Phenylalanine hydroxylase: Difference between revisions
No edit summary |
|||
| Line 39: | Line 39: | ||
==Structure== |
==Structure== |
||
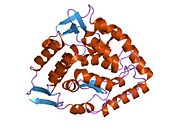
The PheOH monomer (51.9 kDa) consists of three distinct domains: a regulatory N-terminal domain (residues 1-117), the catalytic domain (residues 118-427), and a C-terminal domain (residues 428-453) responsible for oligomerization of identical monomers. Extensive crystallographic analysis has been performed, especially on the pterin- and iron-coordinated catalytic domain to examine the active site. The structure of the N-terminal regulatory domain has also been determined, and together with the solved structure of the homologous tyrosine hydroxylase C-terminal tetramerization domain, a structural model of tetrameric PheOH has been proposed.<ref |
The PheOH monomer (51.9 kDa) consists of three distinct domains: a regulatory N-terminal domain (residues 1-117), the catalytic domain (residues 118-427), and a C-terminal domain (residues 428-453) responsible for oligomerization of identical monomers. Extensive crystallographic analysis has been performed, especially on the pterin- and iron-coordinated catalytic domain to examine the active site. The structure of the N-terminal regulatory domain has also been determined, and together with the solved structure of the homologous tyrosine hydroxylase C-terminal tetramerization domain, a structural model of tetrameric PheOH has been proposed.<ref name=Flatmark1999/> |
||
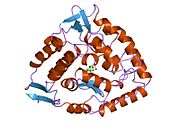
'''Catalytic domain.''' Solved crystal structures of the catalytic domain indicate that the active site consists of an open and spacious pocket lined primarily by hydrophobic residues, though three glutamic acid residues, two histidines, and a tyrosine are also present and critical for pterin- and iron-binding |
'''Catalytic domain.''' Solved crystal structures of the catalytic domain indicate that the active site consists of an open and spacious pocket lined primarily by hydrophobic residues, though three glutamic acid residues, two histidines, and a tyrosine are also present and critical for pterin- and iron-binding.<ref name=Flatmark1999/> Contradictory evidence exists about the coordination state of the ferrous atom and its proximity to BH4 within the active site. According to crystallographic analysis, Fe(II) is coordinated by water, His285, His290 and Glu330 (a 2-his-1-carboxylate facial triad arrangement) with octahedral geometry <ref name=Erlandsen1997>{{cite journal | author=Erlandsen, H et al. |title=Crystal structure of the catalytic domain of human phenylalanine hydroxylase reveals the structural basis for phenylketonuria |journal=Nat. Struct. Biol. |issue= 12|pages= 995-1000 |url=http://www.ncbi.nlm.nih.gov/pubmed/9406548 | volume=4 | year=1997 | pmid=9406548 }}</ref>. Inclusion of a Phe analogue in the crystal structure both changes iron from a six- to a five-coordinated state involving a single water molecule and bidentate coordination to Glu330 and opening a site for oxygen to bind. BH4 is concommitantly shifted towards the iron atom, although the pterin cofactor remains in the second coordination sphere.<ref name=Andersen2002>{{cite journal | author=Andersen, OA et al. |title=Crystal Structure of the Ternary Complex of the Catalytic Domain of Human Phenylalanine Hydroxylase with Tetrahydrobiopterin and 3-(2-Thienyl)-L-alanine, and its Implications for the Mechanism of Catalysis and Substrate Activation |journal=J. Mol. Biol. |issue= 5|pages= 1095-108 |url=http://www.ncbi.nlm.nih.gov/pubmed/12126628 | volume=320 | year=2002 | pmid=9406548 }}</ref> On the other hand, a competing model based on NMR and molecular modeling analyses suggests that all coordinated water molecules are forced out of the active site during the catalytic cycle while BH4 becomes directly coordinated to iron.