Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
August 2
Explain why these 2 statements are not contrary to each other
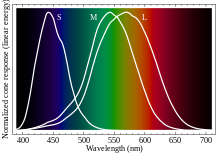
- According to opponent process, there's no such color as reddish green.
- According to RGB color theories, yellow is reddish green. RGB color theories suggest there's no reddish cyan or greenish magenta, but yellow is reddish green. Georgia guy (talk) 23:52, 2 August 2018 (UTC)
- "Even though yellow is a mixture of red and green in the RGB color theory, the eye does not perceive it as such." ←Baseball Bugs What's up, Doc? carrots→ 01:00, 3 August 2018 (UTC)
- I assume that you've read Impossible color. More than fifty years ago, I tried holding a good red filter to one eye and a good green filter to the other, and I saw in yellow (not reddish green). My interpretation was that the brain (not the eye) interprets roughly equal stimulations of L and M cones as being the colour yellow. I haven't tried rapid alternation between the two colours. Perhaps I might see that differently as being "reddish green". Colour perception is partly learnt and partly hard-wired. Dbfirs 08:26, 3 August 2018 (UTC)
- In a graphics program (e.g. MSpaint) if you select a custom color with red and green at max (255) and blue at 0, you get yellow -- this is not a "perception" of either the eye or brain. 2606:A000:1126:4CA:0:98F2:CFF6:1782 (talk) 14:17, 3 August 2018 (UTC)
- No, it's your brain telling you that its yellow. Absolutely no light of the 580nm range is leaving the monitor. The monitor has zero yellow pixels in it. Your perception of yellow from a monitor is a purely psychological phenomenon. Actually, all of your perception of color is a purely psychological phenomenon. See qualia and color perception. If you really want to get down to it, you have never seen yellow, because your eye is only capable of detecting basically 3 colors; red, blue, and green, and your perception of yellow is dependent only on how much of each of those receptors is stimulated by a particular bit of light. You can stimulate all three with a single wavelength of yellow light, OR you can stimulate all three with varying intensities of red, green, and blue light and you can't tell the difference. Because you don't see yellow. --Jayron32 14:58, 3 August 2018 (UTC)
- It depends on your definition of "yellow" -- a can of yellow paint has paint that is yellow in color.— Preceding unsigned comment added by 2606:A000:1126:4CA:0:98F2:CFF6:1782 (talk • contribs)
- Actually, it depends on your definition of "color". If you're defining color as the psychological sensation that looking at the yellow paint produces, then yes. If you're defining color as a specific wavelength of electromagnetic radiation, then maybe not so much. The relationship between color you perceive when looking at something and the wavelength of the light in question is tenuous at best. --Jayron32 15:21, 3 August 2018 (UTC)
- It depends on your definition of "yellow" -- a can of yellow paint has paint that is yellow in color.— Preceding unsigned comment added by 2606:A000:1126:4CA:0:98F2:CFF6:1782 (talk • contribs)
- No, it's your brain telling you that its yellow. Absolutely no light of the 580nm range is leaving the monitor. The monitor has zero yellow pixels in it. Your perception of yellow from a monitor is a purely psychological phenomenon. Actually, all of your perception of color is a purely psychological phenomenon. See qualia and color perception. If you really want to get down to it, you have never seen yellow, because your eye is only capable of detecting basically 3 colors; red, blue, and green, and your perception of yellow is dependent only on how much of each of those receptors is stimulated by a particular bit of light. You can stimulate all three with a single wavelength of yellow light, OR you can stimulate all three with varying intensities of red, green, and blue light and you can't tell the difference. Because you don't see yellow. --Jayron32 14:58, 3 August 2018 (UTC)
- In a graphics program (e.g. MSpaint) if you select a custom color with red and green at max (255) and blue at 0, you get yellow -- this is not a "perception" of either the eye or brain. 2606:A000:1126:4CA:0:98F2:CFF6:1782 (talk) 14:17, 3 August 2018 (UTC)
- While not disagreeing with the general thrust of your comments, the relationship is not really "tenuous" in any sense; there is a direct, causal biophysical relationship between those two things. It's simply that colour as a percept and as a form of qualia is not a product solely of the stimulus of any one photoreceptive cell. Indeed, despite our intuitive feelings to the contrary, as a scientific and ontological matter, both physicists and cognitive scientists working in this area would hold your basic assertion to be correct: "Colour" does not exist as a physical property of light, but rather as a purely mental phenomena. But the relationship between the physical properties of the photon that strikes an opsin chain and the biophysical and neurological series of reactions that result in the ultimate perception of colour is actual and empirically quantifiable in a variety of ways. There is no "colour" encoded in the interaction between the light and the photoreceptor--that, rather, is the product of higher level work in the brain where the information from a vast number of such cells is integrated together--but the perception of colour is absolutely triggered and shaped by the stimulus nevertheless, and the relationship is thus not a tenuous one, but rather one which is highly direct and as consistent as any other property of the interaction of light with matter. Snow let's rap 01:46, 6 August 2018 (UTC)
- Yes, thanks for the additional detail. Good stuff! --Jayron32 15:15, 6 August 2018 (UTC)
- While not disagreeing with the general thrust of your comments, the relationship is not really "tenuous" in any sense; there is a direct, causal biophysical relationship between those two things. It's simply that colour as a percept and as a form of qualia is not a product solely of the stimulus of any one photoreceptive cell. Indeed, despite our intuitive feelings to the contrary, as a scientific and ontological matter, both physicists and cognitive scientists working in this area would hold your basic assertion to be correct: "Colour" does not exist as a physical property of light, but rather as a purely mental phenomena. But the relationship between the physical properties of the photon that strikes an opsin chain and the biophysical and neurological series of reactions that result in the ultimate perception of colour is actual and empirically quantifiable in a variety of ways. There is no "colour" encoded in the interaction between the light and the photoreceptor--that, rather, is the product of higher level work in the brain where the information from a vast number of such cells is integrated together--but the perception of colour is absolutely triggered and shaped by the stimulus nevertheless, and the relationship is thus not a tenuous one, but rather one which is highly direct and as consistent as any other property of the interaction of light with matter. Snow let's rap 01:46, 6 August 2018 (UTC)
- Responding to the OP, it's basically a distinction between the eye and brain. On one hand, the eye contains three types of color-sensitive cones, so the signals leaving the eye can be represented using three independent color dimensions, typically thought of as R, G, and B. However, in the color-processing parts of the visual cortex, the signals are recoded into hue, saturation, and intensity. The hue component can be represented as a circle, with four canonical hues spaced equally around it: red, yellow, green, and blue. Looie496 (talk) 02:38, 4 August 2018 (UTC)
- You can represent hue as anything, I've seen the circle heuristic before, though roundy-triangles seems to be the vogue for more detailed representations of color space. Historically, the CIE 1931 color space was the first attempt to quantify human color perception in such terms; there have been some updates to it over time, but it remains something of a standard. --Jayron32 15:21, 4 August 2018 (UTC)
- I will assume the OP is familiar with the stuff described here (IMO an excellent explanation of RGB and vision) (the IPv6 editor above clearly is not, but answers have been provided to that mini thread-hijack).
- The answer is that the first premise is incorrect.
reddish green
is allowed by the opponent process theory, it just means (going by the diagram in the article) that in neurological processes it is treated as "some luminosity, no R-G, high R+G-B". This is different from other colors, e.g. white (high luminosity, no R-G, low R+G-B). Essentially as long as the brain processes keep three independent coordinates they can describe just as much as RGB. TigraanClick here to contact me 18:22, 4 August 2018 (UTC)
August 3
Material that generates ultrasound, and not sound, when hit
Could a ultrasound drum exists? That is, when hit, it would generate ultrasound and not sound? Could any ultrasound (silent for humans) instrument exist? Does this strike a chord to anyone?--Doroletho (talk) 12:02, 3 August 2018 (UTC)
- A Dog whistle is an ultrasound instrument. ←Baseball Bugs What's up, Doc? carrots→ 12:10, 3 August 2018 (UTC)
- A drum is a membranophone, and there's no reason why one can not be tuned to sounds higher pitched than humans can perceive. The tuning of drums would obey a two-dimensional analogue of Mersenne's laws (which describe the tuning of strings); that is you can alter the diameter, thickness, and tension on the drum head and create any arbitrarily high sound. The problem with doing this with drums is that drums have a very broad musical "bandwidth"; so while you could perhaps create a drum which had significant sound in the ultrasound range would still have some frequency space in the audible range. For an instrument like a whistle, the bandwidth is MUCH tighter, so you only get a narrow band of frequencies. --Jayron32 18:12, 3 August 2018 (UTC)
- And an old Guinness said the lowest musical instrument is a 64 foot tall organ pipe in Atlantic City that makes 8 Hertz. Sagittarian Milky Way (talk) 19:44, 3 August 2018 (UTC)
- A drum is a membranophone, and there's no reason why one can not be tuned to sounds higher pitched than humans can perceive. The tuning of drums would obey a two-dimensional analogue of Mersenne's laws (which describe the tuning of strings); that is you can alter the diameter, thickness, and tension on the drum head and create any arbitrarily high sound. The problem with doing this with drums is that drums have a very broad musical "bandwidth"; so while you could perhaps create a drum which had significant sound in the ultrasound range would still have some frequency space in the audible range. For an instrument like a whistle, the bandwidth is MUCH tighter, so you only get a narrow band of frequencies. --Jayron32 18:12, 3 August 2018 (UTC)
- Jangling a bunch of keys generates plenty of ultrasound, but some audible sound too.
