Lipid

Lipids can be broadly defined as any fat-soluble (hydrophobic), naturally-occurring molecules. The term is more-specifically used to refer to fatty-acids and their derivatives (including tri-, di-, and monoglycerides and phospholipids) as well as other fat-soluble sterol-containing metabolites such as cholesterol.
Lipids have many functions in living organisms including nutrients, energy storage, structural components of cell membranes, and important signaling molecules. Although the term lipid is sometimes used as a synonym for fat, fats are in fact a subgroup of lipids called triglycerides and should not be confused with the term fatty acid.
Fatty acids and glycerides
Chemically, fatty acids can be described as long-chain monocarboxylic acids the saturated examples of which have a general structure of CH3(CH2)nCOOH. The length of the chain usually ranges from 12 to 24, always with an even number of carbon atoms. When the carbon chain contains no double bonds, it is a saturated chain. If it contains one or more such bonds, it is unsaturated. The presence of double bonds reduces the melting point of fatty acids. Furthermore, unsaturated fatty acids can occur either in cis or trans geometric isomers. In naturally occurring fatty acids, the double bonds are in the cis-configuration.
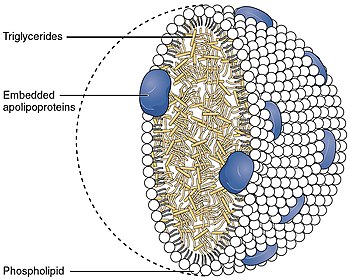
Glycerides are lipids possessing a glycerol (a crude name for which is propan-1, 2, 3-triol) core structure with one or more fatty acyl groups, which are fatty acid-derived chains attached to the glycerol backbone by ester linkages. Glycerides with three acyl groups (triglycerides or neutral fats) are the main form of fatty energy storage in animals and plants.
An important type of glyceride-based molecule found in biological membranes, such as the cell's plasma membrane and the intracellular membranes of organelles, are the phosphoglycerides or glycerophospholipids. These are phospholipids that contain a glycerol core linked to two fatty acid-derived "tails" by ester or, more rarely, ether linkages and to one "head" group by a phosphate ester linkage. The head groups of the phospholipids found in biological membranes are phosphatidylcholine (also known as PC, and lecithin), phosphatidylethanolamine (PE), phosphatidylserine and phosphatidylinositol (PI). These phospholipids are subject to a variety of functions in the cell: for instance, the lipophilic and polar ends can be released from particular phospholipids through enzyme-catalysed hydrolysis to generate secondary messengers involved in signal transduction. In the case of phosphatidylinositol, the head group can be enzymatically modified by the addition of one, two or three phosphate groups, this constituting another mechanism of cell signalling. While phospholipids are the major component of biological membranes, other non-glyceride lipid components like sphingolipids and sterols (such as cholesterol in animal cell membranes) are also found in biological membranes.
A biological membrane is a form of lipid bilayer, as is a liposome. The formation of lipid bilayers is an energetically-preferred process when the glycerophospholipids described above are in an aqueous environment. In an aqueous system, the polar heads of lipids orientate towards the polar, aqueous environment, while the hydrophobic tails minimise their contact with water. The lipophilic tails of lipids (U) tend to cluster together, forming a lipid bilayer (1) or a micelle (2). Other aggregations are also observed and form part of the polymorphism of amphiphile (lipid) behaviour. The polar heads (P) face the aqueous environment, curving away from the water. Phase behaviour is a complicated area within biophysics and is the subject of current academic research.
Micelles and bilayers form in the polar medium by a process known as the lipophilic effect. When dissolving a lipophilic or amphiphilic substance in a polar environment, the polar molecules (i.e. water in an aqueous solution) become more ordered around the dissolved lipophilic substance, since the polar molecules cannot form hydrogen bonds to the lipophilic areas of the amphiphile. So in an aqueous environment the water molecules form an ordered "clathrate" cage around the dissolved lipophilic molecule.