<ref name=Teigen1999>{{cite journal | author=Teigen, K et al. |title= The structural basis of the recognition of phenylalanine and pterin cofactors by phenylalanine hydroxylase: implications for the catalytic mechanism. |journal=J. Mol. Biol. |issue= 3|pages= 807-23 |url=http://www.ncbi.nlm.nih.gov/pubmed/10610798 | volume=294 | year=1999 | pmid=10610798 }}</ref> As discussed above, resolving this discrepancy will be important for determining the exact mechanism of PheOH catalysis. |
||
|journal=J. Mol. Biol. |issue= 3|pages= 807-23 |url=http://www.ncbi.nlm.nih.gov/pubmed/10610798 | volume=294 | pmid=10610798 }}</ref> As discussed above, resolving this discrepancy will be important for determining the exact mechanism of PheOH catalysis. |
|||
[[File:activesite.png]] |
|||
| ⚫ | '''N-terminal regulatory domain'''. The regulatory nature of the N-terminal domain (residues 1-117) is conferred by its structural flexibility. <ref name=Li2010>{{cite journal | author=Li, J et al. |title=Regulation of Phenylalanine Hydroxylase: Conformational Changes Upon Phenylalanine Binding Detected by Hydrogen/Deuterium Exchange and Mass Spectrometry |journal=Biochemistry |issue= 15|pages= 3327-35 |url=http://www.ncbi.nlm.nih.gov/pubmed/20307070 | volume=49| year=2010 | pmid=20307070 }}</ref> Hydrogen/deuterium exchanges analysis indicates that allosteric binding of Phe globally alters the conformation of PheOH such that the active site is less occluded as the interface between the regulatory and catalytic domains is increasingly exposed to solvent.<ref name=Li2011>{{cite journal | author=Li, J et al. |title=Direct evidence for a phenylalanine site in the regulatory domain of phenylalanine hydroxylase |journal=Arch. Biochem. Biophys. |issue= 2|pages= 250-5 |url=http://www.ncbi.nlm.nih.gov/pubmed/20951114 | volume= 505 | year=2011 |pmid=20951114 }}</ref> <ref name=Li2011/> <ref name=Kobe1999/>{{cite journal | author=Kobe, B et al. |title=Structural basis of autoregulation of phenylalanine hydroxylase |journal=Nat. Struct. Biol. |issue= 5|pages= 442-8 |url=http://www.ncbi.nlm.nih.gov/pubmed/10331871 | volume=6| year=1999 | pmid=10331871 }}</ref> This observation is consistent with kinetic studies, which show an initially low rate of tyrosine formation for full-length PheOH. This lag time is not observed, however, for a truncated PheOH lacking the N-terminal domain or if the full-length enzyme is pre-incubated with Phe. Deletion of the N-terminal domain also eliminates the lag time while increasing the affinity for Phe by nearly two-fold; no difference is observed in the Vmax or Km for the tetrahydrobiopterin cofactor.<ref name=Daubner1997>{{cite journal | author=Daubner, SC et al. |title=Expression and Characterization of the Catalytic Domain of Human Phenylalanine Hydroxylase |journal=Arch. Biochem. Biophys. |issue= 2|pages= 295-302 |url=http://www.ncbi.nlm.nih.gov/pubmed/9434741 | volume=348| year=1997 | pmid=9434741 }}</ref> Additional regulation is provided by Ser16; phosphorylation of this residue does not alter enzyme conformation but does reduce the concentration of Phe required for allosteric activation.<ref name=Kobe1999/> This N-terminal regulatory domain is not observed in bacterial PheOHs but shows considerable structural homology to the regulatory domain of phosphogylcerate dehydrogenase, an enzyme in the serine biosynthetic pathway. <ref name=Kobe1999/> |