- Some depends on your definition of "ultrasound". Children can hear frequencies which adults no longer can. Some of the first TV remotes, the 'Zenith Space Command' from the 1950s,[1] used this range of near-ultrasound, generated with by a purely mechanical remote control with mechanical reeds like a thumb piano, and a frequency-sensitive receiver on the set. Andy Dingley (talk) 20:55, 3 August 2018 (UTC)
- Hitting a drum is like subjecting it to an Impulse function, which will then yield an Impulse response. Since the original impulse contains all frequencies, it will include audible sound requencies, so you will need a structure than can act like a high pass filter to only allow ultrasonic frequencies. You will need a mechanical Audio filter. If you can arrange to beat you drum at a rate of 20 kHz or higher you should be able to achieve what you want. Graeme Bartlett (talk) 05:21, 4 August 2018 (UTC)
- Or make 2,001 copies of an mp3 of an AK47 and start them at 1/20010th second intervals? Sagittarian Milky Way (talk) 05:58, 4 August 2018 (UTC)
- Pedantry: MP3, as with any lossy audio codec, removes various parts of the audio signal that humans don't notice significantly. However, this might cause issues in something like that. It would be ideal to use a lossless audio recording. --47.146.63.87 (talk) 09:14, 4 August 2018 (UTC)
- Actually, the chopping of sound from cymbals seems like a good thing. My personal opinion has been that the "ultrasound" of the cymbals so overwhelms the other instruments in a rock performance as to completely overwhelm them in a live concert, while the MP3 will balance them. Barring that, a strategic pillar might be an option (ultrasound is pretty line of sight). [2] at least confirms they go up into "ultrasound". Wnt (talk) 21:51, 5 August 2018 (UTC)
- Ultrasound avoidance is an antibat tactic known to be evolved by insects to escape from predators that echolocate using ultrasound. Evolution of a similar ability in humans, as Wnt's report suggests, is an unexpected byproduct of Rock concerts. During the time it will take to discover whether the ability is inheritable (readers are probably aware of some popular methods) a practical step against concert-induced SHL or NHL is to invest in Earplugs which may be of the passive or active In-ear monitor kind. They won't help much after Tinnitus sets in. DroneB (talk) 13:25, 6 August 2018 (UTC)
- Actually, the chopping of sound from cymbals seems like a good thing. My personal opinion has been that the "ultrasound" of the cymbals so overwhelms the other instruments in a rock performance as to completely overwhelm them in a live concert, while the MP3 will balance them. Barring that, a strategic pillar might be an option (ultrasound is pretty line of sight). [2] at least confirms they go up into "ultrasound". Wnt (talk) 21:51, 5 August 2018 (UTC)
- Pedantry: MP3, as with any lossy audio codec, removes various parts of the audio signal that humans don't notice significantly. However, this might cause issues in something like that. It would be ideal to use a lossless audio recording. --47.146.63.87 (talk) 09:14, 4 August 2018 (UTC)
August 4
the process of communication between the insects of same species
excuse me, I am very eager to know how the insects communicate among themselves. again, many of them have antenna. so how it works would you please tell me.
- Insect antennae are not the same as radio antenna, although they do look similar in some cases. Insect antennae are sensory organs for touch, smell, taste, and some other sensory modalities. I am unaware of any insect species ability to communicate electromagnetically with each-other in the radio frequency range. Insect communication may be chemical (e.g. ants, wasps), acoustic (e.g. crickets, cicadas), visual, tactile, etc; and in many cases antennae are indeed involved. Radio waves are not involved, though :) , at least as far as I know. Dr Dima (talk) 06:59, 4 August 2018 (UTC)
- For more details, you may want to read articles on insect antennae that I already mentioned, as well as articles on animal communication and insect senses and communication. Hope this helps! --Dr Dima (talk) 07:04, 4 August 2018 (UTC)
- See also Ecology and Evolution of Communication in Social Insects. We also have an article on the Waggle dance used by honey bees. Alansplodge (talk) 12:50, 6 August 2018 (UTC)
turtle id in today's featured image

Today's featured image caption says "A Julia butterfly (Dryas iulia) feeding on the tears of a red-headed Amazon River turtle (Podocnemis erythrocephala) ...". Can someone with appropriate expertise please check the turtle identification? The two turtles on the left may be indeed Podocnemis erythrocephala, but the one on the right - the one with the butterfly on its head - doesn't look like P. erythrocephala to me at all. Maybe P. expansa??? I'm not an expert though, so please don't change the featured image caption until an expert has taken a good look at it. I'll also add a note on the image discussion page. Thanks in advance! --Dr Dima (talk) 06:49, 4 August 2018 (UTC)
- The commons description does say expansa, not erythrocephala. The erythrocephala ID seems to have been given by Faendalimas at Wikipedia:Featured_picture_candidates/A_butterfly_feeding_on_the_tears_of_a_turtle_in_Ecuador (ping). I think we should pull the turtle species name for now; will post at WP:ERRORS soon. TigraanClick here to contact me 15:57, 4 August 2018 (UTC)
- The second turtle - the middle one - has a butterfly on its head as well. 194.174.76.21 (talk) 10:32, 6 August 2018 (UTC) Marco Pagliero Berlin
Thermal contraction of helical spring
Imagine you have a helical spring with thickness and unstretched length at room temperature. Then suppose we take the spring into a cryostat and cool it down to something like 10 K. Potentially neglecting the changes to the spring's mechanical properties (its spring constant or Young's modulus, etc.), will the spring stretch due to thermal expansion effects? My intuition tells me that the spring's arclength around the helix will decrease, thus having a small change in the number of turns, but that the length of the spring itself will not change much. Any thoughts would be greatly appreciated! 130.202.62.210 (talk) 17:17, 4 August 2018 (UTC)
- Of each little bit of the spring shrinks the same in every direction then the overall spring will just shrink the same in every direction, so you should just end up with a spring that looks exactly the same but smaller. Dmcq (talk) 17:53, 4 August 2018 (UTC)
- Good explanation. An equally simple question is that if you have a steel donut, when you heat it, does the hole get bigger or smaller? Greglocock (talk) 20:21, 4 August 2018 (UTC)
- Theory or practice?
- Helical springs pretty much couldn't be made (for large strains) until the 1960s. Otherwise they're just extremely inhomogeneous, so that simple assumptions of consistent linear shrinkage are very unrealistic. Andy Dingley (talk) 22:43, 4 August 2018 (UTC)
- The way I heard the classic mechanical engineering question was this: Heat up a doughnut-shaped chunk of metal. Does the hole get bigger or smaller? Now inflate a car-tire inner tube in free air. Does the hole get bigger or smaller? --Guy Macon (talk) 23:11, 4 August 2018 (UTC)
- Doughnuts aren't helical springs. Andy Dingley (talk) 23:13, 4 August 2018 (UTC)
- The atom to atom links get longer. Greglocock (talk) 10:42, 5 August 2018 (UTC)
- I don’t know how to post YouTube videos, but if you search YouTube for ‘New tyres for 257 Squadron’ it shows that the hole for steel gets bigger. Widneymanor (talk) 10:47, 5 August 2018 (UTC)
- The atom to atom links get longer. Greglocock (talk) 10:42, 5 August 2018 (UTC)
- Doughnuts aren't helical springs. Andy Dingley (talk) 23:13, 4 August 2018 (UTC)
- The way I heard the classic mechanical engineering question was this: Heat up a doughnut-shaped chunk of metal. Does the hole get bigger or smaller? Now inflate a car-tire inner tube in free air. Does the hole get bigger or smaller? --Guy Macon (talk) 23:11, 4 August 2018 (UTC)
The OP's intuition about the spring's arclength is correct but the helix diameter changes in proportion, thus the number of turns is unchanged by temperature. To answer Guy Macon's question about a metal doughnut and a car-tire inner, the metal is held in shape by interatomic Van der Waals forces that are 3-dimensional (the hole gets bigger) but the inflated inner is a dual-circular Torus shaped by a thin rubber sheet in 2-dimensional tension. Inflation increases its volume V given by
where R is the radius of revolution of the center of revolved circle radius r. Since R is virtually constant, r increases and the hole gets smaller. DroneB (talk) 09:53, 6 August 2018 (UTC)
August 5
What's the mechanism that allows water and steam to co-exist together, room temperature?
