah11/Sid2 | Cdt1 | DUP | Cdt1/RLF-B |
| Mcm2 | Mcm2/Cdc19/Nda1 | Mcm2 | Mcm2 |
| Mcm3 | Mcm3 | Mcm3 | Mcm3 |
| Cdc54/Mcm4 | Cdc21 | DPA | Mcm4 |
| Cdc46/Mcm5 | Mcm5/Nda4 | Mcm5 | Mcm5 |
| Mcm6 | Mcm6/Mis5 | Mcm6 | Mcm6 |
| Cdc47/Mcm7 | Mcm7 | Mcm7 | mcm7 |
Archaea feature a simplified version of the ORC, Mcm, and as a consequence the combined pre-RC. Instead of using six different mcm proteins to form a pseudo-symmetrical heterohexamer, all six subunits in the archaeal MCM are the same. They usually have multiple proteins that are homologous to both Cdc6 and Orc1, some of which perform the function of both. Unlike eukaryotic Orc, they do not always form a complex. In fact, they have divergent complex structures when these do form. Sulfolobus islandicus also uses a Cdt1 homologue to recognize one of its replication origins.[28]
Autonomously replicating sequences
[edit]Budding yeast
[edit]Autonomously Replicating Sequences (ARS), first discovered in budding yeast, are integral to the success of the ORC. These 100-200bp sequences facilitate replication activity during S phase. ARSs can be placed at any novel location of the chromosomes of budding yeast and will facilitate replication from those sites. A highly conserved sequence of 11bp (known as the A element) is thought to be essential for origin function in budding yeast.[27] The ORC was originally identified by its ability to bind to the A element of the ARS in budding yeast.
Animals
[edit]Animal cells contain a much more cryptic version of an ARS, with no conserved sequences found as of yet. Here, replication origins gather into bundles called replicon clusters. Each cluster's replicons are similar in length, but individual clusters have replicons of varying length. These replicons all have similar basic residues to which the ORC binds, which in many ways mimic the conserved 11bp A element. All of these clusters are simultaneously activated during S phase.[27]
Role in pre-RC assembly
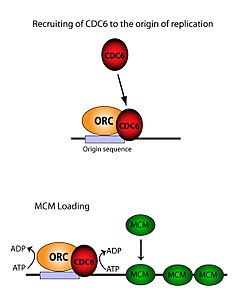
[edit]The ORC is essential for the loading of MCM complexes (Pre-RC) onto DNA. This process is dependent on the ORC, Noc3, Cdc6, and Cdt1 – involving several ATP controlled recruiting events. First, the ORC, Noc3p and Cdc6 form a complex on origin DNA (marked by ARS type regions). New ORC/Noc3/Cdc6 complexes then recruit Cdt1/Mcm2-7 molecules to the site. Once this massive ORC/Noc3/Cdc6/Cdt1/Mcm2-7 complex is formed, the ORC/Noc3/Cdc6/Cdt1 molecules work together to load Mcm2-7 onto the DNA itself by hydrolysis of ATP by Cdc6. Cdc6's phosphorylative activity is dependent on both the ORC and origin DNA. This leads to Cdt1 having decreased stability on the DNA and falling off of the complex leading to Mcm2-7 loading on to the DNA.[29][27][30][31] The structure of the ORC, MCM, as well as the intermediate OCCM complex has been resolved.[32]
Origin binding activity
[edit]Although the ORC is composed of six discrete subunits, only one of these has been found to be significant - ORC1. In vivo studies have shown that Lys-263 and Arg-367 are the basic residues responsible for faithful ORC loading. These molecules represent the above-mentioned ARS.[33] ORC1 interacts with ATP and these basic residues in order to bind the ORC to origin DNA. It has been established that this occurs far before replication, and that the ORC itself is already bound to Origin DNA by the time any Mcm2-7 loading occurs.[31] When Mcm2-7 is first loaded it completely encircles the DNA and helicase activity is inhibited. In S phase, the Mcm2-7 complex interacts with helicase cofactors Cdc45 and GINS to isolate a single DNA strand, unwind the origin, and begin replication down the chromosome. In order to have bidirectional replication, this process happens twice at an origin. Both loading events are mediated by one ORC via an identical process as the first.[34]
See also
[edit]References
[edit]- ^ Origin+Recognition+Complex at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ^ Dutta A, Bell SP (1997). "Initiation of DNA replication in eukaryotic cells". Annual Review of Cell and Developmental Biology. 13: 293–332. doi:10.1146/annurev.cellbio.13.1.293. PMID 9442876.
- ^ Chesnokov IN (2007). Multiple functions of the origin recognition complex. International Review of Cytology. Vol. 256. pp. 69–109. doi:10.1016/S0074-7696(07)56003-1. ISBN 9780123737007. PMID 17241905.