||
| ⚫ | '''N-terminal regulatory domain'''. The regulatory nature of the N-terminal domain (residues 1-117) is conferred by its structural flexibility |
||
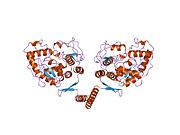
| ⚫ | '''Tetramerization domain.''' Prokaryotic PheOH is monomeric, whereas eukaryotic PheOH exists in an equilibrium between homotetrameric and homodimeric forms. <ref name=Fitzpatrick2003/> <ref name=Flatmark1999/>The dimerization interface is composed of symmetry-related loops that link identical monomers, while the overlapping C-terminal tetramerization domain mediates the association of conformationally distinct dimers that are characterized by a different relative orientation of the catalytic and tetramerization domains (Flatmark, Erlandsen). The resulting distortion of the tetramer symmetry is evident in the differential surface area of the dimerization interfaces and distinguishes PheOH from the tetramerically symmetrical tyrosine hydroxylase.<ref name=Flatmark1999/> A domain swapping mechanism has been proposed to mediate formation of the tetramer from dimers, in which C-terminal alpha-helixes mutually alter their conformation around a flexible C-terminal five-residue hinge region to form a coiled-coil structure, shifting equilibrium towards the tetrameric form.<ref name=Fitzpatrick2003/> <ref name=Flatmark1999/><ref name=Bjorgo2001>{{cite journal | author=Bjorgo, E et al. |title=A comparison of kinetic and regulatory properties of the tetrameric and dimeric forms of wild-type and Thr427-->Pro mutant human phenylalanine hydroxylase: contribution of the flexible hinge region Asp425-Gln429 to the tetramerization and cooperative substrate binding. |journal=Eur J Biochem |issue= 4 |pages= 997-1005 |url=http://www.ncbi.nlm.nih.gov/pubmed/11179966 | volume=268 | year=2001 | pmid=11179966 }}</ref> Although both the homodimeric and homotetrameric forms of PheOH are catalytically active, the two exhibit differential kinetics and regulation. In addition to reduced catalytic efficiency, the dimer does not display positive cooperativity towards L-Phe (which at high concentrations activates the enzyme), suggesting that L-Phe allosterically regulates PheOH by influencing dimer-dimer interaction. <ref name=Bjorgo2001/> |
||
| ⚫ | '''Tetramerization domain.''' Prokaryotic PheOH is monomeric, whereas eukaryotic PheOH exists in an equilibrium between homotetrameric and homodimeric forms |
||
==References== |
==References== |
||
Revision as of 04:31, 17 May 2011
Phenylalanine hydroxylase (PAH) (EC 1.14.16.1) is an enzyme that catalyses the reaction responsible for the addition of a hydroxyl group to the end of the 6-carbon aromatic ring of phenylalanine, such that it becomes tyrosine:
Phenylalanine hydroxylase is the rate-limiting enzyme of the metabolic pathway which degrades excess phenylalanine.
The other substrates in the reaction are molecular oxygen and tetrahydrobiopterin (BH4). Tetrahydrobiopterin is a member of the group of redox biochemicals known as pteridines. PAH is the gene that encodes for phenylalanine hydroxylase.
It was the research on phenylalanine hydroxylase by Seymour Kaufman that led to the discovery of tetrahydrobiopterin as a biological cofactor.[1]
Enzyme Mechanism
The reaction is thought to proceed through the following steps:
1. formation of a Fe(II)-O-O-BH4 bridge.