I always think of boiling point as more subjective than freezing point. You leave a glass of water, even in a refrigerator, and over time it evaporates into steam. I know this is due to a property called volatile. 67.175.224.138 (talk) 13:34, 5 August 2018 (UTC).
- Read Evaporation for an explanation. And note that ice will also evaporate. How much evaporation can occur, if any, is a function of the temperature and also how much moisture is already in the air. ←Baseball Bugs What's up, Doc? carrots→ 16:19, 5 August 2018 (UTC)
- The OP may be confusing the scientific meaning of steam with its common colloquial meaning of water vapour. {The poster formerly known as 87.81.230.195} 90.208.173.225 (talk) 17:16, 5 August 2018 (UTC)
- Take a look at triple point, particularly the part on phase diagrams.
- The point is that water doesn't turn into steam at a single temperature, but rather it's a line bordering two phases on a plane representing temperature and pressure. The "boiling point" presents this as a single temperature, but implicit in that is an assumption about what the pressure is. At atmospheric pressure it's 100°C, but inside a pressurised steam boiler it can be higher than that, and on top of a mountain it can be low enough that you can't even brew a good cup of tea. Andy Dingley (talk) 17:36, 5 August 2018 (UTC)
- I'm surprised no one has linked vapor pressure yet. At any temperature above absolute zero, there exists a non-zero chance of any atom at the surface interface of solids and liquids for a molecule of that substance to break free from its bonds keeping it in the condensed phase and instead move into the gas phase. When the rate of evaporation equals the rate of condensation (the reverse process whereby molecules from the gas phase strike the surface and remain stuck to it) then you have reached dynamic equilibrium, and the gas and condensed phases will be exchanging places at a constant rate, so the relative amounts of each stay constant. But there is always at least some gas. What the boiling point refers to is the temperature at which that equilibrium breaks down, and at which no liquid or solid will remain behind. But gas still exists at temperatures below that because at any given temperature there always exists some molecules whose thermal energy exceeds the intermolecular bonds of the substance in question. Maxwell–Boltzmann distribution would be good reading, though it gets a bit technical. --Jayron32 20:32, 5 August 2018 (UTC)
- Okay this is the answer I am looking for, but how can the rate of evaporation equal the rate of condensation? A glass of cold water at STP can eventually all turn to water vapor. So there is no 50/50 water/gas here. Thanks. 67.175.224.138 (talk) 23:56, 6 August 2018 (UTC).
- What if the room air is already at 100 percent relative humidity? ←Baseball Bugs What's up, Doc? carrots→ 00:27, 7 August 2018 (UTC)
- Okay, I think what you're trying to say here is if water never evaporates below the boiling point, there would be no rain on Earth. So what would be at 0% relative humidity, if that's even possible? 67.175.224.138 (talk) 01:09, 8 August 2018 (UTC).
- What if the room air is already at 100 percent relative humidity? ←Baseball Bugs What's up, Doc? carrots→ 00:27, 7 August 2018 (UTC)
- Think of the glass of water in a smaller closed system, say, a perfectly sealed box. Would all the water still evaporate at STP? Or, think of Earth as a (admittedly, much more complex) system, and note how the water on its surface (lakes, oceans, glaciers etc) remains in dynamic equilibrium with the water vapor in the atmosphere. Abecedare (talk) 00:35, 7 August 2018 (UTC)
- Okay so the glass of water in a box would cause the inside to get moist and up up the humidity, same concept as rainfall. 67.175.224.138 (talk) 01:09, 8 August 2018 (UTC).
- Exactly. And once the relative humidity in the box rises to 100% (someone correct me if this number is wrong) for the box's temperature, the water won't evaporate any further; or rather there will be dynamic equilibrium and the rate of evaporation and condensation will be equal. Abecedare (talk) 01:31, 8 August 2018 (UTC)
- Okay so the glass of water in a box would cause the inside to get moist and up up the humidity, same concept as rainfall. 67.175.224.138 (talk) 01:09, 8 August 2018 (UTC).
- Okay this is the answer I am looking for, but how can the rate of evaporation equal the rate of condensation? A glass of cold water at STP can eventually all turn to water vapor. So there is no 50/50 water/gas here. Thanks. 67.175.224.138 (talk) 23:56, 6 August 2018 (UTC).
Cats and canine questions.
I have a question about the non-pack mentality of cats. Say you used a flying drone to drop off a closed basket full of kittens into the middle of the wild woods, and then half an hour later the basket opens itself and the kittens are free to roam into any direction. My question is, once that happens, are these kittens going to scatter into any direction and branch off on their own, or will they develop a pack mentality and stick together? If it matters, let's make them half male and half female.
2nd question, someone told me that dogs and wolves are only interested in forming packs with themselves. So if a solo wolf found a pack of smaller dogs, and he was much bigger than that alpha dog, that wolf would rather not challenge it and take over the pack, he'd still rather be solo and find a pack of wolves. Does anyone back this statement? Does that mean you won't find a pack consisting of wolves, hyenas, and coyotes all mixed? 67.175.224.138 (talk) 13:39, 5 August 2018 (UTC).
- Kittens may stick together for warmth. Cats will spread out in all directions. The occasional "packs" of cats, such as street cats in Italy that one may have heard of, represent a response to very high density and abundant resources. They have found a way to avoid fighting, but they are not a pack. Abductive (reasoning) 17:06, 5 August 2018 (UTC)
- Cat#Sociability and Coyote#Social_and_reproductive_behaviors discuss the sociability of those animals. Neither form packs as wolves do, but both exhibit other more limited forms of sociable behavior in certain circumstances. Hyenas are not canids, and are not found in the same areas as wolves or coyotes. Rojomoke (talk) 16:00, 5 August 2018 (UTC)
- Coyotes and wolves do not get along. When in packs, they are mostly likely to attempt to kill a lone member of the other species. Abductive (reasoning) 17:03, 5 August 2018 (UTC)
- Also worth emphasising that the original analysis of a wolf pack being a random set of wolves led by an alpha male and/or female because they're the biggest and toughest was mistaken. Usually the alphas are the eldest pair and the other wolves are mostly their offspring. {The poster formerly known as 87.81.230.195} 90.208.173.225 (talk) 17:24, 5 August 2018 (UTC)
- Regarding the kittens: the non-sociability of the domestic cat is greatly oversold in conventional "wisdom" about the species. Provided the right circumstances, cats are quite happy to live in large gregarious groups; indeed, cat colonies tend to be substantially larger than most canine packs. Such cats will even share food resources and be largely non-hostile towards one-another, especially if they are composed mostly of one kin group (though non-castrated males will invariably fight one-another once they get to sexual maturity; even brothers who were litter mates and quite affectionate with one-another up until about the age of 9-12 months will typically become hostile to each-other at this point). However, these groups are ethologically distinct from the social hierarchies of "pack" hunters--and hunting is one of the defining qualities of a pack, as the close coordination for predation is one of the elements of their ecological niche which drives such animals to maintain close proximity to one-another and to act in concert. This kind of behaviour is seen in some species of cat (most famously the lion pride), but is not a part of the lineage of felis cattus, and thus not found in their behavioural make-up; cats will sometimes tag team a particularly unfortunate small prey animal that has become the group's play toy, but they are generally speaking solitary hunters. Nevertheless, domestic cats can and do stake out territories together: in fact, some research has suggested that the more cats are accepted into a mutual living arrangement, the less inclined the individuals are towards aggressive patrolling of the borders of their territory (otherwise a very common behaviour of cats from single-animal homes). Pack-sized groups of domestic cats also tend to lack the strict hierarchical roles of canine packs and rarely take ques from one-another in coordinating their activities in the way canines do. That said, there is typically a pecking order of sorts, generally based on lineage.
- Anyway, bringing all of this back around to your question: if you dropped those kittens in the middle of the woods, they would not be likely to form a hunting pack. But provided an ample source of food, they might stay close together (this would probably have to be artificial cat food supplied at a regular interval, however; domestic cats are not, despite their reputation, well-adapted to subsisting solely on prey, especially in an urban or urban-proximate context). Much of the answer to your question depends upon specifics: particularly the age of the animals and the availability of food. The younger the kittens, the more likely they are to stay together; littermates tend to be very attached to one-another. However, bear in mind that "kitten" is technically any domestic cat under the age of one year, at which time they have generally reached their full mature size and are considered young adults. At the age of four months and older, they become prone to wandering, and in a woods with scarce food they would probably begin to disperse at this point, though some may very well make the effort to tough it out together. They would not, in any event, be behaving like a pack/pride, however; that's just not one of their survival strategies. If they were going to survive in the woods at all (and if you dropped a group of young kittens in the woods, sad to say, but probably most would not survive to maturity) they would need regular food, or they would seek it elsewhere. Anyway, domestic cats do not fare well in actual forests in any event; they are subject to predation from larger carnivore mammals and raptors (against which they have few defenses) and would struggle to compete for their own prey against obligate hunters.
