LY-235959
Appearance
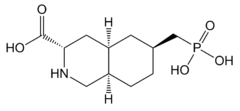 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H20NO5P |
| Molar mass | 277.257 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
LY-235959 is a competitive antagonist at the NMDA receptor.[1] It has analgesic and neuroprotective effects and causes hypothermia in animal models,[2] as well as reducing the development of tolerance to morphine and altering the reinforcing effects of cocaine.[3][4][5][6][7]
References
- ^ Allen RM, Dykstra LA (July 2001). "N-methyl-D-aspartate receptor antagonists potentiate the antinociceptive effects of morphine in squirrel monkeys". The Journal of Pharmacology and Experimental Therapeutics. 298 (1): 288–97. PMID 11408554.
- ^ Rawls SM, Cowan A, Tallarida RJ, Geller EB, Adler MW (October 2002). "N-methyl-D-aspartate antagonists and WIN 55212-2 [4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1-i,j]quinolin-6-one], a cannabinoid agonist, interact to produce synergistic hypothermia". The Journal of Pharmacology and Experimental Therapeutics. 303 (1): 395–402. doi:10.1124/jpet.102.037473. PMID 12235276.
- ^ Allen RM, Carelli RM, Dykstra LA, Suchey TL, Everett CV (October 2005). "Effects of the competitive N-methyl-D-aspartate receptor antagonist, LY235959 [(-)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid], on responding for cocaine under both fixed and progressive ratio schedules of reinforcement". The Journal of Pharmacology and Experimental Therapeutics. 315 (1): 449–57. doi:10.1124/jpet.105.086355. PMID 16024734.
- ^ Allen RM, Dykstra LA, Carelli RM (April 2007). "Continuous exposure to the competitive N-methyl-D: -aspartate receptor antagonist, LY235959, facilitates escalation of cocaine consumption in Sprague-Dawley rats". Psychopharmacology. 191 (2): 341–51. doi:10.1007/s00213-006-0661-3. PMID 17225167.
- ^ Fischer BD, Ward SJ, Henry FE, Dykstra LA (February 2010). "Attenuation of morphine antinociceptive tolerance by a CB(1) receptor agonist and an NMDA receptor antagonist: Interactive effects". Neuropharmacology. 58 (2): 544–50. doi:10.1016/j.neuropharm.2009.08.005. PMC 2813317. PMID 19699755.
- ^ Dykstra LA, Fischer BD, Balter RE, Henry FE, Schmidt KT, Miller LL (September 2011). "Opioid antinociception, tolerance and dependence: interactions with the N-methyl-D-aspartate system in mice". Behavioural Pharmacology. 22 (5–6): 540–7. doi:10.1097/FBP.0b013e328348ed08. PMC 3155647. PMID 21712708.
- ^ Bicca MA, Figueiredo CP, Piermartiri TC, Meotti FC, Bouzon ZL, Tasca CI, et al. (September 2011). "The selective and competitive N-methyl-D-aspartate receptor antagonist, (-)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid, prevents synaptic toxicity induced by amyloid-β in mice". Neuroscience. 192: 631–41. doi:10.1016/j.neuroscience.2011.06.038. PMID 21756976.