2. heterolytic cleavage of the O-O bond to yield the ferryl oxo hydroxylating intermediate Fe(IV)=O
3. attack on Fe(IV)=O to hydroxylate phenylalanine substrate to tyrosine.[2]
Formation and cleavage of the iron-peroxypterin bridge. Although evidence strongly supports Fe(IV)=O as the hydroxylating intermediate,[3] the mechanistic details underlying the formation of the Fe(II)-O-O-BH4 bridge prior to heterolytic cleavage remain controversial. Two pathways have been proposed based on models that differ in the proximity of the iron to the pterin cofactor and the number of water molecules assumed to be iron-coordinated during catalysis. According to one model, an iron dioxygen complex is initially formed and stabilized as a resonance hybrid of Fe2+O2 and Fe3+O2-. The activated O2 then attacks BH4, forming a transition state characterized by charge separation between the electron-deficient pterin ring and the electron-rich dioxygen species.[4] The Fe(II)-O-O-BH4 bridge is subsequently formed. On the other hand, formation of this bridge has been modeled assuming that BH4 is located in iron's first coordination shell and that the iron is not coordinated to any water molecules. This model predicts a different mechanism involving a pterin radical and superoxide as critical intermediates.[5] The critical steps in both proposed mechanisms are diagrammed below.
Hydroxylation of phenylalanine by ferryl oxo intermediate. Once formed, the Fe(II)-O-O-BH4 bridge is broken through heterolytic cleavage of the O-O bond to Fe(IV)=O and 4a-hydroxytetrahydrobiopterin; thus, molecular oxygen is the source of both oxygen atoms used to hydroxylate the pterin ring and phenylalanine. Because the mechanism involves a Fe(IV)=O (as opposed to a peroxypterin) hydroxylating intermediate, oxidation of the BH4 cofactor and hydroxylation of phenylalanine can be decoupled, resulting in unproductive consumption of BH4 and formation of H2O2.[2] When productive, the Fe(IV)=O intermediate is added to phenylalanine in an electrophilic aromatic substitution reaction that reduces iron from the ferryl to the ferrous state.[2] Although initially an arene oxide or radical intermediate was proposed, analyses of the related tryptophan and tyrosine hydroxylases have suggested that the reaction proceeds through a cationic intermediate instead that requires Fe(IV)=O to be coordinated to a water ligand rather than a hydroxo group, as shown below. [2] [6] This cationic intermediate subsequently undergoes a 1,2-hydride NIH shift, yielding a dienone intermediate that then tautomerizes to form the tyrosine product.[7] The pterin cofactor is regenerated by hydration of the carbinolamine product of PheOH to quinonoid dihydrobiopterin (qBH2) and then reduced to BH4. [8]
Clinical significance
Mutations in phenylalanine hydroxylase which result in lower activity are the cause of the disease phenylketonuria, or PKU.
Related enzymes
Phenylalanine hydroxylase is closely related to two other enzymes:
- tryptophan hydroxylase (EC number 1.14.16.4), which controls levels of serotonin in the brain and the gastrointestinal tract
- tyrosine hydroxylase (EC number 1.14.16.2), which controls levels of dopamine, epinephrine, and norepinephrine in the brain and the adrenal medulla.
The three enzymes are homologous, that is, are thought to have evolved from the same ancient hydroxylase.
Structure
The PheOH monomer (51.9 kDa) consists of three distinct domains: a regulatory N-terminal domain (residues 1-117), the catalytic domain (residues 118-427), and a C-terminal domain (residues 428-453) responsible for oligomerization of identical monomers. Extensive crystallographic analysis has been performed, especially on the pterin- and iron-coordinated catalytic domain to examine the active site. The structure of the N-terminal regulatory domain has also been determined, and together with the solved structure of the homologous tyrosine hydroxylase C-terminal tetramerization domain, a structural model of tetrameric PheOH has been proposed.[8]
Catalytic domain. Solved crystal structures of the catalytic domain indicate that the active site consists of an open and spacious pocket lined primarily by hydrophobic residues, though three glutamic acid residues, two histidines, and a tyrosine are also present and critical for pterin- and iron-binding.[8] Contradictory evidence exists about the coordination state of the ferrous atom and its proximity to BH4 within the active site. According to crystallographic analysis, Fe(II) is coordinated by water, His285, His290 and Glu330 (a 2-his-1-carboxylate facial triad arrangement) with octahedral geometry [9]. Inclusion of a Phe analogue in the crystal structure both changes iron from a six- to a five-coordinated state involving a single water molecule and bidentate coordination to Glu330 and opening a site for oxygen to bind. BH4 is concommitantly shifted towards the iron atom, although the pterin cofactor remains in the second coordination sphere.[10] On the other hand, a competing model based on NMR and molecular modeling analyses suggests that all coordinated water molecules are forced out of the active site during the catalytic cycle while BH4 becomes directly coordinated to iron.[11] As discussed above, resolving this discrepancy will be important for determining the exact mechanism of PheOH catalysis.