- Regarding mixed packs of dogs and wolves: This is a much simpler question: generally this does not happen. There is a substantial degree of evolutionary distance between a dog and a wolf now, not withstanding the fact that they can typically interbreed. They are quite aware of their differences, and these extend not just to the obvious traits you and I would pick up on (the drastic differences in body morphology), but also auditory and olfactory identifiers and differences in behaviour with regard to social ques. That said, it's not impossible. Wolfdogs are generally very well accepted into packs of dogs, particularly if they are in a domesticated setting where a human has been adopted as the de facto "head of the pack" and can regulate the entrance of new members. A hybrid individual would probably have to be substantially more wolf than dog to have any chance of being adopted into a wolf pack: even with full-blooded animals, it is exceedingly rare for an alpha to accept a "lone wolf" into the pack--they are typically killed by the pack on sight. I would judge your own proposed scenario--a lone wolf taking over a pack of (presumably feral) dogs--to be even more unlikely. For one, whether we are talking about dogs or wolves, it is not typically a case of an outside alpha coming in and killing or supressing the leadership of the mature breeders; when an animal is accepted into the pack, they are usually a non-aggressive juvenile desperate enough to attempt an approach and willing enough to show complete submission via every method of signaling available--any degree of aggression towards the dominant pack member at this critical moment is likely to set the entire pack on the attack, no matter how much larger the outsider is than the current alpha. Now, would it be possible for a very juvenile lone wolf, at a very young and unintimidating age, to be desperate enough to attempt admission to a feral dog pack, be accepted, and later, after growing to maturity and following the death of the alpha, assume leadership of the pack? I'd have to speculate as to that, but I would say its highly unlikely, but maybe not outside the realm of possibility, canines being as sociable as they are and given the observed variances as to their behaviour. But to the best of my knowledge, this behaviour scenario has never been observed in the wild. Snow let's rap 23:38, 5 August 2018 (UTC)
Ashtabula chemical site
In Ashtabula, Ohio there are some chemical plants:
- ASHTA Chemicals ([3])
- Cristal Global titanium dioxide works ([4], [5])
But there is a third big one, which has no name on Google Maps: Link
Does anybody know the company? --Flinten-Uschi (talk) 23:14, 5 August 2018 (UTC)
- Cristal Plant 2, possibly. Abductive (reasoning) 05:25, 6 August 2018 (UTC)
- That's my guess as well. There's a rectangular building immediately south of the complex the OP is looking at and it seems to be addressed as 1527 Cook Road. There are apparently two businesses there, though - one is K&H Distribution and the other is Millennium-Organic Chemicals, part of the Cristal Global network. I suspect there's something glitched on the address and the Cristal site is further up the road, where the OP is looking; it sure doesn't look like a chemical processing plant whereas the other site certainly does. Still just guesswork, though. Matt Deres (talk) 14:28, 6 August 2018 (UTC)
- I have no knowledge specific to this case, but would observe that chemical and similar manufacturing companies also need a great many non-manufacturing staff, including managers, administrators, marketers, lab researchers, etc. The facilities for these may be housed in some of the buildings on a predominently manufacturing site, or on a separate but fairly adjacent site, or both. I myself have worked at a couple of such twin-site establishments in different industries (pharmaceuticals and atomic weapons, as it happens). By contrast, a petrochemical refinery I worked at housed its office-based staff in smaller buildings amongst or on the periphery of the much more extensive industrial plant, but some of its independent small suppliers had their own smaller sites just down or across the road. {The poster formerly known as 87.81.230.195} 2.219.83.90 (talk) 22:13, 6 August 2018 (UTC)
August 6
Wind speeds on gas and ice giants
I keep hearing about incredible wind speends on gas and ice giants, both inside and outside the solar system, and how destructive the winds could be, but something is bothering me about it. If the wind speed is relative to the average rotational speed of the bulk of the planet, then wouldn't you be just fine so long as you avoid the interaction zones of jet streams? What would turbulence be like within a Neptunian jet stream and would you even notice exciting one, if you had no visual point of reference? I've got the impression that you can easily traverse latitudinally without worrying about being ripped appart by sudden changes in wind direction, but you got little to no control over your latitudinal direction of traverse due to the magnitude of the wind speed. Perhaps, it's a case of 'one does not simply' travel from pole to pole. Plasmic Physics (talk) 10:07, 6 August 2018 (UTC)
- You are referring strictly to wind speed. You can also consider wind force. There is a lot of documentation you can find on Martian wind storms, which you can use to consider the giants. Storms on Mars reach very high speeds, but there is very little atmosphere. So, there is very little force. To do damage, you will need high speeds and a thick atmosphere. Jupiter has that once you get down into the atmosphere. But, it is a gas giant. You should expect that for all gas giants, and looking at Jupiter and Saturn, there is no apparent "calm" spot. The ice giants are different. You need to check the atmosphere to see how thick it is. It could be thick methane. It could be thin hydrogen. 209.149.113.5 (talk) 13:56, 6 August 2018 (UTC)
- You also need a place to stand. If you are traveling at the same speed as the wind you experience no wind. Some of these planets have no well-defined surface. --Guy Macon (talk) 20:00, 6 August 2018 (UTC)
- This depends on wind shear, which may be high near edges of jet streams. Ruslik_Zero 20:04, 6 August 2018 (UTC)
- From the description in the article, I think that wind shear is exactly what I'm refering to. What is the wind shear typically like in those kinds of planets, and is there a way to avoid shear zones when travelling with the air currents or is there too much turbulence? Plasmic Physics (talk) 03:07, 7 August 2018 (UTC)
- Wind shear is a difference in speed and/or direction over a short space. Again, this is speed. You could safely stand on the Moon in the middle of an area with a wind shear of hundreds of miles per hour because there is not enough atmosphere for that wind shear to create substantial force. However, if you are in the thick atmosphere of Jupiter, a small wind shear would be enough to do significant damage. Nearly all of the time, wind speed, shear, and force are used with the assumption that we are discussing sea-level winds on Earth. When discussing other planets, the relationships change. An example many people know is the storm on Mars at the beginning of "The Martian." That wouldn't happen. You could get those winds and dust, but it wouldn't knock anyone over. It would just be an annoying little breeze. If it took place on Earth, it would be deadly. 209.149.113.5 (talk) 13:54, 8 August 2018 (UTC)
- Wind speed doesn't really factor into it, which is the point I raised in the opening statement. Wind shear is measured in Hertz, so the acceleration you'd experience as you travel across an air current is equal to the product of your crossing velocity and the wind shear. Follows, F = ma. Plasmic Physics (talk) 20:52, 8 August 2018 (UTC)
- Wind shear is a difference in speed and/or direction over a short space. Again, this is speed. You could safely stand on the Moon in the middle of an area with a wind shear of hundreds of miles per hour because there is not enough atmosphere for that wind shear to create substantial force. However, if you are in the thick atmosphere of Jupiter, a small wind shear would be enough to do significant damage. Nearly all of the time, wind speed, shear, and force are used with the assumption that we are discussing sea-level winds on Earth. When discussing other planets, the relationships change. An example many people know is the storm on Mars at the beginning of "The Martian." That wouldn't happen. You could get those winds and dust, but it wouldn't knock anyone over. It would just be an annoying little breeze. If it took place on Earth, it would be deadly. 209.149.113.5 (talk) 13:54, 8 August 2018 (UTC)
- From the description in the article, I think that wind shear is exactly what I'm refering to. What is the wind shear typically like in those kinds of planets, and is there a way to avoid shear zones when travelling with the air currents or is there too much turbulence? Plasmic Physics (talk) 03:07, 7 August 2018 (UTC)
- This depends on wind shear, which may be high near edges of jet streams. Ruslik_Zero 20:04, 6 August 2018 (UTC)
- You also need a place to stand. If you are traveling at the same speed as the wind you experience no wind. Some of these planets have no well-defined surface. --Guy Macon (talk) 20:00, 6 August 2018 (UTC)
Inverse flight

In a fixed wing aircraft the flying is made possible because of the wing configuration whereas the lower surface has a flat profile and the upper surface is convex. However there are numerous examples when military aircraft fly upside down. I saw an example on a AHC TV show Top Ten as a post WWII massive 4 engine jet bomber, B-47, flipped during a flight and flew half a minute inverse with the belly facing the sky. Japanese zero fighters during WWII did it routinely for the purpose of showing off as well as observing for a possibility of being attacked from below. Where is the aerodynamics in such cases? How do they do it? Thanks, AboutFace 22 (talk) 15:49, 6 August 2018 (UTC)
- Where you say "made possible", it would be more accurate to say "made more efficient". Lift is generated when the wing forces air downward; it doesn't matter whether it does it because of the shape of the wing (the convex-on-top shape you refer to) or because of its angle of attack. The shape used for wings is one that will force the most air downward with the least friction (drag), so it doesn't need a high angle of attack to get enough lift in cruising flight. But if the airplane is pitched down (the nose is lowered), the angle of attack can reach the point where more air is forced upward for that reason than is forced downward by the shape of the wing, so the "lift" becomes negative. But if the airplane is inverted while doing this, the lift is upward and will keep the plane airborne. However, the drag in this position is much higher than in normal flight, so it requires a high-performance aircraft. --76.69.47.228 (talk) 16:42, 6 August 2018 (UTC)
- Mandatory XKCD. The übernerds at explain-xkcd have a decent explanation of why that explanation is wrong. TigraanClick here to contact me 18:33, 6 August 2018 (UTC)
- Lift (force)#False explanation based on equal transit-time. Also see: List of common misconceptions#Physics. --Guy Macon (talk) 20:05, 6 August 2018 (UTC)
- There are so many details to consider during the study of inverted flight! Some airplanes have symmetric airfoils, and some also have symmetric dihedrals. There are symmetric gravity immersion oil systems for the engines; some airplanes use pumps so the liquid stuff like fuel and oil don't depend on gravity to go where they are needed. All of these engineering details change performance characteristics for the airplane in upright and inverted attitudes.
