Influenza vaccine: Difference between revisions
→History: better list of pandemics |
|||
| Line 508: | Line 508: | ||
[[Influenza research]] includes [[molecular virology]], [[molecular evolution]], [[pathogenesis]], host [[immune response]]s, [[genomics]], and [[epidemiology]]. These help in developing influenza countermeasures such as [[vaccine]]s, therapies and diagnostic tools. Improved influenza countermeasures require basic research on how viruses enter cells, replicate, mutate, evolve into new strains and induce an immune response. The [[Influenza Genome Sequencing Project]] is creating a library of influenza sequences<ref>{{cite web|title=Influenza Genome Sequencing Project - Overview|url=http://www.niaid.nih.gov/LabsAndResources/resources/dmid/gsc/Influenza/Pages/overview.aspx|publisher=National Institutes of Health - National Institute of Allergy and Infectious Diseases|accessdate=27 May 2013}}</ref> that will help us understand what makes one strain more lethal than another, what genetic determinants most affect [[immunogenicity]], and how the virus evolves over time. Solutions to limitations in current{{when|date=August 2012}} vaccine methods are being researched. |
[[Influenza research]] includes [[molecular virology]], [[molecular evolution]], [[pathogenesis]], host [[immune response]]s, [[genomics]], and [[epidemiology]]. These help in developing influenza countermeasures such as [[vaccine]]s, therapies and diagnostic tools. Improved influenza countermeasures require basic research on how viruses enter cells, replicate, mutate, evolve into new strains and induce an immune response. The [[Influenza Genome Sequencing Project]] is creating a library of influenza sequences<ref>{{cite web|title=Influenza Genome Sequencing Project - Overview|url=http://www.niaid.nih.gov/LabsAndResources/resources/dmid/gsc/Influenza/Pages/overview.aspx|publisher=National Institutes of Health - National Institute of Allergy and Infectious Diseases|accessdate=27 May 2013}}</ref> that will help us understand what makes one strain more lethal than another, what genetic determinants most affect [[immunogenicity]], and how the virus evolves over time. Solutions to limitations in current{{when|date=August 2012}} vaccine methods are being researched. |
||
According to VaccineNewsDaily, a recent study published in Vaccines found that by providing a school-located vaccination clinic, [[Influenza vaccine|flu vaccination]] rates among children increased 13.2 percent when compared to children in schools without vaccination clinics. The vaccine can be lifesaving for children and less costly than a doctor's office visit.<ref>{{cite web |url=http://vaccinenewsdaily.com/vaccine_development/325399-vaccination-programs-at-schools-could-reduce-flu-deaths-among-children-vnd/ |title=Vaccination programs at schools could reduce flu deaths among children at VND |last1=Tinder |first1=Paul |date=June 7, 2013 |publisher=VaccineNewsDaily |accessdate=10 June 2013}}</ref> |
According to VaccineNewsDaily, a recent study published in Vaccines found that by providing a school-located vaccination clinic, [[Influenza vaccine|flu vaccination]] rates among children increased 13.2 percent when compared to children in schools without vaccination clinics. The vaccine can be lifesaving for children and less costly than a doctor's office visit.<ref>{{cite web |url=http://vaccinenewsdaily.com/vaccine_development/325399-vaccination-programs-at-schools-could-reduce-flu-deaths-among-children-vnd/ |title=Vaccination programs at schools could reduce flu deaths among children at VND |last1=Tinder |first1=Paul |date=June 7, 2013 |publisher=VaccineNewsDaily |accessdate=10 June 2013}}</ref> Children in low-income and minority families are less likely to be vaccinated against influenza. To address this problem, New York-Presbyterian/Columbia University Medical Center implemented a program in 2010 that demonstrated how text messaging parents with educational messages about influenza and influenza vaccinations could improve vaccination rates for low-income, Latino children.<ref>{{cite web |publisher=Agency for Healthcare Research and Quality | url=http://www.innovations.ahrq.gov/content.aspx?id=3737 |title=Text Messages to Parents Increase Influenza Immunization Rate for Low-Income, Minority Children |date=2012-12-19 |accessdate=2013-07-09}}</ref> |
||
The rapid development, production, and distribution of pandemic influenza vaccines could potentially save millions of lives during an influenza pandemic. Due to the short time frame between identification of a pandemic strain and need for vaccination, researchers are looking at novel technologies for vaccine production that could provide better "real-time" access and be produced more affordably, thereby increasing access for people living in low- and moderate-income countries, where an influenza pandemic may likely originate, such as live attenuated (egg-based or cell-based) technology and recombinant technologies (proteins and virus-like particles).<ref>World Health Organization. Acyte Respiratory Infections: Influenza. 2009. http://www.who.int/vaccine_research/diseases/ari/en/index1.html</ref> As of July 2009, more than 70 known clinical trials have been completed or are ongoing for pandemic influenza vaccines.<ref>World Health Organization. Tables on the Clinical trials of pandemic influenza prototype vaccines. July 2009. http://www.who.int/vaccine_research/immunogenicity/immunogenicity_table.xls</ref> In September 2009, the [[Food and Drug Administration|US Food and Drug Administration]] approved four vaccines against the 2009 H1N1 influenza virus (the 2009 pandemic strain), and expected the initial vaccine lots to be available within the following month.<ref>US Food & Drug Administration. FDA Approves Vaccines for 2009 H1N1 Influenza Virus Approval Provides Important Tool to Fight Pandemic. September 15, 2009. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm182399.htm</ref> A quadrivalent flu vaccine administered by nasal mist was approved by the U.S. [[Food and Drug Administration]] (FDA) in March 2012.<ref>[http://www.medicalnewstoday.com/articles/242385.php "First Quadrivalent Vaccine Against Seasonal Flu Wins FDA Approval"]</ref><ref>[http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm294057.htm "FDA approves first quadrivalent vaccine to prevent seasonal influenza"]</ref> Fluarix Quadrivalent was approved by the FDA in December 2012.<ref>[http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm332484.htm "December 14, 2012 Approval Letter- Fluarix Quadrivalent"]</ref> |
The rapid development, production, and distribution of pandemic influenza vaccines could potentially save millions of lives during an influenza pandemic. Due to the short time frame between identification of a pandemic strain and need for vaccination, researchers are looking at novel technologies for vaccine production that could provide better "real-time" access and be produced more affordably, thereby increasing access for people living in low- and moderate-income countries, where an influenza pandemic may likely originate, such as live attenuated (egg-based or cell-based) technology and recombinant technologies (proteins and virus-like particles).<ref>World Health Organization. Acyte Respiratory Infections: Influenza. 2009. http://www.who.int/vaccine_research/diseases/ari/en/index1.html</ref> As of July 2009, more than 70 known clinical trials have been completed or are ongoing for pandemic influenza vaccines.<ref>World Health Organization. Tables on the Clinical trials of pandemic influenza prototype vaccines. July 2009. http://www.who.int/vaccine_research/immunogenicity/immunogenicity_table.xls</ref> In September 2009, the [[Food and Drug Administration|US Food and Drug Administration]] approved four vaccines against the 2009 H1N1 influenza virus (the 2009 pandemic strain), and expected the initial vaccine lots to be available within the following month.<ref>US Food & Drug Administration. FDA Approves Vaccines for 2009 H1N1 Influenza Virus Approval Provides Important Tool to Fight Pandemic. September 15, 2009. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm182399.htm</ref> A quadrivalent flu vaccine administered by nasal mist was approved by the U.S. [[Food and Drug Administration]] (FDA) in March 2012.<ref>[http://www.medicalnewstoday.com/articles/242385.php "First Quadrivalent Vaccine Against Seasonal Flu Wins FDA Approval"]</ref><ref>[http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm294057.htm "FDA approves first quadrivalent vaccine to prevent seasonal influenza"]</ref> Fluarix Quadrivalent was approved by the FDA in December 2012.<ref>[http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm332484.htm "December 14, 2012 Approval Letter- Fluarix Quadrivalent"]</ref> |
||
Revision as of 19:35, 9 July 2013
- "Flu shot" redirects here. For the 30 Rock episode, see Flu Shot (30 Rock)
| Influenza (flu) |
|---|
 |
The influenza vaccination is an annual vaccination using a vaccine specific for a given year to protect against the highly variable influenza virus.[1] Each seasonal influenza vaccine contains antigens representing three or four influenza virus strains: one influenza type A subtype H1N1 virus strain, one influenza type A subtype H3N2 virus strain, and either one or two influenza type B virus strains.[2] Influenza vaccines may be administered as an injection, also known as a flu shot, or as a nasal spray.
The U.S. Centers for Disease Control and Prevention recommend that everyone over the ages of 6 months should receive the seasonal influenza vaccine.[3] Vaccination campaigns usually focus on people who are at high risk of serious complications if they catch the flu, such as the elderly and people living with chronic illness or those with weakened immune systems, as well as health care workers.[3][4]
Most flu vaccines provide significant protection against the virus.[5] Despite somewhat limited research, the safety of flu vaccines is reassuring; there is no evidence that they can cause serious harm, and no reason for serious side effects to be a concern.[5]
Purpose and benefits of annual flu vaccination
- Influenza vaccines ... cut the risk that elderly people will die of the virus in half and reduced the chance of hospitalization by more than a quarter, according to a study released by the New England Journal of Medicine.[6][7]
- Getting the flu vaccine is the best way to protect yourself against the flu and to help prevent its spread throughout a community. The influenza vaccine can also reduce the severity of the flu should a person contract a strain of the flu that the vaccine did not contain.[8]
Deadly epidemics each winter
An influenza epidemic emerges during flu season each winter. There are two flu seasons annually, corresponding to the occurrence of winter in the Northern and Southern Hemispheres (winter in one hemisphere is at the same time as summer in the other).