N-terminal regulatory domain. The regulatory nature of the N-terminal domain (residues 1-117) is conferred by its structural flexibility. [12] Hydrogen/deuterium exchanges analysis indicates that allosteric binding of Phe globally alters the conformation of PheOH such that the active site is less occluded as the interface between the regulatory and catalytic domains is increasingly exposed to solvent.[13] [13] [14]Kobe, B; et al. (1999). "Structural basis of autoregulation of phenylalanine hydroxylase". Nat. Struct. Biol. 6 (5): 442–8. PMID 10331871. {{cite journal}}: Explicit use of et al. in: |author= (help)</ref> This observation is consistent with kinetic studies, which show an initially low rate of tyrosine formation for full-length PheOH. This lag time is not observed, however, for a truncated PheOH lacking the N-terminal domain or if the full-length enzyme is pre-incubated with Phe. Deletion of the N-terminal domain also eliminates the lag time while increasing the affinity for Phe by nearly two-fold; no difference is observed in the Vmax or Km for the tetrahydrobiopterin cofactor.[15] Additional regulation is provided by Ser16; phosphorylation of this residue does not alter enzyme conformation but does reduce the concentration of Phe required for allosteric activation.[14] This N-terminal regulatory domain is not observed in bacterial PheOHs but shows considerable structural homology to the regulatory domain of phosphogylcerate dehydrogenase, an enzyme in the serine biosynthetic pathway. [14]
Tetramerization domain. Prokaryotic PheOH is monomeric, whereas eukaryotic PheOH exists in an equilibrium between homotetrameric and homodimeric forms. [2] [8]The dimerization interface is composed of symmetry-related loops that link identical monomers, while the overlapping C-terminal tetramerization domain mediates the association of conformationally distinct dimers that are characterized by a different relative orientation of the catalytic and tetramerization domains (Flatmark, Erlandsen). The resulting distortion of the tetramer symmetry is evident in the differential surface area of the dimerization interfaces and distinguishes PheOH from the tetramerically symmetrical tyrosine hydroxylase.[8] A domain swapping mechanism has been proposed to mediate formation of the tetramer from dimers, in which C-terminal alpha-helixes mutually alter their conformation around a flexible C-terminal five-residue hinge region to form a coiled-coil structure, shifting equilibrium towards the tetrameric form.[2] [8][16] Although both the homodimeric and homotetrameric forms of PheOH are catalytically active, the two exhibit differential kinetics and regulation. In addition to reduced catalytic efficiency, the dimer does not display positive cooperativity towards L-Phe (which at high concentrations activates the enzyme), suggesting that L-Phe allosterically regulates PheOH by influencing dimer-dimer interaction. [16]
References
- ^ Kaufman, D (1 February 1958). "A New Cofactor Required for the Enzymatic Conversion of Phenylalanine to Tyrosine". J. Biol. Chem. 230 (2): 931–39. PMID 13525410.
- ^ a b c d e f Fitzpatrick, PF; et al. (2003). "Mechanism of aromatic amino acid hydroxylation". Biochemistry. 42 (48): 1928–33. PMID 14640675.
{{cite journal}}: Explicit use of et al. in:|author=(help); Text "http://www.ncbi.nlm.nih.gov/pubmed/14640675" ignored (help) - ^ Panay, AJ; et al. (2011). "Evidence for a high-spin Fe(IV) species in the catalytic cycle of a bacterial phenylalanine hydroxylase". Biochemistry. 50 (11): 1928–33. PMID 21261288.