- To specifically answer the original question, we should restate the question about the airfoil: how does a symmetric shape like the wing of an Extra 300 generate lift?
- The best way I can understand these physics is to think of the momentum transfer model. My simplified model emphasizes that air is sticky when it's flowing. That means the airfoil's angle of attack - the angle that the chord makes against the incident wind - can be the primary physical mechanism to change the airflow's bulk motion. To first order, the actual shape of the airfoil isn't important for the generation of lift! A flat board, held at an angle to the incident wind, will deflect air, and that will generate a change in momentum. The actual shape of the airfoil becomes important when we look at the secondary details: how efficient is the deflection of air? (This is the L/D parameter, for any aeronautical physics nerds in our audience). And - when does the flow cease to be laminar? (This is the critical angle). Those important numbers can be estimated from fluid dynamics equations; but usually, they are derived experimentally. I can plug them into my mental model to set the boundaries of my operable range.
- These ludicrous oversimplifications give me a good, workable, simplified linear-equation that is accurate enough for my purposes - which are, of course, to maintain aircraft control in all flight attitudes. Put another way - how hard do I need to work the controls to move the airplane where I want it to go? During inverted flight, I can't solve nonlinear aerodynamics equations while the blood is rushing to my feet - I need linear equations that only require me to consider one or two parameters!
- It takes years of study to really grok airfoils; if you want a good book, Aerodynamics for Naval Aviators is available for zero cost and is recommended by the FAA for advanced flight students. This book pulls no punches. The physics and math are difficult and if you aren't a domain-expert, you'll easily get lost in the details. But it is one of the best, most thorough, practical physics books that doesn't simplify the real science of aerodynamic analysis.
- Nimur (talk) 14:52, 7 August 2018 (UTC)
- Most airplane wings have a convex upper surface and a less-convex, or even flat, lower surface but these two features are not essential for an airfoil; they are used to achieve the most efficient design possible, not to guarantee that lift can be generated. Many airfoils have a symmetric profile.
- The essential feature of any airfoil is that it has a sharp trailing edge - see Kutta condition. Whether a wing is operating with its “usual side” up, or upside down, its trailing edge is sharp so it functions as an airfoil and generates lift. (If the airplane was falling backwards, the wing would not generate lift, for the obvious reason.) Dolphin (t) 22:05, 7 August 2018 (UTC)
- Nonsense. I can take a 1" x 12" x 12" wood plank, hold it out of the window of a car going 60 MPH, and depending how I angle it, get plenty of lift.
- Also see: Here's What It Takes To Fly a Model Plane Backwards]. --Guy Macon (talk) 21:19, 8 August 2018 (UTC)
- Understandably, most people imagine an airfoil must have a rounded leading edge with a large radius. The fact that a flat plate will generate lift proves that a rounded leading edge is not required. The essence of an airfoil is not the rounded leading edge - that is provided on all subsonic airfoils to maximise the stalling angle and, therefore, the lift coefficient.
- Designers of all fixed-wing aircraft would love to be able to use a wing with a generously rounded trailing edge. (The wing profile of a Rankine body!) It would provide so much extra volume for a deep rear spar, extra fuel storage space, and extra volume into which the undercarriage could be retracted. Unfortunately a wing with a generously rounded trailing edge will not generate much lift so designers must make use of airfoil sections with what is always called a sharp trailing edge.
- One of the first successful attempts to analyse, mathematically, the properties of airfoil sections was the Joukowski airfoil which has a cusped trailing edge. A cusped trailing edge is not essential for a good airfoil - a so-called sharp trailing edge is sufficient. Dolphin (t) 06:20, 9 August 2018 (UTC)
Use of millimeter paper
Do people still use millimeter paper in a non-educational setting? Are there scenarios where it's a better choice than a laptop, tablet or the like?--Doroletho (talk) 15:59, 6 August 2018 (UTC)
- Yes. Some technical professionals may use graphing software that is limited to particular subject matter or scales, and would use graph paper for graphs that aren't within the domain of their graphing software.
- General purpose graphing software can be expensive, or hard to use, so a person who only needs to create an occasional graph may use graphing paper.
- Also, sometimes it may be necessary to make a graph that is larger than the available printers. If this need only occurs once in a while, it may be more appropriate to use large paper. Jc3s5h (talk) 16:21, 6 August 2018 (UTC)
- I've not come across the phrase "millimeter paper" before but the spelling is American. Knowing the aversion some Americans have to the metric system I would imagine they would use sheets printed with a grid of one-inch squares, each square divided into 100 smaller squares. 95.150.52.197 (talk) 09:49, 8 August 2018 (UTC)
- 1/100-inch graph paper is fairly unusable. Can one even visualize that small a space once the width of the line itself is accounted-for? 1/16 in or rarely 1/32 in is about the smallest ruler I've ever used in technical settings. But 1 mm is easily seen. "Millimeter paper" is a redirect where you can learn about it and the inch-fractions that are commonly found. DMacks (talk) 13:49, 8 August 2018 (UTC)
- Or did "divided into 100" mean a 10x10 grid of 1/10-in squares? Those are available ("Engineering paper" entry on the graph-paper article). DMacks (talk) 13:51, 8 August 2018 (UTC)
- 1/100-inch graph paper is fairly unusable. Can one even visualize that small a space once the width of the line itself is accounted-for? 1/16 in or rarely 1/32 in is about the smallest ruler I've ever used in technical settings. But 1 mm is easily seen. "Millimeter paper" is a redirect where you can learn about it and the inch-fractions that are commonly found. DMacks (talk) 13:49, 8 August 2018 (UTC)
- I've not come across the phrase "millimeter paper" before but the spelling is American. Knowing the aversion some Americans have to the metric system I would imagine they would use sheets printed with a grid of one-inch squares, each square divided into 100 smaller squares. 95.150.52.197 (talk) 09:49, 8 August 2018 (UTC)
Why are all notable nearby stars getting nearer?