- ^ a b c Matsuda K, Makise M, Sueyasu Y, Takehara M, Asano T, Mizushima T (December 2007). "Yeast two-hybrid analysis of the origin recognition complex of Saccharomyces cerevisiae: interaction between subunits and identification of binding proteins". FEMS Yeast Research. 7 (8): 1263–9. doi:10.1111/j.1567-1364.2007.00298.x. PMID 17825065.
- ^ a b Bell SP, Stillman B (May 1992). "ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex". Nature. 357 (6374): 128–34. Bibcode:1992Natur.357..128B. doi:10.1038/357128a0. PMID 1579162. S2CID 4346767.
- ^ a b Bell SP, Mitchell J, Leber J, Kobayashi R, Stillman B (November 1995). "The multidomain structure of Orc1p reveals similarity to regulators of DNA replication and transcriptional silencing". Cell. 83 (4): 563–8. doi:10.1016/0092-8674(95)90096-9. PMID 7585959.
- ^ a b c Gibson DG, Bell SP, Aparicio OM (June 2006). "Cell cycle execution point analysis of ORC function and characterization of the checkpoint response to ORC inactivation in Saccharomyces cerevisiae". Genes to Cells. 11 (6): 557–73. doi:10.1111/j.1365-2443.2006.00967.x. PMID 16716188. S2CID 22439595.
- ^ Zhang Y, Yu Z, Fu X, Liang C (June 2002). "Noc3p, a bHLH Protein, Plays an Integral Role in the Initiation of DNA Replication in Budding Yeast". Cell. 109 (7): 849–860. doi:10.1016/s0092-8674(02)00805-x. PMID 12110182.
- ^ Rao H, Stillman B (March 1995). "The origin recognition complex interacts with a bipartite DNA binding site within yeast replicators". Proceedings of the National Academy of Sciences of the United States of America. 92 (6): 2224–8. Bibcode:1995PNAS...92.2224R. doi:10.1073/pnas.92.6.2224. PMC 42456. PMID 7892251.
- ^ Rowley A, Cocker JH, Harwood J, Diffley JF (June 1995). "Initiation complex assembly at budding yeast replication origins begins with the recognition of a bipartite sequence by limiting amounts of the initiator, ORC". The EMBO Journal. 14 (11): 2631–41. doi:10.1002/j.1460-2075.1995.tb07261.x. PMC 398377. PMID 7781615.
- ^ a b Speck C, Chen Z, Li H, Stillman B (November 2005). "ATPase-dependent cooperative binding of ORC and Cdc6 to origin DNA". Nature Structural & Molecular Biology. 12 (11): 965–71. doi:10.1038/nsmb1002. PMC 2952294. PMID 16228006.
- ^ Kelly TJ, Brown GW (2000). "Regulation of chromosome replication". Annual Review of Biochemistry. 69: 829–80. doi:10.1146/annurev.biochem.69.1.829. PMID 10966477.
- ^ Bell SP, Dutta A (2002). "DNA replication in eukaryotic cells". Annual Review of Biochemistry. 71: 333–74. doi:10.1146/annurev.biochem.71.110601.135425. PMID 12045100.
- ^ Stillman B (February 2005). "Origin recognition and the chromosome cycle". FEBS Letters. 579 (4): 877–84. doi:10.1016/j.febslet.2004.12.011. PMID 15680967. S2CID 33220937.
- ^ Weinreich M, Liang C, Chen HH, Stillman B (September 2001). "Binding of cyclin-dependent kinases to ORC and Cdc6p regulates the chromosome replication cycle". Proceedings of the National Academy of Sciences of the United States of America. 98 (20): 11211–7. doi:10.1073/pnas.201387198. PMC 58709. PMID 11572976.
- ^ Nguyen VQ, Co C, Li JJ (June 2001). "Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms". Nature. 411 (6841): 1068–73. Bibcode:2001Natur.411.1068N. doi:10.1038/35082600. PMID 11429609. S2CID 4393812.
- ^ Archambault V, Ikui AE, Drapkin BJ, Cross FR (August 2005). "Disruption of mechanisms that prevent rereplication triggers a DNA damage response". Molecular and Cellular Biology. 25 (15): 6707–21. doi:10.1128/MCB.25.15.6707-6721.2005. PMC 1190345. PMID 16024805.
- ^ Triolo T, Sternglanz R (May 1996). "Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing". Nature. 381 (6579): 251–3. Bibcode:1996Natur.381..251T. doi:10.1038/381251a0. PMID 8622770. S2CID 4309206.
- ^ Fox CA, Ehrenhofer-Murray AE, Loo S, Rine J (June 1997). "The origin recognition complex, SIR1, and the S phase requirement for silencing". Science. 276 (5318): 1547–51. doi:10.1126/science.276.5318.1547. PMID 9171055.
- ^ Klemm RD, Austin RJ, Bell SP (February 1997). "Coordinate binding of ATP and origin DNA regulates the ATPase activity of the origin recognition complex". Cell. 88 (4): 493–502. doi:10.1016/S0092-8674(00)81889-9. PMID 9038340.