Although difficult to assess, these annual epidemics are thought to result in between three and five million cases of severe illness and between 250,000 and 500,000 deaths every year around the world.[9] Tens of thousands of Americans die in a typical flu season, but there are notable variations from year to year. In 2010 the Centers for Disease Control and Prevention (CDC) in the United States changed the way it reports the 30-year estimates for deaths from influenza. They are reported as a range from a low of about 3,300 deaths to a high of 49,000 per year over the past 30 years.[10][11]
The majority of influenza-caused deaths in the industrialized world occur in adults aged 65 and over.[4] A review at the National Institute of Allergy and Infectious Diseases (NIAID) division of the National Institutes of Health (NIH) in 2008 concluded that "Seasonal influenza causes more than 200,000 hospitalizations and 41,000 deaths in the U.S. each year, and is the seventh leading cause of death in the U.S."[12] The average total economic costs caused by the annual influenza outbreak in the U.S. have been estimated at over $80 billion.[13][14]
The number of annual influenza-related hospitalizations is many times the number of deaths.[15] "The high costs of hospitalizing young children for influenza creates a significant economic burden in the United States, underscoring the importance of preventive flu shots for children and the people with whom they have regular contact..."[16] The CDC has projected that a total of 38 million days of school were missed by American students due to the flu.[17]
In 2006, the United States began recommending influenza vaccinations for preschoolers, but Canada did not follow suit until 2010, "thereby creating a natural experiment to evaluate the effect of the policy in the United States."[18] Studying the interim from the 2006 recommendation by the US and until 2010 when the Canadian recommendation to vaccinate preschoolers was initiated, a Canadian study found emergency room (ER) visits significantly lower for 2- to 4-year-olds in Boston than in Montreal (34% fewer ER trips). Vaccination of preschoolers may have reduced their likelihood of transmission of flu to older siblings and raised the chances that their parents would vaccinate older children as well, since there were also 18 percent fewer emergency room visits by 5- to 18-year-olds in Boston than Montreal during the study period.[19]
In another six-year observational study, vaccination of children aged six months through five years was found to prevent illness in more than half.[20]
National advice on flu vaccination
In 2008, the National Advisory Committee on Immunization, the group that advises the Public Health Agency of Canada, recommended that everyone aged 2 to 64 years be encouraged to receive annual influenza vaccination, and that children between the age of six and 24 months, and their household contacts, should be considered a high priority for the flu vaccine.[21]
In the United States, "Routine influenza vaccination is recommended for all persons aged ≥ 6 months."[22][23]
Within its blanket recommendation for general vaccination in the United States, the Centers for Disease Control and Prevention (CDC), who began recommending the influenza vaccine to health care workers in 1981, emphasizes to clinicians the special urgency of vaccination for members of certain vulnerable groups, and their caregivers:
- Vaccination is especially important for people at higher risk of serious influenza complications or people who live with or care for people at higher risk for serious complications.[24]
Influenza vaccine has been demonstrated to prevent disease and death, both in numerous controlled studies and in painstaking scientific reviews of these studies. The CDC reports that studies demonstrate that vaccination is a cost-effective counter-measure to seasonal outbreaks of influenza.[25] The CDC in 2010, after a review of extant studies, extended its guidelines to recommend that every child over 6 months be given the influenza vaccine.[26]
Benefits of vaccination
According to research published in July 2010, vaccination against influenza is also thought to be important for members of high-risk groups who would be likely to suffer complications from influenza, for example pregnant women[22][27] and children and teenagers from six months to 18 years of age;[28]
- In raising the upper age limit to 18 years, the aim is to reduce both the time children and parents lose from visits to pediatricians and missing school and the need for antibiotics for complications[29]
- An added expected benefit would be indirect: reducing the number of influenza cases among parents and other household members, and possibly spread to the general community.[29]
Vaccination of school-age children has a strong protective effect on the adults and elderly with whom the children are in contact.[30] Children born to mothers who received flu vaccination while pregnant are strongly protected from having to be hospitalized with the flu. "The effectiveness of influenza vaccine given to mothers during pregnancy in preventing hospitalization among their infants, adjusted for potential confounders, was 91.5%."[31]
For healthy, working adults, influenza vaccines can provide moderate protection against virologically confirmed influenza, but such protection is greatly reduced or absent in some seasons. Evidence for protection in adults aged 65 years or older is lacking. New vaccines with improved clinical efficacy and effectiveness are needed to further reduce influenza-related morbidity and mortality.[32]
Influenza vaccination has been shown highly effective in health care workers (HCW), with minimal adverse effects. In a study of forty matched nursing homes, staff influenza vaccination rates were 69.9% in the vaccination arm versus 31.8% in the control arm. The vaccinated staff experienced a 42% reduction in sick leave from work (P=.03).[33] A review of eighteen studies likewise found a strong net benefit to health care workers.[34] Of these eighteen HCW studies, only two also assessed the relationship of patient mortality relative to staff influenza vaccine uptake; both found that higher rates of health care worker vaccination correlated with reduced patient deaths.[34] An analysis of data and patient population health in New Mexico's 75 long-term care facilities nursing homes found that as vaccination rates of health care personnel with direct patient contact rose from 51 to 75 percent, the chances of a flu outbreak among patients in that facility went down by 87 percent. The New Mexico study showed that vaccinating health care personnel provided more protection to residents than vaccinating the residents themselves.[35]
In a 2010 survey of United States healthcare workers, 63.5% reported that they received the flu vaccine during the 2010–11 season, an increase from 61.9% reported the previous season. Health professionals with direct patient contact had higher vaccination uptake, such as physicians and dentists (84.2%) and nurse practitioners (82.6%).[36][37][38]
It is important to note that the flu vaccine takes about two weeks to build up enough antibodies to protect against the flu (thus making the vaccinated person protected against the disease),[2] and that the vaccine does not protect against every strain of the flu.[2]
Safety
Side effects of the flu vaccine injection include:
- mild soreness, redness, and swelling where the shot was given
- fever
- aches
These problems usually begin soon after the injection, and last 1–2 days.[39]
Side effects of the activated/live/LAIV flu nasal spray vaccine:
Some children and adolescents 2–17 years of age have reported:[40]
- runny nose, nasal congestion or cough
- fever
- headache and muscle aches
- wheezing
- abdominal pain or occasional vomiting or diarrhea
Some adults 18–49 years of age have reported:[40]
- runny nose or nasal congestion
- sore throat
- cough, chills, tiredness/weakness
- headache
More severe, but very rare side effects include:[40]
- life-threatening allergic reaction
Some injection-based flu vaccines intended for adults in the United States contain thiomersal (also known as thimerosal), a mercury-based preservative. Despite some controversy in the media,[41] the World Health Organization (WHO) has concluded that there is no evidence of toxicity from thiomersal in vaccines and no reason on grounds of safety to change to more-expensive single-dose administration.[42]
Although Guillain-Barré syndrome had been feared as a complication of vaccination, the CDC states that most studies on modern influenza vaccines have seen no link with Guillain-Barré.[43][44]
A review has concluded that the 2009 H1N1 ("swine flu") vaccine has a safety profile similar to that of seasonal vaccine.[45] Although one review gives an incidence of about one case per million vaccinations,[46] a large study in China, reported in The New England Journal of Medicine covering close to 100 million doses of vaccine against the 2009 H1N1 "swine" flu found only eleven cases of Guillain-Barre syndrome, (0.1 per million doses) total incidence in persons vaccinated, actually lower than the normal rate of the disease in China, and no other notable side effects; "The risk-benefit ratio, which is what vaccines and everything in medicine is about, is overwhelmingly in favor of vaccination."[47] Getting infected by influenza itself increases both the risk of death (up to 1 in 10,000) and increases the risk of developing Guillain-Barré syndrome to a much higher level than the highest level of suspected vaccine involvement (approx. 10 times higher by 2009 estimates).[48][49]
Efficacy and effectiveness
A vaccine is assessed by its efficacy, the extent to which it reduces risk of disease under controlled conditions, and its effectiveness, the observed reduction in risk after the vaccine is put into use.[50] In the case of influenza, effectiveness is expected to be lower than the efficacy because it is measured using the rates of influenza-like illness, which is not always caused by influenza.[51] Influenza vaccines generally show high efficacy, as measured by the antibody production induced in animal models or vaccinated people,[52] or most rigorously, by immunizing healthy adult volunteers and then challenging them with virulent influenza virus.[53] However, studies on the effectiveness of flu vaccines in the real world are uniquely difficult; vaccines may be imperfectly matched, virus prevalence varies widely between years, and influenza is often confused with other influenza-like illnesses.[54] However, in most years (16 of the 19 years before 2007), the flu vaccine strains have been a good match for the circulating strains,[55] and even a mis-matched vaccine can often provide cross-protection.[56]
Nevertheless, multiple clinical trials of both live and inactivated influenza vaccines against seasonal influenza have been performed and their results pooled and analyzed in several 2012 meta-analyses. Studies on live vaccines have very limited data, but these preparations may be more effective than inactivated vaccines.[53] The meta-analyses examined the efficacy and effectiveness of inactivated vaccines against seasonal influenza in adults,[51] children,[57] and the elderly.[58][59] In adults, vaccines show a three-quarters reduction in risk of contracting influenza (4% influenza rate among the unvaccinated versus 1% among vaccinated persons) when the vaccine is perfectly matched to the virus and a one-half reduction (2% get flu without vaccine versus 1% with vaccine) when it is not, but no significant effect on the rate of hospitalization.[51] However, the risk of serious complications from influenza is small in adults, so unless the effect from vaccination is large it might not have been detected. In children, vaccines again showed high efficacy, but low effectiveness in preventing "flu-like illness". In children under the age of two the data are extremely limited, but vaccination appeared to confer no measurable benefit.[57] In the elderly, while many individual studies show effectiveness,[60][61][62] the overall evidence is still insufficient evidence to draw clear conclusions on the effectiveness of vaccination,[58][59][63][64] including a new high-dose flu vaccine specificially formulated to provide a larger immune response.[65] Available evidence indicates that the high-dose vaccine produces a stronger immune response, and a study designed to determine the effectiveness of Fluzone High-Dose in preventing illness from influenza compared with Fluzone is expected to be completed in 2014–2015.[66]
During an influenza pandemic, where a single strain of virus is responsible for illnesses, an effective vaccine could produce a large decrease in the number of cases and be highly effective in controlling an epidemic.[67] However, such a vaccine would have to be produced and distributed rapidly to have maximum effect.[68] Such distribution challenges may be met, with good success.[citation needed] Overall, vaccines against the 2009 H1N1 influenza pandemic were found to be effective in a Scottish study.[69]
A 2011 meta-study published in the journal The Lancet, "Efficacy and Effectiveness of Influenza Vaccines," analyzed 31 prior studies on the effectiveness of influenza vaccination trials conducted between 1967 and 2011. The analysis found that flu shots were efficacious 67 percent of the time; the populations that benefited the most were HIV-positive adults ages 18 to 55 (76 percent), healthy adults ages 18 to 46 (approximately 70 percent) and healthy children ages 6 to 24 months (66 percent).[64]
The group most vulnerable to non-pandemic flu, the elderly, is also the least to benefit from the vaccine. There are multiple reasons behind this steep decline in vaccine efficacy, the most common of which are the declining immunological function and frailty associated with advanced age.[70] In a non-pandemic year, a person in the United States aged 50–64 is nearly ten times more likely to die an influenza-associated death than a younger person, and a person over age 65 is over ten times more likely to die an influenza-associated death than the 50–64 age group.[71]
As mortality is also high among infants who contract influenza, the household contacts and caregivers of infants should be vaccinated to reduce the risk of passing an influenza infection to the infant.[4] Data from the years when Japan required annual flu vaccinations for school-aged children indicate that vaccinating children—the group most likely to catch and spread the disease—has a strikingly positive effect on reducing mortality among older people, due to herd immunity: one life saved for every 420 children who received the flu vaccine.[72] However, a 2010 Cochrane review found that the same benefit did not extend to vaccinating health care workers working with elderly patients in long-term care facilities.[73] In working adults, by contrast, Cochrane found that vaccination reduced both influenza symptoms and working days lost, without affecting transmission or influenza-related complications.[51]
Duration of protection
According to work published in 1973, 1983, and 2004, after vaccination against seasonal flu, antibody titres peak after typically two to four weeks. They decrease by about 50% over the next six months (the decrease is less for older adults), then remain stable for two to three years; protection without revaccination persists for at least three years for children and young adults.[74]
It was previously thought that vaccination provided lifelong protection against specific strains.[75] This is not totally untrue; a 2010 study found a significantly enhanced immune response against the 2009 pandemic H1N1 in study participants who had received vaccination against the swine flu in 1976.[76] Also, a study, published in Nature, found that 90 years after the 1918 pandemic, survivors had antibody-producing cells that produced antibodies with "remarkable power to block 1918 flu virus infection in mice, proving that, even nine decades after infection with this virus, survivors retain protection from it".[77] (This immunity was a consequence of infection, not vaccination.)