In this graph (original), every star shown has negative slope at time zero, implying that every one coming closer to Earth (before turning around sometime in the future), where I'd expect half of them to be approaching and half to be receding. Is there any reason this is so? Or are we just better at detecting or care more about stars coming towards us? Thanks, cmɢʟee⎆τaʟκ 17:39, 6 August 2018 (UTC)
- Any object smaller than about a galaxy cluster would be under enough gravity to overcome the metric expansion of space, which means that their relative motions of those objects is determined primarily by non-Big Bang related motion (things like gravity and inertia). Many of the closest stars to our star are probably going to be ever-so-slightly attracted to each other due to gravity, while galaxies within our Local Group also moving together; the Andromeda Galaxy is rushing at us at a pretty good clip. There are going to be some stars which are moving away, but I would suspect that most of the closest stars are gravitationally bound in such a way as to be drifting generally closer. --Jayron32 17:53, 6 August 2018 (UTC)

- No need to invoke the big bang or expansion on the scale of a few light years... The figure is incomplete in the sense that it does not include all stars currently within 10 lyr, with Sirius at 8.6 lyr a notable omission. The figure on the right is more complete and shows that roughly half of the stars are indeed receding. The other figure was drawn after a figure in a German popular astronomy magazine, and seems to refer to this article, which deals with the successors to Proxima Centauri as the clostest star to the solar system. --Wrongfilter (talk) 18:13, 6 August 2018 (UTC)
- Yes, to the original poster, sorry, but you've misinterpreted the graph. That graph depicts stars that will be nearest to the Sun in the astronomically-near future. List of nearest stars and brown dwarfs shows there are many more nearby stars, and not all of them are moving towards the Sun. --47.146.63.87 (talk) 00:11, 7 August 2018 (UTC)
- Thanks, everyone. Now I understand that "Nearest Stars..." should be interpreted as "Stars that will hold the title of nearest star..." In this case, shouldn't Lalande 21185 be excluded? Thanks, cmɢʟee⎆τaʟκ 12:35, 8 August 2018 (UTC)
- In 20,000 years Lalande 21185 will hold third place on the "nearest stars" chart, so I think it's worth a mention. — PhilHibbs | talk 13:57, 8 August 2018 (UTC)
- Thanks, everyone. Now I understand that "Nearest Stars..." should be interpreted as "Stars that will hold the title of nearest star..." In this case, shouldn't Lalande 21185 be excluded? Thanks, cmɢʟee⎆τaʟκ 12:35, 8 August 2018 (UTC)
- Yes, to the original poster, sorry, but you've misinterpreted the graph. That graph depicts stars that will be nearest to the Sun in the astronomically-near future. List of nearest stars and brown dwarfs shows there are many more nearby stars, and not all of them are moving towards the Sun. --47.146.63.87 (talk) 00:11, 7 August 2018 (UTC)
- No need to invoke the big bang or expansion on the scale of a few light years... The figure is incomplete in the sense that it does not include all stars currently within 10 lyr, with Sirius at 8.6 lyr a notable omission. The figure on the right is more complete and shows that roughly half of the stars are indeed receding. The other figure was drawn after a figure in a German popular astronomy magazine, and seems to refer to this article, which deals with the successors to Proxima Centauri as the clostest star to the solar system. --Wrongfilter (talk) 18:13, 6 August 2018 (UTC)
August 7
How long does it take for the Heisenberg uncertainty principle to scramble the weather?
Knowing every law of physics and particle in the observable universe to the quantum level and having a real-time Planck length and Planck time-scale physics model wouldn't let you predict Earth's weather beyond x weeks in advance right? What is x?
2. At what point does the 2018 level of knowledge of the current state of the atmosphere become the weakest link? There should be a level of weather computer power where no amount of extra computing power could help much without better weather satellites or something right? It might be orders of magnitude better than current weather computers but more computing power can't keep making predictions better forever at the current (or any finite) level of input detail. Sagittarian Milky Way (talk) 03:31, 7 August 2018 (UTC)
- There is no need to invoke quantum mechanics here; weather is way waaaaaayyyy beyond the limit where classical mechanics applies. The problem is that weather is a chaotic system. x is the Lyapunov time. Without knowing everything about the initial conditions of the system, you can only predict its future state out so far. In practice there are other limitations as well, like our lack of complete understanding of the Navier–Stokes equations. There's a Millennium Prize in it for you if you can fix that! --47.146.63.87 (talk) 04:20, 7 August 2018 (UTC)
- So how far could God predict the weather if he turned off his omniscience for the dice rolling? 3 weeks? 4? More? Sagittarian Milky Way (talk) 05:18, 7 August 2018 (UTC)
- You should start here and then read the references cited in that paper. Someguy1221 (talk) 05:37, 7 August 2018 (UTC)
- So how far could God predict the weather if he turned off his omniscience for the dice rolling? 3 weeks? 4? More? Sagittarian Milky Way (talk) 05:18, 7 August 2018 (UTC)
- How do you know the weather is not exactly the way God plans it? ←Baseball Bugs What's up, Doc? carrots→ 05:37, 7 August 2018 (UTC)
- No one can know but this seems to be a fire-and-forget God. Maybe there's an afterlife though. We'll find out one day. Sagittarian Milky Way (talk) 05:47, 7 August 2018 (UTC)
- Well, that's the religion of causality. As I understand it, the creed is roughly that there were some kind of small random variations a very long time ago, which may or may not have been planned by somebody, and these "caused" our current situation, which is therefore only planned in a "watchmaker God" model if there is a deterministic physics. I would think it would make sense that the boundary conditions would be set at present or future points of spacetime, but the whole notion of the past "resulting" from mathematical consistency with future events seems to conflict with the dogma. Wnt (talk) 12:32, 7 August 2018 (UTC)
- No one can know but this seems to be a fire-and-forget God. Maybe there's an afterlife though. We'll find out one day. Sagittarian Milky Way (talk) 05:47, 7 August 2018 (UTC)
- The Heisenberg Uncertainty Principle only applies in situations where the DeBroglie wavelength is on scale with the size of the object in question. For anything larger than a molecule, like say "the entire planet Earth", the DeBroglie wavelength is so insanely small that both the uncertainty in position and momentum can be close enough to zero as to make no difference. The general equation is Δx•mΔv ≥ hbar/2. The deal is, because the mass of any macroscopic object is so large, you can set both Δx (uncertainty in position) and Δv (uncertainty in velocity) to be about 15 decimal places to the right of the zero, and STILL get it to be greater than hbar/2, which is a TINY number. The only time uncertainty comes into it is when the mass is on the same scale as hbar, which only happens at the molecular scale and below. So no, the Heisenberg Uncertainly Principle doesn't even come into play on processes whose scale is as large as the weather. Good old chaos theory are sufficient to screw up long-term predictions. --Jayron32 13:44, 7 August 2018 (UTC)
- As you predict further and further in advance you need to know the state of the atmosphere at time 0 to greater greater accuracy for it to work. Wouldn't you eventually need to know where every molecule is and its momentum, and then need to know where every molecule is and its momentum to more detail than is possible? Sagittarian Milky Way (talk) 16:40, 7 August 2018 (UTC)
- You can't know that because the behavior of gases is an emergent behavior which does not depend on the individual motion of the molecules, but rather on the statistical behavior of the entirety of the system. Concepts like temperature, pressure, entropy, fluid behavior, etc. don't come out of the individual behavior of the individual molecules, but rather on the bulk behavior of the system. You can't understand weather by looking at the individual molecules even if quantum mechanics wasn't a thing. This sort of statistical thermodynamics was developed by people like Ludwig Boltzmann decades before Max Planck even thought of fixing the ultraviolet catastrophe with his quantum kludge. If you want a really good overview over the difference between individual molecular behavior (time independent) and the time-dependant statistical behavior of bulk systems, google the Feynman lecture of Entropy. It's in 2 parts, and explains it pretty well. --Jayron32 16:50, 7 August 2018 (UTC)
- As you predict further and further in advance you need to know the state of the atmosphere at time 0 to greater greater accuracy for it to work. Wouldn't you eventually need to know where every molecule is and its momentum, and then need to know where every molecule is and its momentum to more detail than is possible? Sagittarian Milky Way (talk) 16:40, 7 August 2018 (UTC)
Back of the envelope estimate. Let's take the largest Lyapunov exponent to correspond to a doubling of the uncertainty of the state of the weather per 3 days. Then we assume that this exponential increase in error holds for the smallest perturbations, so not just for the uncertainties in specifying the initial thermodynamic state of the and flow velocities, but also the way the thermal noise in specifying the exact state and the fluctuations in the forcing would affect the evolution. So, on top of the exact deterministic flow equation there is a stochastic component due to Brownian motion of the molecules. The average effect of this Brownian motion is already accounted for by the viscosity and the thermal conductivity. But on top of this average there is a fluctuation, and part of that fluctuation will be due to quantum effects.
To answer the question we can reason as follows. The quantum uncertainty in the position of molecules is of the order of the thermal deBroglie wavelength, which is given by . This fundamental uncertainty present in the position of all the molecules of the system will then double every 3 days. Relative to a classical picture where the system is described as a deterministic N-body problem where we take all effects including Brownian motion into account exactly, the system will thus drift away in a random way. If we take 100 km random displacement of all moleculues to be a totally new configuraion of the system, then this should take about 152 days. Count Iblis (talk) 00:19, 8 August 2018 (UTC)
Shortest person
Who is the shortest person in the world without some kind of genetic abnormality?
