Pyrazolam
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, Sublingual, rectal |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 17 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
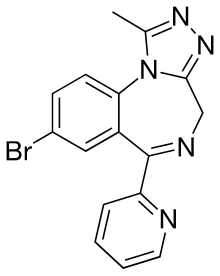
| Formula | C16H12BrN5 |
| Molar mass | 354.204 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pyrazolam is a benzodiazepine derivative originally developed by a team lead by Leo Sternbach at Hoffman-La Roche in the 1970s,[1] and subsequently "rediscovered" and sold as a research chemical starting in 2012.[2] It is mainly an anxiolytic, but it has also shown anticonvulsant and hypnotic effects at high doses. Pyrazolam has structural similarities to alprazolam[3] and bromazepam. Unlike other benzodiazepines pyrazolam does not appear to undergo metabolism, instead being excreted unchanged in the urine, no metabolites have been found in the urine of volunteers [4][5] and has anxiolytic activity 12x stronger than diazepam while causing little ataxia and sedation when used in its anxiolytic dose range. It is most selective for the α2 and α3 receptor subtypes[6]
Binding data (GABA):[citation needed]
- α1 3.84±0.25
- α2 1.31±0.19
- α3 1.48±0.21
- α5 3.72±0.32
References
- ^ US Patent 3954728 Preparation of triazolo benzodiazepines and novel compounds
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1007/s11419-013-0187-4, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1007/s11419-013-0187-4instead. - ^ 6-Phenyl-4H-s-triazolo[4,3-a][l,4]benzodiazepines Which Have Central Nervous System Depressant Activity J. Med. CHem Vol 14, No 11 Pages 1078-1081
- ^ http://www.uniklinik-freiburg.de/fileadmin/mediapool/08_institute/rechtsmedizin/pdf/Poster2013/Mossmann_DPHG2013.pdf>
- ^ Characterization of the designer benzodiazepine pyrazolam and its detectability in human serum and urine - Forensic Toxicology July 2013, Volume 31, Issue 2, pp 263-271
- ^ 6-Aryl-4H-s-triazolo[4,3-a][1,4]benzodiazepines. Influence of 1-substitution on pharmacological activity - J. Med. Chem., 1979, 22 (11), pp 1390–1398
Further reading
List of relevant reviews at Pubmed