Injection versus nasal spray
Flu vaccines are available either as
- TIV (flu shot (injection) of trivalent (three strains; usually A/H1N1, A/H3N2, and B) inactivated (killed) vaccine) or
- LAIV; Q/LAIV (nasal spray (mist) of live attenuated influenza vaccine.)
TIV induces protection after injection (typically intramuscular, though subcutaneous and intradermal routes are also immunogenic)[78] based on an immune response to the antigens present on the inactivated virus, while cold-adapted LAIV works by establishing infection in the nasal passages.[79]
LAIV is not recommended for individuals under age 2 or over age 50,[80] but might be comparatively more effective among children over age 2.[81]
A study of military personnel showed that flu shots yielded less illness than nasal spray. This study was based on one of the largest head-to-head studies comparing LAIV and TIV. It was conducted by the U.S. Armed Forces Surveillance Center, on military personnel stationed in the U.S. during three flu seasons from 2004 through 2007. Investigators concluded that: "It may be prudent to use TIV in patients who were vaccinated at least once in the past 2 years [...] but LAIV against pandemic strains may be more protective than inactivated vaccines, because the population will probably lack preexisting immunity."[82]
Cross-protection
Annual seasonal flu vaccination provide some protection against flu viruses that the vaccine was not designed for, including novel viruses.[83] The CDC made the following statement in relation to the 2007-2008 vaccine:
- ...[A]ntibodies made in response to vaccination with one strain of influenza viruses can provide protection against different, but related strains. A less than ideal match may result in reduced vaccine effectiveness against the variant viruses, but it still can provide enough protection to prevent or lessen illness severity and prevent flu-related complications. In addition, it is important to remember that the influenza vaccine contains three virus strains so the vaccine can also protect against the other two viruses. For these reasons, even during seasons when there is a less than ideal match, CDC continues to recommend influenza vaccination. This is particularly important for people at high risk for serious flu complications and their close contacts.[56]
Vaccination recommendations

Various public health organizations, including the World Health Organization, have recommended that yearly influenza vaccination be routinely offered to patients at risk of complications of influenza and those individuals who live with or care for high-risk individuals, including:
- the elderly (UK recommendation is those aged 65 or above)
- patients with chronic lung diseases (asthma, COPD, etc.)
- patients with chronic heart diseases (congenital heart disease, chronic heart failure, ischaemic heart disease)
- patients with chronic liver diseases (including cirrhosis)
- patients with chronic renal diseases (such as the nephrotic syndrome)
- patients who are immunosuppressed (those with HIV or who are receiving drugs to suppress the immune system such as chemotherapy and long-term steroids) and their household contacts
- people who live together in large numbers in an environment where influenza can spread rapidly, such as prisons, nursing homes, schools, and dormitories.
- people who plan to attend or participate in a high profile important event with large amounts of people from various places (such as the Olympic Games, FIFA World Cup, and the World's Fair).
- people who are in the armed forces.
- healthcare workers (both to prevent sickness and to prevent spread to patients)[84]
- pregnant women. However, a 2009 review concluded that there was insufficient evidence to recommend routine use of trivalent influenza vaccine during the first trimester of pregnancy.[85] Influenza vaccination during flu season is part of recommendations for influenza vaccination of pregnant women in the United States.[86]
- children from ages six months to two years
Both types of flu vaccines are contraindicated for those with severe allergies to egg proteins and people with a history of Guillain-Barré syndrome.[87]
Cost-effectiveness
The cost-effectiveness of seasonal influenza vaccination has been widely evaluated for different groups and in different settings. In the elderly (aged over 65 years) the majority of published studies have found that vaccination is cost saving, with the cost savings associated with influenza vaccination (e.g. prevented health care visits) outweighing the cost of vaccination.[88] In older adults (aged 50–64 years), several published studies have found that influenza vaccination is likely to be cost-effective, however the results of these studies were often found to be dependent on key assumptions used in the economic evaluations.[89] The uncertainty in influenza cost-effectiveness models can partially be explained by the complexities involved in estimating the disease burden,[90] as well as the seasonal variability in the circulating strains and the match of the vaccine.[91] In healthy working adults (aged 18–49 years), a 2012 review found that vaccination was generally not cost-saving, with the suitability for funding being dependent on the willingness to pay to obtain the associated health benefits.[92] In children, the majority of studies have found that influenza vaccination was cost-effective, however many of the studies included (indirect) productivity gains, which may not be given the same weight in all settings.[93] Several studies have attempted to predict the cost-effectiveness of interventions (including prepandemic vaccination) to help protect against a future pandemic, however estimating the cost-effectiveness has been complicated by uncertainty as to the severity of a potential future pandemic and the efficacy of measures against it.[94]
H5N1
 |
Vaccines have been formulated against several of the avian H5N1 influenza varieties. Vaccination of poultry against the ongoing H5N1 epizootic is widespread in certain countries. Some vaccines also exist for use in humans, and others are in testing, but none have been made available to civilian populations, nor produced in quantities sufficient to protect more than a tiny fraction of the Earth's population in the event of an H5N1 pandemic.
Manufacturing
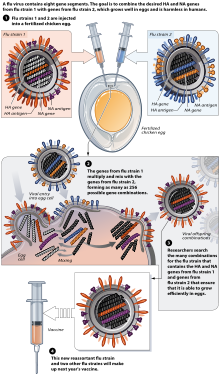
Flu vaccine is usually grown by vaccine manufacturers in fertilized chicken eggs.[95][96] In the Northern hemisphere, the manufacturing process begins following the announcement (typically in February) of the WHO recommended strains for the winter flu season.[95][97] Three strains (representing an H1N1, an H3N2, and a B strain) of flu are selected and chicken eggs inoculated separately, these monovalent harvests are then combined to make the trivalent vaccine.[98]
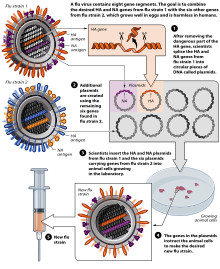
As of November 2007[update], both the conventional injection and the nasal spray are manufactured using chicken eggs.[96] The European Union has also approved Optaflu, a vaccine produced by Novartis using vats of animal cells.[96] This technique is expected to be more scalable and avoid problems with eggs, such as allergic reactions and incompatibility with strains that affect avians like chickens.[96] Research continues into the idea of a "universal" influenza vaccine that would not require tailoring to a particular strains, but would be effective against a broad variety of influenza viruses. However, no vaccine candidates had been announced by Nov 2007.[96]
A DNA-based vaccination, which is hoped to be even faster to manufacture, is as of 2011 in clinical trials, determining safety and efficacy.[99]
On November 20, 2012, Novartis received FDA approval for the first cell-culture vaccine.[100][101][102]
In a 2007 report, the global capacity of approximately 826 million seasonal influenza vaccine doses (inactivated and live) was double the production of 413 million doses. In an aggressive scenario of producing pandemic influenza vaccines by 2013, only 2.8 billion courses could be produced in a six-month time frame. If all high- and upper-middle-income countries sought vaccines for their entire populations in a pandemic, nearly 2 billion courses would be required. If China pursued this goal as well, more than 3 billion courses would be required to serve these populations.[103] Vaccine research and development is ongoing to identify novel vaccine approaches that could produce much greater quantities of vaccine at a price that is affordable to the global population.