- Living or dead? People were generally much shorter in the past. ~Anachronist (talk) 06:15, 7 August 2018 (UTC)
- 0.1 mm is about the smallest for a human. Most are this size immediately after conception. Graeme Bartlett (talk) 10:02, 7 August 2018 (UTC)
- Even less impressively, we all once weighed about 0.004 of a milligram. InedibleHulk (talk) 11:32, August 7, 2018 (UTC)
- Ah, mass. A 609 picogram sphere of styrofoam is a lot more not microscopic than it sounds, 10kg of iridium is a lot smaller than it sounds. Sagittarian Milky Way (talk) 17:38, 7 August 2018 (UTC)
- According to polystyrene, "expanded polystyrene" (in corporatese) has density 0.016 g/cm^3; iridium is at 22.56. That's a ratio of 1410, which means that the size of spheres of the two differ by a linear factor of 11.2. Compress a styrofoam sphere by a factor of 11.2 in all directions, and it's dense as iridium/osmium! Density really has a lot less range to work with than a lot of other physical properties. Wnt (talk) 21:10, 7 August 2018 (UTC)
- How do you compress styrofoam that much? With explosives? It's still made of a compound with a density of about 1 g/cm^3. It'd be neat to see what happens if you compress it to the highest pressure diamond vices can make though (slowly enough that it doesn't melt or something) Sagittarian Milky Way (talk) 22:06, 7 August 2018 (UTC)
- According to polystyrene, "expanded polystyrene" (in corporatese) has density 0.016 g/cm^3; iridium is at 22.56. That's a ratio of 1410, which means that the size of spheres of the two differ by a linear factor of 11.2. Compress a styrofoam sphere by a factor of 11.2 in all directions, and it's dense as iridium/osmium! Density really has a lot less range to work with than a lot of other physical properties. Wnt (talk) 21:10, 7 August 2018 (UTC)
- Ah, mass. A 609 picogram sphere of styrofoam is a lot more not microscopic than it sounds, 10kg of iridium is a lot smaller than it sounds. Sagittarian Milky Way (talk) 17:38, 7 August 2018 (UTC)
- Even less impressively, we all once weighed about 0.004 of a milligram. InedibleHulk (talk) 11:32, August 7, 2018 (UTC)
- You may also be interested in List of the verified shortest people and Preterm birth#Notable cases where 20cm is given for a live born person. Graeme Bartlett (talk) 10:05, 7 August 2018 (UTC)
- 0.1 mm is about the smallest for a human. Most are this size immediately after conception. Graeme Bartlett (talk) 10:02, 7 August 2018 (UTC)
- Presumably the OP (193.64.221.25) meant the shortest adult person. Obviously, everyone starts out as a single-celled entity. ←Baseball Bugs What's up, Doc? carrots→ 18:28, 7 August 2018 (UTC)
- Not an answerable question, given that there is no-one without some kind of genetic abnormality ... or, thinking it of the other way, people are just different rather than abnormal. Klbrain (talk) 22:57, 7 August 2018 (UTC)
- The op says "without some kind of genetic abnormality", which is another vague statement but presumably means someone affected by dwarfism. ←Baseball Bugs What's up, Doc? carrots→ 00:49, 8 August 2018 (UTC)
- I read the list of shortest people here, and almost all of them listed some named disorder or abnormality.
- The op says "without some kind of genetic abnormality", which is another vague statement but presumably means someone affected by dwarfism. ←Baseball Bugs What's up, Doc? carrots→ 00:49, 8 August 2018 (UTC)
- Not an answerable question, given that there is no-one without some kind of genetic abnormality ... or, thinking it of the other way, people are just different rather than abnormal. Klbrain (talk) 22:57, 7 August 2018 (UTC)
- Being somewhat pedantic, note that dwarfism (or "short stature") is a descriptive term, not a diagnostic term. Our article says "short stature is clinically defined as a height within the lowest 2.3% of those in the general population", which roughly corresponds to an adult height of less than 147 centimetres (4 ft 10 in). Short stature can have a wide variety of causes, some of which are not genetic (although most are). Gandalf61 (talk) 11:52, 9 August 2018 (UTC)
Mars and Moon

When I observed the recent lunar eclipse in Poland, Mars was below and to the right from the Moon, but on this photo from Australia it's above and to the left. Why was that? Inverted photo? Thanks. --212.180.235.46 (talk) 19:42, 7 August 2018 (UTC)
- A person standing in Australia is oriented differently (relative to the stars) than a person standing in Poland, because the Earth is a sphere. CodeTalker (talk) 20:30, 7 August 2018 (UTC)
- The picture is inverted because the camera was inverted, the camera was inverted because the photographer was inverted and the photographer was inverted because that's what happens when you're down under. PiusImpavidus (talk) 07:55, 8 August 2018 (UTC)
- No, no, no. We Aussies are the right way up. It's you northerners who are upside down. HiLo48 (talk) 08:27, 8 August 2018 (UTC)
- On your typical Aussie map, which edge is northward? ←Baseball Bugs What's up, Doc? carrots→ 10:42, 8 August 2018 (UTC)
- Whichever edge the mapmaker randomly decided to make it. --Jayron32 17:45, 8 August 2018 (UTC)
- I was supposing there was a standard approach inside Australia. How often do you see American mapmakers print a map with Texas at the top? ←Baseball Bugs What's up, Doc? carrots→ 19:09, 8 August 2018 (UTC)
- Whichever edge the mapmaker randomly decided to make it. --Jayron32 17:45, 8 August 2018 (UTC)
- On your typical Aussie map, which edge is northward? ←Baseball Bugs What's up, Doc? carrots→ 10:42, 8 August 2018 (UTC)
- No, no, no. We Aussies are the right way up. It's you northerners who are upside down. HiLo48 (talk) 08:27, 8 August 2018 (UTC)
- When observing the moon, we tend to be facing the equator, which is north of Australia and south of the USA. To make it look in the northern hemisphere the way it does in the southern hemisphere, look at the moon when facing the north pole. (Also, have something to lean on so you won't fall over backwards.) ←Baseball Bugs What's up, Doc? carrots→ 10:42, 8 August 2018 (UTC)
- This is also why in Australia the horns of the moon point the other way, as this diagram illustrates:
CRESCENT MOON (EVENING VISIBILITY)
Northern hemisphere Southern hemisphere
E S W W N E
) ☼ ☼ (
So the mnemonic of adding a line to visualise the first letter of the French word premier, "first [quarter]", or dernier, "last [quarter]", does not hold. 95.150.52.197 (talk) 13:47, 8 August 2018 (UTC)
- Although the other french mnemonic of visualising the first letter of croissant (growing) or décroissant (shrinking) does work (when in applying this in France, people need to add 'but the moon is lieing') 89.225.227.82 (talk) 07:35, 9 August 2018 (UTC)
- "This is also why in Australia the horns of the moon point the other way". They point the same way east or west in both emispheres, but you are rotated 180 degrees so you see east and west swapped in the first place. Or you can say that down under the sun turns the other way round? 194.174.76.21 (talk) 12:35, 9 August 2018 (UTC) Marco Pgliero Berlin
- Although the other french mnemonic of visualising the first letter of croissant (growing) or décroissant (shrinking) does work (when in applying this in France, people need to add 'but the moon is lieing') 89.225.227.82 (talk) 07:35, 9 August 2018 (UTC)
bisexuals, heterosexuals, and tall women
I read a forum post a while back saying that heterosexuals differ from bisexuals in their attraction to women because heterosexuals greatly undervalue tall women. I feel like that has to be spot on, but is there evidence? Wnt (talk) 21:14, 7 August 2018 (UTC)
- Completely anecdotal and bad sample, but as only a 1 on the Kinsey scale and 6'2"/190 cm, this scene comes to my mind whenever I see tall and/or strong women. However, I've noticed that's an eccentricity on my part.
- I'm having total source amnesia, but I would assume based on prior reading of long since closed articles that relevant sources would discuss:
- long-term evolutionary explanations for why men favor small women focused on neoteny or... ancient likelihood of physically seizing a mate... (ugh)
- sociological or psychological explanations focused on gender roles, security in manhood, etc
- explanations combining evolution and sociology, such as how the average population height tends to shrink during and after especially terrible plagues/famines/wars because smaller people require fewer calories to function (counterbalanced with need for larger men even during these times)
- But I get the feeling that the matter really hasn't been completely settled (although some of the sources would probably present their views as something that should just be taken for granted) and that it's probably a combination of factors.
- I'm not as certain there will be sources regarding the specific issue that straight men tend to favor short women while bisexual men are more open to taller partners. Ian.thomson (talk) 21:46, 7 August 2018 (UTC)
- I think we should start by acknowledging that its difficult even to empirically validate both premises put forward here (that men tend to favour shorter women on the average and that for bisexual men the average preferred height is a little taller). Given the variances in behaviour between both cultures and individuals--as well as difficulties in formulating psychological research procedures which validate such a difficult-to-access mental processes as attraction--its difficult to know the degree to which these phenomena even exist, let alone to come up with concrete explanations for why it occurs that will be widely affirmed by the research community of cognitive scientists working in this area.
