Rociletinib: Difference between revisions
Appearance
Content deleted Content added
→Medical uses: +1 ref |
there was never a clinical use - it was only in clinical research |
||
| Line 56: | Line 56: | ||
}} |
}} |
||
'''Rociletinib''' is a medication developed to treat [[non-small cell lung carcinoma]]s with a specific mutation. It is a third-generation [[epidermal growth factor receptor]] [[tyrosine kinase inhibitor]].<ref>{{cite journal|doi=10.2147/OTT.S97644|pmc=5063481|title=New developments in the management of non-small-cell lung cancer, focus on rociletinib: What went wrong?|journal=OncoTargets and Therapy|pages=6065|year=2016|last1=Van Der Steen|first1=Nele|last2=Caparello|first2=Chiara|last3=Rolfo|first3=Christian|last4=Pauwels|first4=Patrick|last5=Peters|first5=Godefridus|last6=Giovannetti|first6=Elisa|pmid=27785053|volume=9}}</ref> It was being developed by [[Clovis Oncology]] |
'''Rociletinib''' is a medication developed to treat [[non-small cell lung carcinoma]]s with a specific mutation. It is a third-generation [[epidermal growth factor receptor]] [[tyrosine kinase inhibitor]].<ref name=Oncotargets2016>{{cite journal|doi=10.2147/OTT.S97644|pmc=5063481|title=New developments in the management of non-small-cell lung cancer, focus on rociletinib: What went wrong?|journal=OncoTargets and Therapy|pages=6065|year=2016|last1=Van Der Steen|first1=Nele|last2=Caparello|first2=Chiara|last3=Rolfo|first3=Christian|last4=Pauwels|first4=Patrick|last5=Peters|first5=Godefridus|last6=Giovannetti|first6=Elisa|pmid=27785053|volume=9}}</ref> It was being [[drug development|developed]] by [[Clovis Oncology]] as a potential treatment for [[non-small-cell lung cancer]].<ref name=Oncotargets2016/> In May 2016, development of rociletinib was halted, along with its associated clinical trials, and Clovis Oncology withdrew its [[marketing authorisation application]] from the [[European Medicines Agency]].<ref name=Oncotargets2016/> |
||
==Medical uses== |
|||
Rociletinib was used to treat locally advanced or metastatic [[non-small-cell lung cancer]] (NSCLC), if the cancer cells were positive for the [[T790M]] mutation in the gene coding for [[epidermal growth factor receptor|EGFR]].<ref>{{Cite news|url=https://clinicaltrials.gov/ct2/show/study/NCT01526928|title=Study to Evaluate Safety, Pharmacokinetics, and Efficacy of Rociletinib (CO-1686) in Previously Treated Mutant Epidermal Growth Factor Receptor (EGFR) in Non-Small Cell Lung Cancer (NSCLC) Patients - Full Text View - ClinicalTrials.gov|access-date=2018-03-15|language=en}}</ref> The T790M mutation is usually acquired following first-line treatment with other tyrosine kinase inhibitors, such as [[gefitinib]] and [[afatinib]]. |
|||
==References== |
==References== |
||
Revision as of 18:40, 15 March 2018
 | |
| Clinical data | |
|---|---|
| Trade names | Xegafri |
| Other names | CO-1686, AVL-301 |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
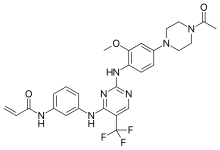
| Formula | C27H28F3N7O3 |
| Molar mass | 555.562 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Rociletinib is a medication developed to treat non-small cell lung carcinomas with a specific mutation. It is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor.[1] It was being developed by Clovis Oncology as a potential treatment for non-small-cell lung cancer.[1] In May 2016, development of rociletinib was halted, along with its associated clinical trials, and Clovis Oncology withdrew its marketing authorisation application from the European Medicines Agency.[1]
References
- ^ a b c Van Der Steen, Nele; Caparello, Chiara; Rolfo, Christian; Pauwels, Patrick; Peters, Godefridus; Giovannetti, Elisa (2016). "New developments in the management of non-small-cell lung cancer, focus on rociletinib: What went wrong?". OncoTargets and Therapy. 9: 6065. doi:10.2147/OTT.S97644. PMC 5063481. PMID 27785053.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