Methods of vaccine generation that bypass the need for eggs include the construction of influenza virus-like particles (VLP). VLP resemble viruses, but there is no need for inactivation, as they do not include viral coding elements, but merely present antigens in a similar manner to a virion. Some methods of producing VLP include cultures of Spodoptera frugiperda Sf9 insect cells and plant-based vaccine production (e.g., production in Nicotiana benthamiana). There is evidence that some VLPs elicit antibodies that recognize a broader panel of antigenically distinct viral isolates compared to other vaccines in the hemagglutination-inhibition assay (HIA).[104]
Annual reformulation
Each year, three strains are chosen for selection in that year's flu vaccination by the WHO Global Influenza Surveillance Network. The chosen strains are the H1N1, H3N2, and Type-B strains thought most likely to cause significant human suffering in the coming season. Starting with the 2012-2013 Northern Hemisphere influenza season (coincident with the approval of quadrivalent influenza vaccines) the WHO has also recommended a 2nd B-strain for use in quadrivalent vaccines. The World Health Organization coordinates the contents of the vaccine each year to contain the most likely strains of the virus to attack the next year.
- "The WHO Global Influenza Surveillance Network was established in 1952. The network comprises 4 WHO Collaborating Centres (WHO CCs) and 112 institutions in 83 countries, which are recognized by WHO as WHO National Influenza Centres (NICs). These NICs collect specimens in their country, perform primary virus isolation and preliminary antigenic characterization. They ship newly isolated strains to WHO CCs for high level antigenic and genetic analysis, the result of which forms the basis for WHO recommendations on the composition of influenza vaccine for the Northern and Southern Hemisphere each year."[105]
The Global Influenza Surveillance Network's selection of viruses for the vaccine manufacturing process is based on its best estimate of which strains will predominate the next year, amounting in the end to well-informed but fallible guesswork.[106]
Formal WHO recommendations first issued in 1973; beginning 1999 there have been two recommendations per year, one for the northern hemisphere (N) and the other for the southern hemisphere (S).[107]
Historical annual reformulations of the influenza vaccine are listed in a separate article. Recent[update] WHO seasonal influenza vaccine composition recommendations:
2013 Southern Hemisphere influenza season
The composition of virus vaccines for use in the 2013 Southern Hemisphere influenza season recommended by the World Health Organization in September, 2012 was:
- an A/California/7/2009 (H1N1)pdm09-like virus;
- an A/Victoria/361/2011 (H3N2)-like virus;
- a B/Wisconsin/1/2010-like virus.
It is recommended that quadrivalent vaccines containing two influenza B viruses contain the above three viruses and a B/Brisbane/60/2008-like virus.[108]
2013-2014 Northern Hemisphere influenza season
The composition of virus vaccines for use in the 2013-2014 Northern Hemisphere influenza season recommended by the World Health Organization on February 20, 2013 was:
- an A/California/7/2009 (H1N1)pdm09-like virus;[109]
- an A(H3N2) virus antigenically like the cell-propagated prototype virus A/Victoria/361/2011†;
- B/Massachusetts/2/2012-like virus.[110]
It is recommended that quadrivalent vaccines containing two influenza B viruses contain the above three viruses and a B/Brisbane/60/2008-like virus[110]
†It is recommended that A/Texas/50/2012 is used as the A(H3N2) vaccine component because of antigenic changes in earlier A/Victoria/361/2011-like vaccine viruses (such as IVR-165) resulting from adaptation to propagation in eggs.
The H1N1 strain used in these compositions is the same strain used in the 2009 flu pandemic vaccine, now known as A(H1N1)pdm09.[111]
History
Vaccines are used in both humans and nonhumans. Human vaccine is meant unless specifically identified as a veterinary, poultry or livestock vaccine.
The first influenza pandemic was recorded in 1580.[112] However, the etiological cause of influenza, the orthomyxoviridae was discovered by the Medical Research Council (MRC) of the United Kingdom in 1933.[113]
| Name of pandemic | Date | Deaths | Case fatality rate | Subtype involved | Pandemic Severity Index |
|---|---|---|---|---|---|
| 1889–1890 flu pandemic (Asiatic or Russian Flu)[117] |
1889–1890 | 1 million | 0.15% | possibly H3N8 or H2N2 |
NA |
| 1918 flu pandemic (Spanish flu)[118] |
1918–1920 | 20 to 100 million | 2% | H1N1 | 5 |
| Asian Flu | 1957–1958 | 1 to 1.5 million | 0.13% | H2N2 | 2 |
| Hong Kong Flu | 1968–1969 | 0.75 to 1 million | <0.1% | H3N2 | 2 |
| Russian flu | 1977–1978 | no accurate count | N/A | H1N1 | N/A |
| 2009 flu pandemic[119][120] | 2009–2010 | 18,000 | 0.03% | H1N1 | NA |
Origins and development
In the world wide Spanish flu pandemic of 1918, "Physicians tried everything they knew, everything they had ever heard of, from the ancient art of bleeding patients, to administering oxygen, to developing new vaccines and sera (chiefly against what we now call Hemophilus influenzae—a name derived from the fact that it was originally considered the etiological agent—and several types of pneumococci). Only one therapeutic measure, transfusing blood from recovered patients to new victims, showed any hint of success."[121]
In 1931, viral growth in embryonated hens' eggs was reported by Ernest William Goodpasture and colleagues at Vanderbilt University. The work was extended to growth of influenza virus by several workers, including Thomas Frances, Wilson Smith and Macfarlane Burnet, leading to the first experimental influenza vaccines.[122] In the 1940s, the US military developed the first approved inactivated vaccines for influenza, which were used in the Second World War.[123] Hen's eggs continued to be used to produce virus used in influenza vaccines, but manufacturers made improvements in the purity of the virus by developing improved processes to remove egg proteins and to reduce systemic reactivity of the vaccine.[124] Recently the US FDA has approved influenza vaccines made by growing virus in cell cultures[125] and influenza vaccines made from recombinant proteins[126] have been approved, with plant-based influenza vaccines being tested in clinical trials.[127]
Acceptance
According to the CDC: "Influenza vaccination is the primary method for preventing influenza and its severe complications. [...] Vaccination is associated with reductions in influenza-related respiratory illness and physician visits among all age groups, hospitalization and death among persons at high risk, otitis media among children, and work absenteeism among adults. Although influenza vaccination levels increased substantially during the 1990s, further improvements in vaccine coverage levels are needed".[128]
The egg-based technology (still in use as of 2005) for producing influenza vaccine was created in the 1950s.[129] In the U.S. swine flu scare of 1976, President Gerald Ford was confronted with a potential swine flu pandemic. The vaccination program was rushed, yet plagued by delays and public relations problems. Meanwhile, maximum military containment efforts succeeded unexpectedly in confining the new strain to the single army base where it had originated. On that base a number of soldiers fell severely ill, but only one died. The program was canceled, after about 24% of the population had received vaccinations. An excess in deaths of twenty-five over normal annual levels as well as 400 excess hospitalizations, both from Guillain-Barré syndrome, were estimated to have occurred from the vaccination program itself, illustrating that vaccine itself is not free of risks. The result has been cited to stoke lingering doubts about vaccination.[130] In the end, however, even the maligned 1976 vaccine may have saved lives. A 2010 study found a significantly enhanced immune response against the 2009 pandemic H1N1 in study participants who had received vaccination against the swine flu in 1976.[76]
Research
Influenza research includes molecular virology, molecular evolution, pathogenesis, host immune responses, genomics, and epidemiology. These help in developing influenza countermeasures such as vaccines, therapies and diagnostic tools. Improved influenza countermeasures require basic research on how viruses enter cells, replicate, mutate, evolve into new strains and induce an immune response. The Influenza Genome Sequencing Project is creating a library of influenza sequences[131] that will help us understand what makes one strain more lethal than another, what genetic determinants most affect immunogenicity, and how the virus evolves over time. Solutions to limitations in current[when?] vaccine methods are being researched.
According to VaccineNewsDaily, a recent study published in Vaccines found that by providing a school-located vaccination clinic, flu vaccination rates among children increased 13.2 percent when compared to children in schools without vaccination clinics. The vaccine can be lifesaving for children and less costly than a doctor's office visit.[132] Children in low-income and minority families are less likely to be vaccinated against influenza. To address this problem, New York-Presbyterian/Columbia University Medical Center implemented a program in 2010 that demonstrated how text messaging parents with educational messages about influenza and influenza vaccinations could improve vaccination rates for low-income, Latino children.[133]
The rapid development, production, and distribution of pandemic influenza vaccines could potentially save millions of lives during an influenza pandemic. Due to the short time frame between identification of a pandemic strain and need for vaccination, researchers are looking at novel technologies for vaccine production that could provide better "real-time" access and be produced more affordably, thereby increasing access for people living in low- and moderate-income countries, where an influenza pandemic may likely originate, such as live attenuated (egg-based or cell-based) technology and recombinant technologies (proteins and virus-like particles).[134] As of July 2009, more than 70 known clinical trials have been completed or are ongoing for pandemic influenza vaccines.[135] In September 2009, the US Food and Drug Administration approved four vaccines against the 2009 H1N1 influenza virus (the 2009 pandemic strain), and expected the initial vaccine lots to be available within the following month.[136] A quadrivalent flu vaccine administered by nasal mist was approved by the U.S. Food and Drug Administration (FDA) in March 2012.[137][138] Fluarix Quadrivalent was approved by the FDA in December 2012.[139]
Prospects for universal flu vaccines
Many groups world wide are working on a universal flu vaccine that doesn't require changing each year.[140] Companies pursuing the vaccine as of 2009 and 2010 include BiondVax,[141] Theraclone,[142] Dynavax Technologies Corporation,[143] VaxInnate,[144] Crucell NV,[145] Inovio Pharmaceuticals,[146] and Immune Targeting Systems (ITS)[147]
In 2008 Acambis announced work on a universal flu vaccine (ACAM-FLU-ATM) based on the less variable M2 protein component of the flu virus shell.[148] The vaccine was tested in a human trial in the United States, where it was reported in 2008 to have developed antibodies against flu virus in 90% of individuals; further human trials were planned.[149] See also H5N1 vaccines.