- That said, I think your shortlist of broad categories of likely factors is not bad. However, there is a much more specific and well understood factor which would certainly be playing a role here, as it is one of the strongest known influences on mate selection and attraction--and one of the best researched for that matter. All mammals, humans most assuredly included, tend to favour mates which look similar to those upon whom they imprinted when young. For example, if you dye a family of mice, recently birthed young included, all pink, then the offspring will later show a preferential attraction to other pink mice, but the parents will continue to prefer more conventionally coloured partners. The same effect can be seen in the fact that humans tend to choose partners who look like their siblings (and thus themselves); if you compare small details of body and facial morphology, you will find that most husbands and wives have features (length of the bridge of the nose, contour of the ear lobe, depth of the brow, ect.) which are substantially closer to eachother than they are to the average for any immediate local population that they belong to. It is by no means the only factor by which people choose their partners (and there's reason to believe the effect has itself been influenced by increases in ethnic integration in modern societies as well as media exposure to paradigms of physical beauty that youthful individuals would not otherwise be exposed to in their immediate environment), and so not absolutely determinative of what any given individual's partner will look like, but as a general factor operating over large groups of people, it is empirically undeniable.
- To the extent that this propensity maps to height as well, this goes a long way to explaining why the average man would, at a minimum, typically prefer a woman who is shorter than him, because the women in a family are almost always shorter than the men; indeed, baring developmental abnormality, it is virtually certain that male offspring in a family will be taller than their full-blooded female siblings. Of course, smaller than a given male individual does not necessarily equate to smaller than average, but it is a starting point for looking at population trends. Now, all that would make for a difficult analysis of the question of why bisexual men deviate from this norm with regard to their female partners (if that is indeed the case); one can speculate that there is some bleed-through of the traits which they find attractive in their partners, male and female. It might also be (and has been) argued that our preferred model for decoding sexual attraction is backward and upside-down and that rather than trying view individual features as components that conform to archetypes (including maleness and femaleness) we ought instead to be looking at the indvidual features as the drivers of attraction, on to to which the composite archetypes are later stamped. If that's the case, it would make perfect sense that an individual attracted to both men and women might have a span of heights that they find attractive and to which either male or female partners may conform. But that's as far as I can go into the matter based on the current state of research without striking off into a degree of speculation which would be inappropriate for the reference desk. Snow let's rap 03:44, 8 August 2018 (UTC)
- It could be related to sexual dimorphism and general attraction for the opposite sex for reproductive reasons: among other traits that display sexual identity, women on average tend to be shorter than men, although their are exceptions. —PaleoNeonate – 04:38, 8 August 2018 (UTC)
- Adding: if this is correct, for the same reasons many women would be attracted to tall males. —PaleoNeonate – 04:40, 8 August 2018 (UTC)
- Anne Meara was taller than her husband Jerry Stiller. Lauren Bacall was taller than Humphrey Bogart. ←Baseball Bugs What's up, Doc? carrots→ 10:44, 8 August 2018 (UTC)
What's the approximate height of the world's tallest building that can fit on 20x100 feet of land?
- 25x100 feet?
- 45x100 feet?
- 60x100 feet?
Are there any taller towers that start above the ground floor? That is, they have a tower of stuff that can stand small floors (like condos) sticking out of wider floors that might have stuff that hates small floors even more (like parking, supermarkets, offices, casinos). (in some areas zoning even has to encourage wide base/thin tower or everyone would just block the sun with a lot more floors that fill their land)
What about in 1983? Reading about a zoning law passed in 1983 has made me curious about this. Sagittarian Milky Way (talk) 21:26, 7 August 2018 (UTC)
- Trying to answer your last question, I think you are asking if there are any buildings which are narrower at the base than higher up; University Hall (University of Illinois at Chicago) is, and it's one of the tallest buildings in Chicago outside of the loop. The building also has an open ground floor; just pylons/stairways/elevators that you can enter from the courtyard. --Jayron32 16:17, 8 August 2018 (UTC)
RfC Announce: Should the EmDrive be labeled as Pseudoscience?
Talk:RF resonant cavity thruster#RfC: Should the EmDrive be labeled as Pseudoscience? --Guy Macon (talk) 22:56, 7 August 2018 (UTC)
August 8
Chemical formulas (C25H35NO5)

Hi, I was recently given some Mebeverine to take by my doctor and was reading about it on WP. I note the Chemical formula of this drug is C25H35NO5. As I know nothing about chemistry I was just wondering, does this mean my medicine contains Nobelium? or some kind of Nitrogen/Oxygen mix? I am a complete newbie to chemistry so apologies if this seems a very elementary, or even ridiculous question to be asking. I am just trying to understand what this formula means in reality, so any, even simplistic pointers would be most appreciated. Uhooep (talk) 10:57, 8 August 2018 (UTC)
- No, it does not. The symbol for Nobelium is No, not NO. The distinction between capital letters and lower case letters is VERY IMPORTANT. A two-letter symbol is always written with a capital letter first and a lower case letter second. If you see two capital letters in a row, that means two different elements. A capital letter in the formula indicates a distinct element, and the number 1 is not written if there are just one of that element in the compound. So C25H35NO5 means twenty-five carbons, thirty-five hydrogens, one nitrogen, and five oxygens. Wikipedia has articles titled Symbol (chemistry) and Chemical formula if you want more information. --Jayron32 12:45, 8 August 2018 (UTC)
- The diagram of this chemical in the Mebeverine (top–right of the whole article) helps illustrate the location of the one nitrogen and five oxygens (and no nobeliums). These sorts of skeletal diagrams might not be generally meaningful to lay readers, but they can help reinforce some formula details. DMacks (talk) 13:44, 8 August 2018 (UTC)
- I've added this above. Wnt (talk) 15:18, 8 August 2018 (UTC)
- The chemical formula tells you basically nothing about the molecule. Virtually all organic molecules are just carbon, hydrogen, oxygen, and maybe nitrogen arranged in different ways. Sometimes there's an extra element or two thrown in for variety. To know anything about how it behaves, both on its own and in vivo, you need to analyze its structure. This is why chemists use things like structural formulas. As the article states, mebeverine is an analog of papaverine. It is believed to work by antagonizing muscarinic acetylcholine receptors and probably also blocking some calcium channels in smooth muscle. It may have other targets as well—not surprising as papaverine appears similarly "dirty". --47.146.63.87 (talk) 07:05, 9 August 2018 (UTC)
Can anybody identify this insect?

It was approximately 5cm in length, and would occasionally buzz its wings. It did not actually fly whilst I was observing it, which was not for long as someone stood on it. — PhilHibbs | talk 13:47, 8 August 2018 (UTC)
- Mole cricket most likely. They typically hang around in summer. Brandmeistertalk 14:15, 8 August 2018 (UTC)
- Thanks, looks very like Gryllotalpa africana. — PhilHibbs | talk 14:55, 8 August 2018 (UTC)
- Could also be a Jerusalem cricket. --Jayron32 14:56, 8 August 2018 (UTC)
- Nah, not in the south of France (see notation under picture); besides the morphology is all wrong. Looking at the distinctive features of the head and prothorax in particular, I would say Brandmeister hit the nail on the head. Based on the aggregate clues, we seem to have two likely contenders for the exact species: the very common European Mole Cricket, Gryllotalpa gryllotalpa, and the less common (but notably also a native of southern France) Gryllotalpa vinae. The afore-mentioned Gryllotalpa africana is sometimes found in Europe, but I can find no source that reports sightings in France (they seem to be confined to rare sightings in Portugal in the West and the Caucus region and Eastward). Snow let's rap 01:42, 9 August 2018 (UTC)
- Could also be a Jerusalem cricket. --Jayron32 14:56, 8 August 2018 (UTC)
- Thanks, looks very like Gryllotalpa africana. — PhilHibbs | talk 14:55, 8 August 2018 (UTC)
August 9
Please explain this power plant availability formula specially the summation part
thermal generating station for any period means the average of the daily average declared capacities for all the days during that period expressed as a percentage of the installed capacity (in MW) of the generating station minus normative auxiliary consumption as specified in these regulations and shall be computed in accordance with the following formula; N Availability = 10000 × Σ DCi
/ {N × IC × (100 – AUXn
)} % i = 1 Where — N = Number of time blocks in the given period as may decided by the Commission from time to time; DCi
= Average Declared ex-bus Capacity in MW for the ith time block in such period;
IC = Installed Capacity of the generating station in MW; AUXn
= Normative auxiliary consumption as a percentage of gross generation;45.120.17.7 (talk) 04:22, 9 August 2018 (UTC)