{{cite journal}}: Explicit use of et al. in:|author=(help); Text "http://www.ncbi.nlm.nih.gov/pubmed/21261288" ignored (help) - ^ Bassan, A; et al. (2003). "Mechanism of Dioxygen Cleavage in Tetrahydrobiopterin-Dependent Amino Acid Hydroxylases". Chemistry. 9 (1): 106–15. PMID 12506369.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Olsson, E; et al. (2011). "Formation of the iron-oxo hydroxylating species in the catalytic cycle of aromatic amino acid hydroxylases". Chemistry. 17 (13): 3746–58. PMID 21351297.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Bassan, A; et al. (2003). "Mechanism of Aromatic Hydroxylation by an Activated FeIV=O Core in Tetrahydrobiopterin-Dependent Hydroxylases". Chemistry. 9 (17): 4055–67. PMID 12953191.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Pavon, JA; et al. (2006). "Insights into the Catalytic Mechanisms of Phenylalanine and Tryptophan Hydroxylase from Kinetic Isotope Effects on Aromatic Hydroxylation". Biochemistry. 12 (45): 11030–7. PMID 16953590.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b c d e f Flatmark, T; et al. (1999). "Structural Insight into the Aromatic Amino Acid Hydroxylases and Their Disease-Related Mutant Forms". Chem. Rev. 99 (8): 2137–2160. PMID 11849022.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Erlandsen, H; et al. (1997). "Crystal structure of the catalytic domain of human phenylalanine hydroxylase reveals the structural basis for phenylketonuria". Nat. Struct. Biol. 4 (12): 995–1000. PMID 9406548.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Andersen, OA; et al. (2002). "Crystal Structure of the Ternary Complex of the Catalytic Domain of Human Phenylalanine Hydroxylase with Tetrahydrobiopterin and 3-(2-Thienyl)-L-alanine, and its Implications for the Mechanism of Catalysis and Substrate Activation". J. Mol. Biol. 320 (5): 1095–108. PMID 9406548.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Teigen, K; et al. (1999). "The structural basis of the recognition of phenylalanine and pterin cofactors by phenylalanine hydroxylase: implications for the catalytic mechanism". J. Mol. Biol. 294 (3): 807–23. PMID 10610798.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Li, J; et al. (2010). "Regulation of Phenylalanine Hydroxylase: Conformational Changes Upon Phenylalanine Binding Detected by Hydrogen/Deuterium Exchange and Mass Spectrometry". Biochemistry. 49 (15): 3327–35. PMID 20307070.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b Li, J; et al. (2011). "Direct evidence for a phenylalanine site in the regulatory domain of phenylalanine hydroxylase". Arch. Biochem. Biophys. 505 (2): 250–5. PMID 20951114.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b c Cite error: The named reference
Kobe1999was invoked but never defined (see the help page). - ^ Daubner, SC; et al. (1997). "Expression and Characterization of the Catalytic Domain of Human Phenylalanine Hydroxylase". Arch. Biochem. Biophys. 348 (2): 295–302. PMID 9434741.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b Bjorgo, E; et al. (2001). "A comparison of kinetic and regulatory properties of the tetrameric and dimeric forms of wild-type and Thr427-->Pro mutant human phenylalanine hydroxylase: contribution of the flexible hinge region Asp425-Gln429 to the tetramerization and cooperative substrate binding". Eur J Biochem. 268 (4): 997–1005. PMID 11179966.
{{cite journal}}: Explicit use of et al. in:|author=(help)
External links
- GeneReviews/NCBI/NIH/UW entry on Phenylalanine Hydroxylase Deficiency
- PAHdb - online locus-specific mutation database of the human phenylalanine hydroxylase gene
Further reading