In 2009, the Wistar Institute received a patent for using "a variety of peptides" in a flu vaccine, and announced it was seeking a corporate partner.[150]
In 2010, the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. NIH announced a breakthrough; the effort targets the stem, which mutates less often than the head of the virus.[151]
DNA vaccines such as VGX-3400X (aimed at multiple H5N1 strains) contain DNA fragments (plasmids).[146][152] Inovio's SynCon DNA vaccines include H5N1 and H1N1 subtypes.[153]
In July 2011, researchers created an antibody, which targets a protein found on the surface of all influenza A viruses called haemagglutinin.[154][155][156] F16 is the only known antibody that binds (its neutralizing activity is controversial) to all 16 subtypes of the influenza A virus hemagglutinin and might be the lynchpin for a universal influenza vaccine.[154][155][156] The subdomain of the hemagglutinin that is targeted by FI6, namely the stalk domain, was actually successfully used earlier as universal influenza virus vaccine by Peter Palese's research group at Mount Sinai School of Medicine.[157]
Other vaccines are polypeptide based.[158]
Some universal flu vaccines have started early stage clinical trials.
- BiondVax are targeting the less variable stalk of the haemagglutinin molecule[159] with Multimeric-001.[160] This is aimed at type A (inc H1N1) and Type B influenza and has started a phase IIa study.[161]
- Dynavax have developed a vaccine N8295 based on two highly conserved antigens NP and M2e[162] and their TLR9 agonist, and started clinical trials in June 2010.[163]
- ITS's fp01[164] includes 6 peptide antigens to highly conserved segments of the PA, PB1, PB2, NP & M1 proteins, and has started phase I trials.
Based on the results of animal studies, a universal flu vaccine may use a two-step vaccination strategy — priming with a DNA-based HA vaccine followed by a second dose with an inactivated, attenuated, or adenovirus-vector–based vaccine.[165]
Some people given a 2009 H1N1 flu vaccine have developed broadly protective antibodies, raises hopes for a universal flu vaccine.[166][167][168]
On February 13, 2013, U.S. Food and Drug Administration (FDA) Chief Scientist Jesse Goodman predicted that a universal flu vaccine was still 5 to 10 years away. When asked about the prospects of a universal flu vaccine in a hearing before House Energy and Commerce Subcommittee on Oversight and Investigations, Goodman replied "Nature is very tricky and as this is a very crafty virus, so I'd be very hesitant to predict... I think the earliest we'd begin to see something with clinical benefit might be 5 to 10 years."[169]
Veterinary use
"Vaccination in the veterinary world pursues four goals: (i) protection from clinical disease, (ii) protection from infection with virulent virus, (iii) protection from virus excretion, and (iv) serological differentiation of infected from vaccinated animals (so-called DIVA principle). In the field of influenza vaccination, neither commercially available nor experimentally tested vaccines have been shown so far to fulfil all of these requirements."[170]
Horses
Horses with horse flu can run a fever, have a dry hacking cough, have a runny nose, and become depressed and reluctant to eat or drink for several days but usually recover in two to three weeks. "Vaccination schedules generally require a primary course of 2 doses, 3–6 weeks apart, followed by boosters at 6–12 month intervals. It is generally recognised that in many cases such schedules may not maintain protective levels of antibody and more frequent administration is advised in high-risk situations."[171]
It is a common requirement at shows in the United Kingdom that horses be vaccinated against equine flu and a vaccination card must be produced; the International Federation for Equestrian Sports (FEI) requires vaccination every six months.[172][173]
Poultry
Poultry vaccines for bird flu are made on the cheap and are not filtered and purified like human vaccines to remove bits of bacteria or other viruses. They usually contain whole virus, not just hemagglutinin as in most human flu vaccines. Purification to standards needed for humans is far more expensive than the original creation of the unpurified vaccine from eggs. There is no market for veterinary vaccines that are that expensive. Another difference between human and poultry vaccines is that poultry vaccines are adjuvated with mineral oil, which induces a strong immune reaction but can cause inflammation and abscesses. "Chicken vaccinators who have accidentally jabbed themselves have developed painful swollen fingers or even lost thumbs, doctors said. Effectiveness may also be limited. Chicken vaccines are often only vaguely similar to circulating flu strains — some contain an H5N2 strain isolated in Mexico years ago. 'With a chicken, if you use a vaccine that's only 85 percent related, you'll get protection,' Dr. Cardona said. 'In humans, you can get a single point mutation, and a vaccine that's 99.99 percent related won't protect you.' And they are weaker [than human vaccines]. 'Chickens are smaller and you only need to protect them for six weeks, because that's how long they live till you eat them,' said Dr. John J. Treanor, a vaccine expert at the University of Rochester. Human seasonal flu vaccines contain about 45 micrograms of antigen, while an experimental A(H5N1) vaccine contains 180. Chicken vaccines may contain less than 1 microgram. 'You have to be careful about extrapolating data from poultry to humans,' warned Dr. David E. Swayne, director of the agriculture department's Southeast Poultry Research Laboratory. 'Birds are more closely related to dinosaurs.'"[174]
Researchers, led by Nicholas Savill of the University of Edinburgh in Scotland, used mathematical models to simulate the spread of H5N1 and concluded that "at least 95 percent of birds need to be protected to prevent the virus spreading silently. In practice, it is difficult to protect more than 90 percent of a flock; protection levels achieved by a vaccine are usually much lower than this."[175] The Food and Agriculture Organization of the United Nations has issued recommendations on the prevention and control of avian influenza in poultry, including the use of vaccination.[176]
A filtered and purified Influenza A vaccine for humans is being developed[when?] and many countries have recommended it be stockpiled so if an Avian influenza pandemic starts jumping to humans, the vaccine can quickly be administered to avoid loss of life. Avian influenza is sometimes called avian flu, and commonly bird flu.[177]
Pigs
Swine origin influenza virus (SoIV) vaccines are extensively used in the swine industry in Europe and North America. Most swine flu vaccine manufacturers include an H1N1 and an H3N2 SoIV strains.
Swine influenza has been recognized as a greater problem since the outbreak in 1976. Evolution of the virus has resulted in inconsistent responses to traditional vaccines. Standard commercial swine origin flu vaccines are effective in controlling the problem when the virus strains match enough to have significant cross-protection and custom (autogenous) vaccines made from the specific viruses isolated are created and used in the more difficult cases.[178] SoIV vaccine manufacture Novartis paints this picture: "A strain of swine origin influenza virus (SoIV) called H3N2, first identified in the US in 1998, has brought exasperating production losses to swine producers. Abortion storms are a common sign. Sows go off feed for two or three days and run a fever up to 106°F. Mortality in a naïve herd can run as high as 15%."[179]
Dogs
In 2004, Influenza A virus subtype H3N8 was discovered to cause canine influenza. Because of the lack of previous exposure to this virus, dogs have no natural immunity to this virus. However a vaccine is now available.[180]
References
- ^ Couch, RB (2008). "Seasonal Inactivated Influenza Virus Vaccines". Vaccine. 26 Suppl 4 (Suppl 4): D5–9. doi:10.1016/j.vaccine.2008.05.076. PMC 2643340. PMID 18602728.
- ^ a b c "Key Facts About Seasonal Flu Vaccine"
- ^ a b [No authors listed] (September 9, 2011). "Who Should Get Vaccinated Against Influenza". U.S. Centers for Disease Control and Prevention. Retrieved April 7, 2013.
- ^ a b c World Health Organization. Seasonal Influenza
- ^ a b Manzoli L, Ioannidis JP, Flacco ME, De Vito C, Villari P (2012). "Effectiveness and harms of seasonal and pandemic influenza vaccines in children, adults and elderly: a critical review and re-analysis of 15 meta-analyses". Hum Vaccin Immunother. 8 (7): 851–62. doi:10.4161/hv.19917. PMC 3495721. PMID 22777099.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ pid=20601081&sid=aoDQn_PvtPGw&refer=Australia[verification needed]
- ^ McNeil, Donald G. Jr. (March 28, 2008). "A Multitude of Vaccine Benefits, Yet Controversy Persists,". The New York Times.
Public health experts generally agree that after clean water and flush toilets, the most important health advances in history have been vaccinations
- ^ Center for Disease Control and Prevention. "Key Facts About Seasonal Flu Vaccine". Retrieved February 7, 2013.
- ^ WHO Influenza Overview
- ^ "Estimates of deaths associated with seasonal influenza --- United States, 1976-2007" (PDF). MMWR Morb. Mortal. Wkly. Rep. 59 (33). Centers for Disease Control and Prevention (CDC): 1057–62. 2010. PMID 20798667.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Julie Steenhuysen (August 26, 2010). "CDC backs away from decades-old flu death estimate". Reuters. Retrieved September 13, 2010.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18679129, please use {{cite journal}} with
|pmid=18679129instead. - ^ WHO Influenza Overview
http://www.who.int/mediacentre/factsheets/2003/fs211/en/
- "In the United States of America, for example, recent estimates put the cost of influenza epidemics to the economy at US$ 71-167 billion per year."
- ^ Molinari NA, Ortega-Sanchez IR, Messonnier ML; et al. (2007). "The annual impact of seasonal influenza in the US: measuring disease burden and costs". Vaccine. 25 (27): 5086–96. doi:10.1016/j.vaccine.2007.03.046. PMID 17544181.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) (Estimates that in the United States, annual influenza epidemics result in approximately 600,000 life-years lost, 3 million hospitalized days, and 30 million outpatient visits, resulting in direct medical costs of $10 billion annually. Lost earnings due to illness and loss of life added over $15 billion in direct costs annually and the average of all direct and indirect economic burdens of annual influenza epidemics (funeral expenses, lost productivity, etc.) amounts to over $80 billion. - ^ Huge flu vaccine trial to be held. March 31, 2008 :"Professor Robert Booy, of the National Centre for Immunisation Research and Surveillance at Westmead in Sydney, said influenza put 15,000 Australians in hospital each year."
- ...
- "About 1,500 deaths annually are attributed to the virus and it costs our country millions of dollars annually in lost productivity, Professor Robert Booy said.
- ^ "Young Children Hospitalized for Flu Associated With Higher Costs and Higher Risk Illness" (Press release). Cincinnati Children's Hospital Medical Center. May 4, 2008.
After analyzing data in three U.S. cities over the course of three flu seasons (2003-2006), the researchers found that 90 percent of the highest-cost hospitalizations for children were linked to influenza, or flu with a co-infection of the respiratory tract
- ^ CDC (November 17, 2011). "Adolescent School Health".