- ^ Klemm RD, Bell SP (July 2001). "ATP bound to the origin recognition complex is important for preRC formation". Proceedings of the National Academy of Sciences of the United States of America. 98 (15): 8361–7. Bibcode:2001PNAS...98.8361K. doi:10.1073/pnas.131006898. PMC 37444. PMID 11459976.
- ^ Bowers JL, Randell JC, Chen S, Bell SP (December 2004). "ATP hydrolysis by ORC catalyzes reiterative Mcm2-7 assembly at a defined origin of replication". Molecular Cell. 16 (6): 967–78. doi:10.1016/j.molcel.2004.11.038. PMID 15610739.
- ^ Randell JC, Bowers JL, Rodríguez HK, Bell SP (January 2006). "Sequential ATP hydrolysis by Cdc6 and ORC directs loading of the Mcm2-7 helicase". Molecular Cell. 21 (1): 29–39. doi:10.1016/j.molcel.2005.11.023. PMID 16387651.
- ^ Semple JW, Da-Silva LF, Jervis EJ, Ah-Kee J, Al-Attar H, Kummer L, et al. (November 2006). "An essential role for Orc6 in DNA replication through maintenance of pre-replicative complexes". The EMBO Journal. 25 (21): 5150–8. doi:10.1038/sj.emboj.7601391. PMC 1630405. PMID 17053779.
- ^ Yin YC, Prasanth SG (July 2021). "Replication initiation: Implications in genome integrity". DNA Repair. 103: 103131. doi:10.1016/j.dnarep.2021.103131. PMC 8296962. PMID 33992866.
- ^ Amin A, Wu, R, Cheung MH, Scott JF, Wang Z, Zhou Z, Liu C, Zhu G, Wong KC, Yu Z, Liang C (March 2020). "An Essential and Cell-Cycle-Dependent ORC Dimerization Cycle Regulates Eukaryotic Chromosomal DNA Replication". Cell Reports. 30 (10): 3323–3338.e6. doi:10.1016/j.celrep.2020.02.046. PMID 32160540.
- ^ a b c d Morgan, David (2007). The Cell Cycle: Principles of Control. Primers in Biology. pp. 62–75. ISBN 978-0878935086.
- ^ Ausiannikava D, Allers T (January 2017). "Diversity of DNA Replication in the Archaea". Genes. 8 (2): 56. doi:10.3390/genes8020056. PMC 5333045. PMID 28146124.
- ^ Fernández-Cid A, Riera A, Tognetti S, Herrera MC, Samel S, Evrin C, et al. (May 2013). "An ORC/Cdc6/MCM2-7 complex is formed in a multistep reaction to serve as a platform for MCM double-hexamer assembly". Molecular Cell. 50 (4): 577–88. doi:10.1016/j.molcel.2013.03.026. hdl:10044/1/19289. PMID 23603117.
- ^ Randell JC, Bowers JL, Rodríguez HK, Bell SP (January 2006). "Sequential ATP hydrolysis by Cdc6 and ORC directs loading of the Mcm2-7 helicase". Molecular Cell. 21 (1): 29–39. doi:10.1016/j.molcel.2005.11.023. PMID 16387651.
- ^ a b Speck C, Chen Z, Li H, Stillman B (November 2005). "ATPase-dependent cooperative binding of ORC and Cdc6 to origin DNA". Nature Structural & Molecular Biology. 12 (11): 965–71. doi:10.1038/nsmb1002. PMC 2952294. PMID 16228006.
- ^ Yuan Z, Riera A, Bai L, Sun J, Nandi S, Spanos C, et al. (March 2017). "Structural basis of Mcm2-7 replicative helicase loading by ORC-Cdc6 and Cdt1". Nature Structural & Molecular Biology. 24 (3): 316–324. doi:10.1038/nsmb.3372. PMC 5503505. PMID 28191893.
- ^ Kawakami H, Ohashi E, Kanamoto S, Tsurimoto T, Katayama T (October 2015). "Specific binding of eukaryotic ORC to DNA replication origins depends on highly conserved basic residues". Scientific Reports. 5: 14929. Bibcode:2015NatSR...514929K. doi:10.1038/srep14929. PMC 4601075. PMID 26456755.
- ^ Chistol G, Walter JC (April 2015). "Single-Molecule Visualization of MCM2-7 DNA Loading: Seeing Is Believing". Cell. 161 (3): 429–430. doi:10.1016/j.cell.2015.04.006. PMID 25910200.
Further reading
[edit]- Bell SP, Dutta A (July 2002). "DNA replication in eukaryotic cells". Annual Review of Biochemistry. 71. Annual Reviews: 333–74. doi:10.1146/annurev.biochem.71.110601.135425. PMID 12045100.
A comprehensive review of molecular DNA replication