The self-organisation depends on the concentration of the lipid present in solution. Below the critical micelle concentration, the lipids form a single layer on the liquid surface and are (sparingly) dispersed in the solution. At the first critical micelle concentration (CMC-I), the lipids organise in spherical micelles, at given points above this concentration, other phases are observed (see lipid polymorphism).
Nutrition and health
Lipids play diverse and important roles in nutrition and health. Many lipids are absolutely essential for life, however, there is also considerable awareness that abnormal levels of certain lipids, particularly cholesterol (in hypercholesterolemia) and, more recently, fatty acids with trans fatty acids, are risk factors for heart disease amongst others. We need fats in our bodies and certain types in our diet. Animals in general use fat for energy storage because fat stores 9 KCal/g of energy. Plants, which do not require energy for movement, can afford to store food for energy in a less compact but more easily accessible form, so they have evolved to use starch, a carbohydrate, (not a lipid) for energy storage. Carbohydrates and proteins store only 4 KCal/g of energy, so fat stores over twice as much energy/gram as other sources of energy. Furthermore, lipids can be stored in an anhydrous form whereas carbohydrates typically cannot, which means that anhydrous lipid stores about 6 times as much energy per weight as hydrated carbohydrates. As an example, a typical 70 kg man would have to weigh approximately 125 kg if his energy stores were converted from triacylglycerol to glycogen.
The baby formula brands Enfamil and Similac offer versions with lipids (DHA and ARA) added to the base formula.
Types of Lipids
- Fat - Fats consist of a wide group of compounds that are generally soluble in organic solvents and largely insoluble in water. Chemically, fats are generally triesters of glycerol and fatty acids. Fats may be either solid or liquid at normal room temperature, depending on their structure and composition. Although the words "oils", "fats" and "lipids" are all used to refer to fats, "oils" is usually used to refer to fats that are liquids at normal room temperature, while "fats" is usually used to refer to fats that are solids at normal room temperature. "Lipids" is used to refer to both liquid and solid fats. The word "oil" is used for any substance that does not mix with water and has a greasy feel, such as petroleum (or crude oil) and heating oil, regardless of its chemical structure.
- Phospholipid are a class of lipids, and a major component of all biological membranes, along with glycolipids, cholesterol and proteins. Understanding of the aggregation properties of these molecules is known as lipid polymorphism and forms part of current academic research.
- Steroids - A steroid is a terpenoid lipid characterized by a carbon skeleton with four fused rings, generally arranged in a 6-6-6-5 fashion.
- Waxes - Fatty acids and an alcohol
References
- Chapter 12 in "Biochemistry" by Jeremy M. Berg, John L. Tymoczko and Lubert Stryer (2002) W. H. Freeman and Co.
- B. Alberts, et al. (2004) "Essential Cell Biology, 2nd Edition." Garland Science. ISBN 0-8153-3480-X
- E. P. Solomon, et al. (2005) "Biology, 7th Edition." Thomson, Brooks/Cole.
- "Advanced Biology 2 - Principles and Applications." C.J. Clegg and D.G. Mackean. ISBN 0-7195-7670-9
- J. M. Seddon, R. H. Templer. Polymorphism of Lipid-Water Systems, from the Handbook of Biological Physics, Vol. 1, ed. R. Lipowsky, and E. Sackmann. (c) 1995, Elsevier Science B.V. ISBN 0-444-81975-4
See also
External links
- Euro Fed Lipid – The European Federation for the Science and Technology of Lipids
- ApolloLipids - Provides dyslipidemia and cardiovascular disease prevention and treatment information as well as continuing medical education programs.
- Lipids, Membranes and Vesicle Trafficking - The Virtual Library of Biochemistry and Cell Biology
- The Lipid library - provides information on the chemistry, analysis and biochemistry of lipids
- LIPID MAPS: LIPID Metabolites and Pathways Strategy
- IUPAC glossary entry for the lipid class of molecules what is IUPAC?
- "A comprehensive classification system for lipids"
- Science aid: Lipids Resource for students