- ^ Hoen AG (2011). "Effect of expanded US recommendations for seasonal influenza vaccination: comparison of two pediatric emergency departments in the United States and Canada". CMAJ. 183 (13): E1025–32. doi:10.1503/cmaj.110241. PMC 3176865. PMID 21930745.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Nicholas Bakalar (September 19, 2011). "Prevention: Flu Shots' Benefits Seen in Hospital Study". The New York Times.
- ^ Katayose M (2011). "The effectiveness of trivalent inactivated influenza vaccine in children over six consecutive influenza seasons". Vaccine. 29 (9): 1844–9. doi:10.1016/j.vaccine.2010.12.049. PMID 21195802.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ "U.S. panel recommends all kids get the flu shot". CTV. February 27, 2008.
In Canada, the National Advisory Committee on Immunization (NACI), the group that advises the Public Health Agency of Canada, currently says that children between the age of six and 24 months should be considered a high priority for the flu vaccine.
- ^ a b Fiore, AE; Uyeki, TM; Broder, K; Finelli, L; Euler, GL; Singleton, JA; Iskander, JK; Wortley, PM; Shay, DK (2010). "Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010" (PDF). MMWR Recomm Rep. 59 (RR–8): 1–62. PMID 20689501.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)--United States, 2012-13 influenza season" (PDF). MMWR Morb. Mortal. Wkly. Rep. 61 (32): 613–8. 2012. ISSN 0149-2195. PMID 22895385.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ CDC - Influenza (Flu) | Vaccination: Summary for Clinicians
- ^ Comparisons of LAIV and TAIV Efficacy Centers for Disease Control and Prevention (CDC)
- ^ "Children, the Flu, and the Flu Vaccine"
- ^ Centers for Disease Control and Prevention (CDC) (2010). "Seasonal influenza and 2009 H1N1 influenza vaccination coverage among pregnant women--10 states, 2009-10 influenza season" (PDF). MMWR Morb Mortal Wkly Rep. 59 (47): 1541–5. PMID 21124293.
Because pregnant women are at increased risk for severe disease associated with influenza infection, the American College of Obstetricians and Gynecologists and the Advisory Committee on Immunization Practices have recommended seasonal influenza vaccination for women while pregnant, regardless of trimester (1,2). In 2009, a novel strain of influenza A (H1N1) virus was identified (3), and pregnant women also were found at greater risk for influenza-related complications from this new virus (4). As a result, during the 2009--10 influenza season, two separate influenza vaccines were recommended to pregnant women: inactivated trivalent 2009--10 seasonal vaccine and influenza A (H1N1) 2009 monovalent vaccine (2,5)
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Fiore, AE; Shay, DK; Broder, K; Iskander, JK; Uyeki, TM; Mootrey, G; Bresee, JS; Cox, NJ; Centers for Disease Control and Prevention (2009). "Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009" (PDF). MMWR Recomm Rep. 58 (RR–8): 1–52. PMID 19644442.
Annual vaccination of all children aged 6 months--18 years should begin as soon as the 2009--10 influenza vaccine is available. Annual vaccination of all children aged 6 months--4 years (59 months) and older children with conditions that place them at increased risk for complications from influenza should continue to be a primary focus of vaccination efforts as providers and programs transition to routinely vaccinating all children.
- ^ a b Altman, Lawrence K. (February 28, 2008). "Panel Advises Flu Shots for Children Up to Age 18". The New York Times.
- ^ McNeil, Donald G. Jr. (March 9, 2010). "Flu Shots in Children Can Help Community". The New York Times.
- ^ Benowitz, I.; Esposito, D. B.; Gracey, K. D.; Shapiro, E. D.; Vázquez, M. (2010). "Influenza Vaccine Given to Pregnant Women Reduces Hospitalization Due to Influenza in Their Infants". Clin Infect Dis. 51 (12): 1355–61. doi:10.1086/657309. PMC 3106242. PMID 21058908.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Osterholm MT, Kelley NS, Sommer A, Belongia EA. (2012). "Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis". Lancet Infect Dis. 12 (1): 36–44. doi:10.1016/S1473-3099(11)70295-X. PMID 22032844.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19682118, please use {{cite journal}} with
|pmid=19682118instead. - ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16546308, please use {{cite journal}} with
|pmid=16546308instead. - ^ Wendelboe AM, Avery C, Andrade B, Baumbach J, Landen MG (2011). "Importance of employee vaccination against influenza in preventing cases in long-term care facilities". Infect Control Hosp Epidemiol. 32 (10): 990–7. doi:10.1086/661916. PMID 21931249.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Coverage in healthcare workers
- ^ "Influenza Vaccination Coverage Among Health-Care Personnel --- United States, 2010--11 Influenza Season". Morbidity and Mortality Weekly Report (MMWR)
- ^ "Influenza vaccination coverage among health-care personnel: 2011-12 influenza season, United States" (PDF). MMWR Morb. Mortal. Wkly. Rep. 61 (38): 753–7. 2012. ISSN 0149-2195. PMID 23013720.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ CDC - Inactivated Influenza Vaccine 2007-2008 - What You Need To Know
- ^ a b c Flu - LAIV
- ^ Offit PA (2007). "Thimerosal and vaccines—a cautionary tale" (PDF). N Engl J Med. 357 (13): 1278–9. doi:10.1056/NEJMp078187. PMID 17898096.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Global Advisory Committee on Vaccine Safety (July 14, 2006). "Thiomersal and vaccines". World Health Organization. Retrieved November 20, 2007.
- ^ Haber P, Sejvar J, Mikaeloff Y, DeStefano F (2009). "Vaccines and Guillain-Barré syndrome". Drug Safety. 32 (4): 309–23. doi:10.2165/00002018-200932040-00005. PMID 19388722.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kaplan JE, Katona P, Hurwitz ES, Schonberger LB (1982). "Guillain-Barré syndrome in the United States, 1979-1980 and 1980-1981. Lack of an association with influenza vaccination". JAMA. 248 (6): 698–700. doi:10.1001/jama.248.6.698. PMID 7097920.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Denise Grady (December 4, 2009). "Review Shows Safety of H1N1 Vaccine, Officials Say". The New York Times.
No substantial differences between H1N1 and seasonal influenza vaccines were noted in the proportion or types of serious adverse events reported
- ^ Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K, Haber P (2009). "Safety of trivalent inactivated influenza vaccines in adults: Background for pandemic influenza vaccine safety monitoring". Vaccine. 27 (15): 2114–2120. doi:10.1016/j.vaccine.2009.01.125. PMID 19356614.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Steven Reinberg (February 2, 2011). "Last Year's H1N1 Flu Vaccine Was Safe, Study Finds". U.S.News & World Report.
- ^ Stowe J, Andrews N, Wise L, Miller E (2009). "Investigation of the temporal association of Guillain-Barre syndrome with influenza vaccine and influenzalike illness using the United Kingdom General Practice Research Database". Am. J. Epidemiol. 169 (3): 382–8. doi:10.1093/aje/kwn310. PMID 19033158.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sivadon-Tardy V; Orlikowski D; Porcher R; et al. (2009). "Guillain-Barré syndrome and influenza virus infection". Clin. Infect. Dis. 48 (1): 48–56. doi:10.1086/594124. PMID 19025491.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ Fedson DS (1998). "Measuring protection: efficacy versus effectiveness". Dev Biol Stand. 95: 195–201. PMID 9855432.
- ^ a b c d Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E (2010). "Vaccines for preventing influenza in healthy adults". Cochrane Database Syst Rev (7): CD001269. doi:10.1002/14651858.CD001269.pub4. PMID 20614424.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stephenson, I.; Zambon, M. C.; Rudin, A.; Colegate, A.; Podda, A.; Bugarini, R.; Del Giudice, G.; Minutello, A.; Bonnington, S. (2006). "Phase I Evaluation of Intranasal Trivalent Inactivated Influenza Vaccine with Nontoxigenic Escherichia coli Enterotoxin and Novel Biovector as Mucosal Adjuvants, Using Adult Volunteers". J Virol. 80 (10): 4962–70. doi:10.1128/JVI.80.10.4962-4970.2006. PMC 1472052. PMID 16641287.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Treanor, J.; Kotloff, K.; Betts, R.; Belshe, R.; Newman, F.; Iacuzio, D.; Wittes, J.; Bryant, M. (1999). "Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses". Vaccine. 18 (9–10): 899–906. doi:10.1016/S0264-410X(99)00334-5. PMID 10580204.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Jefferson, T. (2006). "Influenza vaccination: policy versus evidence". BMJ. 333 (7574): 912–5. doi:10.1136/bmj.38995.531701.80. PMC 1626345. PMID 17068038.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ CDC - Influenza (Flu) | Q & A: 2007-08 Flu Season
- ^ a b CDC - seasonal flu
- ^ a b Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V, Ferroni E (2012). "Vaccines for preventing influenza in healthy children". Cochrane Database Syst Rev. 8: CD004879. doi:10.1002/14651858.CD004879.pub4. PMID 22895945.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE (2010). "Vaccines for preventing influenza in the elderly". Cochrane Database Syst Rev (2): CD004876. doi:10.1002/14651858.CD004876.pub3. PMID 20166072.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V (2005). "Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review". Lancet. 366 (9492): 1165–74. doi:10.1016/S0140-6736(05)67339-4. PMID 16198765.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nichol, K.L.; Nordin, J.D.; Nelson, D.B.; Mullooly, J.D.; Hak, E. (2007). "Effectiveness of Influenza Vaccine in the community-dwelling elderly". The New England Journal of Medicine. 357 (14): 1373–1381. doi:10.1056/NEJMoa070844. PMID 17914038.
- ^ Vu, T.; Farish, S.; Jenkins, M.; Kelly, H. (2002). "A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community". Vaccine. 20 (13–14): 1831–1836. doi:10.1016/S0264-410X(02)00041-5. PMID 11906772.
- ^ Nordin, J.; Mullooly, J.; Poblete, S.; Strikas, R.; Petrucci, R.; Wei, F.; Rush, B; Safirstein, B; Wheeler, D (2001). "Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: Data from 3 health plans". The Journal of Infectious Diseases. 184 (6): 665–670. doi:10.1086/323085. PMID 11517426.
