Manganese(II) chloride
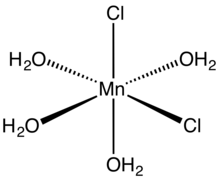 molecular structure
| |
 Tetrahydrate
| |
| Names | |
|---|---|
| IUPAC names
Manganese(II) chloride
Manganese dichloride | |
| Other names
Manganous chloride
hyperchloride of manganese | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.972 |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| MnCl2 | |
| Molar mass | 125.844 g/mol (anhydrous) 161.874 g/mol (dihydrate) 197.91 g/mol (tetrahydrate) |
| Appearance | pink solid (tetrahydrate) |
| Density | 2.977 g/cm3 (anhydrous) 2.27 g/cm3 (dihydrate) 2.01 g/cm3 (tetrahydrate) |
| Melting point | 654 °C (1,209 °F; 927 K) (anhydrous) dihydrate dehydrates at 135 °C tetrahydrate dehydrates at 58 °C |
| Boiling point | 1,225 °C (2,237 °F; 1,498 K) |
| 63.4 g/100 ml (0 °C) 73.9 g/100 ml (20 °C) 88.5 g/100 ml (40 °C) 123.8 g/100 ml (100 °C) | |
| Solubility | slightly soluble in pyridine, soluble in ethanol insoluble in ether |
| +14,350·10−6 cm3/mol | |
| Structure | |
| CdCl2 | |
| octahedral | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
250-275 mg/kg (rat, oral)[citation needed] 1715 mg/kg (mouse, oral)[1] |
| Related compounds | |
Other anions
|
Manganese(II) fluoride Manganese(II) bromide Manganese(II) iodide |
Other cations
|
Manganese(III) chloride Technetium(IV) chloride Rhenium(III) chloride Rhenium(IV) chloride Rhenium(V) chloride Rhenium(VI) chloride |
Related compounds
|
Chromium(II) chloride Iron(II) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Manganese(II) chloride is the dichloride salt of manganese, MnCl2. This inorganic chemical exists in the anhydrous form, as well as the dihydrate (MnCl2·2H2O) and tetrahydrate (MnCl2·4H2O), with the tetrahydrate being the most common form. Like many Mn(II) species, these salts are pink, with the paleness of the color being characteristic of transition metal complexes with high spin d5 configurations.[2]
Preparation
Manganese chloride is produced by treating manganese(IV) oxide with concentrated hydrochloric acid.
- MnO2 + 4 HCl → MnCl2 + 2 H2O + Cl2
This reaction was once used for the manufacture of chlorine. By carefully neutralizing the resulting solution with MnCO3, one can selectively precipitate iron salts, which are common impurities in manganese dioxide.[3]

In the laboratory, manganese chloride can be prepared by treating manganese metal or manganese(II) carbonate with hydrochloric acid:
- Mn + 2 HCl + 4 H2O → MnCl2(H2O)4 + H2
- MnCO3 + 2 HCl + 3 H2O → MnCl2(H2O)4 + CO2
Structures
Anhydrous MnCl2 adopts a layered cadmium chloride-like structure. The tetrahydrate consists of octahedral cis-Mn(H2O)4Cl2 molecules. The trans isomer, which is metastable, is also known.[4][5] The dihydrate MnCl2(H2O)2 is a coordination polymer. Each Mn center is coordinated to four doubly bridging chloride ligands. The octahedron is completed by a pair of mutually trans aquo ligands.[6]

Chemical properties
The hydrates dissolve in water to give mildly acidic solutions with a pH of around 4. These solutions consist of the metal aquo complex [Mn(H2O)6]2+.
It is a weak Lewis acid, reacting with chloride ions to produce a series of solids containing the following ions [MnCl3]−, [MnCl4]2−, and [MnCl6]4−. Both [MnCl3]− and [MnCl4]2− are polymeric.
Upon treatment with typical organic ligands, manganese(II) undergoes oxidation by air to give Mn(III) complexes. Examples include [Mn(EDTA)]−, [Mn(CN)6]3−, and [Mn(acetylacetonate)3]. Triphenylphosphine forms a labile 2:1 adduct:
- MnCl2 + 2 Ph3P → [MnCl2(Ph3P)2]
Anhydrous manganese(II) chloride serves as a starting point for the synthesis of a variety of manganese compounds. For example, manganocene is prepared by reaction of MnCl2 with a solution of sodium cyclopentadienide in THF.
- MnCl2 + 2 NaC5H5 → Mn(C5H5)2 + 2 NaCl
NMR
Aqueous solutions of manganese(II) chloride are used in 31P-NMR to determine the size and lamellarity of phospholipid vesicles.[7] When manganese chloride is added to a vesicular solution, Mn2+ paramagnetic ions are released, perturbing the relaxation time of the phospholipids' phosphate groups and broadening the resulting 31P resonance signal. Only phospholipids located in the outermost monolayer exposed to Mn2+ experience this broadening. The effect is negligible for multilamellar vesicles, but for large unilamellar vesicles, a ~50% reduction in signal intensity is observed.[8]
Natural occurrence
Scacchite is the natural, anhydrous form of manganese(II) chloride.[9] The only other currently known mineral systematized as manganese chloride is kempite - a representative of the atacamite group, a group of hydroxide-chlorides.[10]
Applications
Manganese chloride is mainly used in the production of dry cell batteries. It is the precursor to the antiknock compound methylcyclopentadienyl manganese tricarbonyl.[3]
Precautions
Manganism, or manganese poisoning, can be caused by long-term exposure to manganese dust or fumes.
References
- ^ "Manganese compounds (as Mn)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- ^ a b Reidies, Arno H. (2002), "Manganese Compounds", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a16_123, ISBN 978-3-527-30385-4.
- ^ Zalkin, Allan; Forrester, J. D.; Templeton, David H. (1964). "Crystal structure of manganese dichloride tetrahydrate". Inorganic Chemistry. 3 (4): 529–33. doi:10.1021/ic50014a017.
- ^ A. F. Wells, Structural Inorganic Chemistry, 5th ed., Oxford University Press, Oxford, UK, 1984.
- ^ Morosin, B.; Graeber, E. J. (1965). "Crystal structures of manganese(II) and iron(II) chloride dihydrate". Journal of Chemical Physics. 42 (3): 898–901. Bibcode:1965JChPh..42..898M. doi:10.1063/1.1696078.
- ^ Frohlich, Margret; Brecht, Volker; Peschka-Suss, Regine (January 2001), "Parameters influencing the determination of liposome lamellarity by 31P-NMR", Chemistry and Physics of Lipids, 109 (1): 103–112, doi:10.1016/S0009-3084(00)00220-6, PMID 11163348
- ^ Hope M, Bally M, Webb G, Cullis P (April 10, 1984), "Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential" (PDF), Biochimica et Biophysica Acta (BBA) - Biomembranes, 812 (1): 55–65, doi:10.1016/0005-2736(85)90521-8, PMID 23008845
- ^ https://www.mindat.org/min-3549.html
- ^ https://www.mindat.org/min-2183.html