- ^ Simonsen L, Viboud C, Taylor RJ, Miller MA, Jackson L (2009). "Influenza vaccination and mortality benefits: new insights, new opportunities". Vaccine. 27 (45): 6300–4. doi:10.1016/j.vaccine.2009.07.008. PMID 19840664.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Osterholm, MT; Kelley, NS; Sommer, A; Belongia, EA (2012). "Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis". The Lancet Infectious Diseases. 12 (1): 36–44. doi:10.1016/S1473-3099(11)70295-X. PMID 22032844. Also discussed in "Efficacy and Effectiveness of Influenza Vaccines: A Systematic Review and Meta-analysis". JournalistsResource.org, retrieved February 2, 2012
- ^ http://www.sciencebasedmedicine.org/index.php/high-dose-flu-vaccine-for-the-elderly/
- ^ http://www.cdc.gov/flu/protect/vaccine/qa_fluzone.htm
- ^ Nuño, M.; Chowell, G.; Gumel, A. B. (2007). "Assessing the role of basic control measures, antivirals and vaccine in curtailing pandemic influenza: scenarios for the US, UK and the Netherlands". Journal of the Royal Society Interface. 4 (14): 505–521. doi:10.1098/rsif.2006.0186. PMC 2373400. PMID 17251132.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Ferguson, N. M.; Cummings, D. A.; Fraser, C.; Cajka, J. C.; Cooley, P. C.; Burke, D. S. (2006). "Strategies for mitigating an influenza pandemic". Nature. 442 (7101): 448–452. Bibcode:2006Natur.442..448F. doi:10.1038/nature04795. PMID 16642006.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Simpson, CR; Ritchie, LD; Robertson, C; Sheikh, A; McMenamin, J (2012). "Effectiveness of H1N1 vaccine for the prevention of pandemic influenza in Scotland, UK: A retrospective observational cohort study". The Lancet Infectious Diseases. 12 (9): 696–702. doi:10.1016/S1473-3099(12)70133-0. PMID 22738894.
- ^ Simonsen, L.; Taylor, R.J.; Viboud, C.; Miller, M.A.; Jackson, L.A. (2007). "Mortality benefits of influenza vaccination in elderly people: An ongoing controversy". The Lancet Infectious Diseases. 7 (10): 658–666. doi:10.1016/S1473-3099(07)70236-0. PMID 17897608.
- ^ Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K (2003). "Mortality associated with influenza and respiratory syncytial virus in the United States". The Journal of the American Medical Association. 289 (2): 179–186. doi:10.1001/jama.289.2.179. PMID 12517228.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ NEJM - The Japanese Experience with Vaccinating Schoolchildren against Influenza
- ^ Thomas RE, Jefferson T, Lasserson TJ (2010). "Influenza vaccination for healthcare workers who work with the elderly". Cochrane Database Syst Rev (2): CD005187. doi:10.1002/14651858.CD005187.pub3. PMID 20166073.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Duration of Serum Antibody Response to Seasonal Influenza Vaccines: Summary, presentation citing references
- ^ New York Times: IDEAS & TRENDS; Flu, 1 December 1991. "Once infected with a strain of flu, people develop lifelong immunity to it." Example of earlier belief in lifelong immunity.]
- ^ a b McCullers JA, Van De Velde LA, Allison KJ, Branum KC, Webby RJ, Flynn PM (2010). "Vaccinees against the 1976 "swine flu" have enhanced neutralization responses to the 2009 novel H1N1 influenza virus". Clin. Infect. Dis. 50 (11): 1487–92. doi:10.1086/652441. PMC 2946351. PMID 20415539.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ e! Science News,Survivors of 1918 flu pandemic protected with a lifetime immunity to virus
- ^ Plotkin & Mortimer (1988). Vaccines. Philadelphia: W.B. Saunders Company. ISBN 0-7216-1946-0.
- ^ Product Monograph: Flumist, Astrazeneca Canada Inc., 2011
- ^ immunize.org article Summary of Recommendations for Adult Immunization, which is linked from CDC website CDC
- ^ "The Nasal-Spray Flu Vaccine (Live Attenuated Influenza Vaccine [LAIV])" Centers for Disease Control and Prevention (CDC)
- ^ Wang, Z.; Tobler, S.; Roayaei, J.; Eick, A. (2009). "Live attenuated or inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel" (PDF). JAMA: the Journal of the American Medical Association. 301 (9): 945–53. doi:10.1001/jama.2009.265. PMID 19255113.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Xie H, Jing X, Li X; et al. (2011). "Immunogenicity and cross-reactivity of 2009-2010 inactivated seasonal influenza vaccine in US adults and elderly". PLoS ONE. 6 (1): e16650. doi:10.1371/journal.pone.0016650. PMC 3031605. PMID 21304946.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Thomas RE, Jefferson TO, Demicheli V, Rivetti D (2006). "Influenza vaccination for health-care workers who work with elderly people in institutions: a systematic review". Lancet Infect Dis. 6 (5): 273–279. doi:10.1016/S1473-3099(06)70462-5. PMID 16631547.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Skowronski DM, De Serres G (2009). "Is routine influenza immunization warranted in early pregnancy?". Vaccine. 27 (35): 4754–70. doi:10.1016/j.vaccine.2009.03.079. PMID 19515466.
- ^ Health Care Guideline: Routine Prenatal Care. Fourteenth Edition. By the Institute for Clinical Systems Improvement. July 2010.
- ^ CDC
- ^ Postma, M.J (2006). "Further evidence for favorable cost-effectiveness of elderly influenza vaccination". Expert Review of Pharmacoeconomics and Outcomes Research. 6 (2). doi:10.1586/14737167.6.2.215. PMID 20528557.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Newall, AT; Kelly, H; Harsley, S; Scuffham, PA (2009). "Cost Effectiveness of Influenza Vaccination in Older Adults". PharmacoEconomics. 27 (6): 439–50. doi:10.2165/00019053-200927060-00001. PMID 19640008.
- ^ Newall, AT; Viboud, C; Wood, JG (2009). "Influenza-attributable mortality in Australians aged more than 50 years: A comparison of different modelling approaches". Epidemiology and Infection. 138 (6): 836–42. doi:10.1017/S095026880999118X. PMID 19941685.
- ^ Newall, A.T. (2011). "Uncertainty and variability in influenza cost-effectiveness models". Australian and New Zealand Journal of Public Health. 35 (6). doi:10.1111/j.1753-6405.2011.00788.x. PMID 22151168.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Gatwood, J; Meltzer, MI; Messonnier, M; Ortega-Sanchez, IR; Balkrishnan, R; Prosser, LA (2012). "Seasonal Influenza Vaccination of Healthy Working-Age Adults". Drugs. 72 (1): 35–48. doi:10.2165/11597310-000000000-00000. PMID 22191794.
- ^ Newall, Anthony T. (August 1, 2012). "Economic Evaluations of Childhood Influenza Vaccination". PharmacoEconomics. 30 (8): 647–660. doi:10.2165/11599130-000000000-00000.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Newall, A.T. (2010). "Cost-effectiveness of pharmaceutical-based pandemic influenza mitigation strategies". Emerging Infectious Diseases. 16 (2). doi:10.3201/eid1602.090571.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b how it's made
- ^ a b c d e New and Old Ways to Make Flu Vaccines, November 8, 2007, National Public Radio.
- ^ WHO:Influenza vaccine viruses and reagents
- ^ "Recommendations for the production and control of influenza vaccine (inactivated)" (PDF). World Health Organization. Retrieved May 27, 2013.
- ^ "Priming with DNA vaccine makes avian flu vaccine work better (NIH News)". October 3, 2011.
- ^ "Novartis receives FDA approval for Flucelvax, the first cell-culture vaccine in US to help protect against seasonal influenza" (Press release). Novartis. November 20, 2012.
- ^ "FDA approves first seasonal influenza vaccine manufactured using cell culture technology"
- ^ "November 20, 2012 Approval Letter- Flucelvax"
- ^ PATH, Oliver Wyman. Influenza Vaccine Strategies for Broad Global Access. 2007. http://www.path.org/files/VAC_infl_publ_rpt_10-07.pdf
- ^ Bright, R. A.; Carter, D. M.; Daniluk, S.; Toapanta, F. R.; Ahmad, A.; Gavrilov, V.; Massare, M.; Pushko, P.; Mytle, N. (2007). "Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin". Vaccine. 25 (19): 3871–8. doi:10.1016/j.vaccine.2007.01.106. PMID 17337102.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ WHO article Global influenza surveillance[verification needed]
- ^ Keeping ahead of flu comes down to guessing game : Health and Fitness : Knoxville News Sentinel
- ^ WHO Report on Global Surveillance of Epidemic-prone Infectious Diseases (pdf)
- ^ WHO | Recommended composition of influenza virus vaccines for use in the 2013 southern hemisphere influenza season.
- ^ WHO | Standardization of terminology of the pandemic A(H1N1)2009 virus
- ^ a b WHO | Recommended composition of influenza virus vaccines for use in the 2013-2014 northern hemisphere influenza season
- ^ Update on Influenza A (H1N1) 2009 Monovalent Vaccines
- ^ Webster, Robert; Walker, Elizabeth (2003). "Influenza". American Scientist. 91 (2): 122. doi:10.1511/2003.2.122.
- ^ Blakemore, C. (April 9, 2006). "Battle of time, luck and science". The Sunday Times. London. Retrieved June 22, 2006.
- ^ Hilleman, M (2002). "Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control". Vaccine. 20 (25–26): 3068–87. doi:10.1016/S0264-410X(02)00254-2. PMID 12163258.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Cite error: The named reference
Potterwas invoked but never defined (see the help page). - ^ "Ten things you need to know about pandemic influenza". World Health Organization. October 14, 2005. Archived from the original on September 23, 2009. Retrieved September 26, 2009.
- ^ Valleron AJ, Cori A, Valtat S, Meurisse S, Carrat F, Boëlle PY (2010). "Transmissibility and geographic spread of the 1889 influenza pandemic". Proc. Natl. Acad. Sci. U.S.A. 107 (19): 8778–81. Bibcode:2010PNAS..107.8778V. doi:10.1073/pnas.1000886107. PMC 2889325. PMID 20421481.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mills CE, Robins JM, Lipsitch M (2004). "Transmissibility of 1918 pandemic influenza". Nature. 432 (7019): 904–6. Bibcode:2004Natur.432..904M. doi:10.1038/nature03063. PMID 15602562.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Donaldson LJ; Rutter PD; Ellis BM; et al. (2009). "Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study". BMJ. 339: b5213. doi:10.1136/bmj.b5213. PMC 2791802. PMID 20007665.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ "ECDC Daily Update – Pandemic (H1N1) 2009 – January 18, 2010" (PDF). European Centre for Disease Prevention and Control. January 18, 2010. Retrieved January 18, 2010.
- ^ The Threat of Pandemic Influenza: Are We Ready? Workshop Summary (2005) (free online book) page 62
- ^ Plotkin, S.L. and Plotkin, S.A. "A short history of vaccination." In: Vaccines, Stanley A. Plotkin, Walter A. Orenstein, Paul A. Offit, eds. Elsevier Health Sciences, 2008, pp. 6-7.
- ^ Artenstein, A.W. "Influenza" In: Vaccines: A Biography Andrew W. Artenstein, ed. pp. 191-205.
- ^ Hampson, AW. Vaccines for Pandemic Influenza. The History of our Current Vaccines, their Limitations and the Requirements to Deal with a Pandemic Threat. Ann Acad Med Singapore 2008;37:510-7.
- ^ USFDA FDA approves first seasonal influenza vaccine manufactured using cell culture technology. November 20, 2012.
- ^ USFDA FDA approves new seasonal influenza vaccine made using novel technology. Jan. 16, 2013.
- ^ Landry, Nathalie (December 22, 2010). "Preclinical and Clinical Development of Plant-Made Virus-Like Particle Vaccine against Avian H5N1 Influenza". PLOS One. 5 (12). doi:10.1371/journal.pone.0015559. Retrieved May 14, 2013.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ CDC report Prevention and Control of Influenza published April 12, 2002.
- ^ Osterholm, Michael T. (2005). "Preparing for the Next Pandemic". New England Journal of Medicine. 352 (18): 1839–42. doi:10.1056/NEJMp058068. PMID 15872196.
- ^ The Sky is Falling: An Analysis of the Swine Flu Affair of 1976
- ^ "Influenza Genome Sequencing Project - Overview". National Institutes of Health - National Institute of Allergy and Infectious Diseases. Retrieved May 27, 2013.
- ^ Tinder, Paul (June 7, 2013). "Vaccination programs at schools could reduce flu deaths among children at VND". VaccineNewsDaily. Retrieved June 10, 2013.
- ^ "Text Messages to Parents Increase Influenza Immunization Rate for Low-Income, Minority Children". Agency for Healthcare Research and Quality. December 19, 2012. Retrieved July 9, 2013.
- ^ World Health Organization. Acyte Respiratory Infections: Influenza. 2009. http://www.who.int/vaccine_research/diseases/ari/en/index1.html
- ^ World Health Organization. Tables on the Clinical trials of pandemic influenza prototype vaccines. July 2009. http://www.who.int/vaccine_research/immunogenicity/immunogenicity_table.xls
- ^ US Food & Drug Administration. FDA Approves Vaccines for 2009 H1N1 Influenza Virus Approval Provides Important Tool to Fight Pandemic. September 15, 2009. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm182399.htm
- ^ "First Quadrivalent Vaccine Against Seasonal Flu Wins FDA Approval"
- ^ "FDA approves first quadrivalent vaccine to prevent seasonal influenza"
- ^ "December 14, 2012 Approval Letter- Fluarix Quadrivalent"
- ^ Du, Lanying; Zhou, Yusen; Jiang, Shibo (2010). "Research and development of universal influenza vaccines". Microbes and Infection. 12 (4): 280–6. doi:10.1016/j.micinf.2010.01.001. PMID 20079871.
- ^ <http://www.fiercebiotech.com/press-releases/biondvax-begins-phase-iia-study-universal-flu-vaccine-0>
- ^ Seattle's Theraclone makes a 'first step' on long road to universal flu vaccine. The Seattle Times.
- ^ Dynavax Reports Positive Data on Universal Flu Vaccine Candidate. Rita Biotech.
- ^ VaxInnate's Universal Flu Vaccine Candidate Shown Safe and Immunogenic in Phase I Clinical Study. Fierce Biotech.
- ^ Johnson & Johnson pursues vaccine firm. Charleston Gazette.
- ^ a b "Inovio Pharmaceuticals, Inc. Immunizes First Subject In U.S. Influenza DNA Vaccine Clinical Trial". Reuters.
- ^ Immune Targeting Systems - About Us
- ^ "Universal Influenza Vaccine Tested Successfully In Humans".
- ^ "Universal flu jab works in people". BBC News Online. January 4, 2008.
- ^ The Wistar Institute obtains patent for universal flu vaccine technology. Wistar Institute.
- ^ NIH Scientists Advance Universal Flu Vaccine. NIH.
- ^ Inovio Biomedical's SynCon preventive DNA vaccine receives approval in Korea for Phase I clinical trial
- ^ "Scientific Paper on Inovio Pharmaceuticals SynCon(TM) DNA Vaccines and Intradermal DNA Delivery Technology One of Most Cited Articles in the Journal Vaccine". October 14, 2010.
- ^ a b BBC: 'Super antibody' fights off flu
- ^ a b Independent: Scientists hail the prospect of a universal vaccine for flu
- ^ a b "Universal Flu Vaccine On The Horizon: Researchers Find 'Super Antibody'" The Huffington Post. July 28, 2011
- ^ Influenza Virus Vaccine Based on the Conserved Hemagglutinin Stalk Domain
- ^ Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Wilson IA, García-Sastre A, Moran TM (2010). "Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes". Proc Natl Acad Sci U S A. 107 (44): 18979–84. doi:10.1073/pnas.1013387107. PMID 20956293.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hidden weakness could yield universal flu vaccine - health - February 24, 2009 - New Scientist
- ^ http://biopharminternational.findpharma.com/biopharm/News/Phase-1-2-Completed-for-Universal-Influenza-Vaccin/ArticleStandard/Article/detail/651691
- ^ "BiondVax Begins Phase IIa Study for Universal Flu Vaccine". October 14, 2010.
- ^ "Dynavax Presents Data From Novel Universal Flu Vaccine Candidate".
- ^ MarketWatch.com
- ^ Immune Targeting Systems - FP01 Influenza
- ^ Lambert and Fauci; Fauci, Anthony S. (2010). "Influenza Vaccines for the Future". NEJM. 363 (21): 2036–44. doi:10.1056/NEJMra1002842. PMID 21083388.
- ^ "H1N1 Gives Clues to Universal Flu Vaccine". January 18, 2011.
- ^ Wrammert; Koutsonanos, D.; Li, G.-M.; et al. (2011). "Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection" (PDF). JEM. 208: 181–193. doi:10.1084/jem.20101352.
{{cite journal}}: Unknown parameter|author-separator=ignored (help); Unknown parameter|month=ignored (help) - ^ "A vaccine for all flu seasons". Spring 2011.
- ^ Roos, Robert. "FDA expert: Universal flu vaccine still 5-10 years off". Center for Infectious Disease Research and Policy (CIDRAP). Retrieved February 13, 2013.
- ^ Influenza Report (online book) chapter Avian Influenza by Timm C. Harder and Ortrud Werner
- ^ equiflunet_vaccines
- ^ UAE Equestrian & Racing Federation
- ^ FEI guidelines
- ^ McNeil, Donald G. Jr. (May 2, 2006). "Turning to Chickens in Fight With Bird Flu". The New York Times.
- ^ SciDev.Net article Bird flu warning over partial protection of flocks published August 16, 2006
- ^ FAO Recommendations on the Prevention, Control and Eradication of Highly Pathogenic Avian Influenza (HPAI) in Asia http://www.fao.org/docs/eims/upload/246982/aj126e00.pdf
- ^ Yen, Hui-Ling (June 21, 2012). "Virology: Bird flu in mammals". Nature. 486: 332–333. Retrieved May 27, 2013.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ National Hog Farmer article Swine Flu Virus Turns Endemic published September 15, 2007
- ^ livestock.novartis.com Custom Vaccines: Swine
- ^ Karaca K, Dubovi E, Siger L, Robles A, Audonnet J, Jiansheng Y, Nordgren R, Minke J (2007). "Evaluation of the ability of canarypox-vectored equine influenza virus vaccines to induce humoral immune responses against canine influenza viruses in dogs". Am. J. Vet. Res. 68 (2): 208–12. doi:10.2460/ajvr.68.2.208. PMID 17269888.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- An Innovative Evolution in Influenza Vaccine Manufacturing
- The Compelling Need for Game-Changing Influenza Vaccines CIDRAP Comprehensive Influenza Vaccine Initiative (CCIVI)
- Vaccine Strains vs. Circulating Strains
- NHS seasonal flu vaccination campaign NHS flu fighter
- History of past WHO seasonal influenza vaccine composition recommendations
- Vaccine Research Center Information from NIAID regarding preventative vaccine research studies
- About the flu jab (NHS Direct)
- World Health Organization's Initiative for Vaccine Research Influenza web page
- Influenza Strategies for Broad Global Access
- PATH's Vaccine Resource Library influenza resources
- Orr, Pamela (2000). "Influenza vaccination for health care workers: A duty of care". Canadian Journal of Infectious Diseases. 11 (5): 225–6. PMC 2094777. PMID 18159292.
- CDC 2009 H1N1 Influenza Vaccine Supply Status
- CDC Vaccine Information Statement Inactivated Influenza Vaccine
- Read Congressional Research Service (CRS) Reports regarding Influenza and vaccines
- Influenza Research Database – Database of influenza genomic sequences and related information.
- Health-EU portal EU response to influenza
- European Commission - Public Health EU coordination on Pandemic (H1N1) 2009
- Centers for Disease Control and Prevention - Vaccines and Preventable Diseases - Seasonal Influenza (Flu) Vaccination
- Centers for Disease Control and Prevention - Misconceptions about Seasonal Influenza and Influenza Vaccines
- Influenza Vaccine (NYTimes / Adam)
- "FluMist Quadrivalent Prescribing Information"
- "Fluarix Quadrivalent Prescribing Information"
- "Flucelvax Prescribing Information"
