Carbohydrate: Difference between revisions
ClueBot NG (talk | contribs) m Reverting possible vandalism by Whynotlamacha to version by Lugia2453. False positive? Report it. Thanks, ClueBot NG. (1187439) (Bot) |
No edit summary Tag: repeating characters |
||
| Line 2: | Line 2: | ||
[[Image:Lactose.svg|thumb |right|310px|[[Lactose]] is a [[disaccharide]] found in [[milk]]. It consists of a molecule of [[galactose|D-galactose]] and a molecule of [[glucose|D-glucose]] bonded by beta-1-4 [[glycosidic linkage]]. It has a formula of C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>.]] |
[[Image:Lactose.svg|thumb |right|310px|[[Lactose]] is a [[disaccharide]] found in [[milk]]. It consists of a molecule of [[galactose|D-galactose]] and a molecule of [[glucose|D-glucose]] bonded by beta-1-4 [[glycosidic linkage]]. It has a formula of C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>.]] |
||
A '''carbohydrate''' is an [[ |
A '''carbohydrate''' is an [[inorganic compound]] that consists only of [[carbon]], [[hydrogen]], and [[oxygen]], usually with a hydrogen:oxygen [[atom]] ratio of 2:1 (as in [[water]]); in other words, with the empirical formula {{nowrap|C<sub>''m''</sub>(H<sub>2</sub>O)<sub>''n''</sub>}}. (Some exceptions exist; for example, [[deoxyribose]], a component of DNA, has the empirical formula C<sub>5</sub>H<sub>10</sub>O<sub>4</sub>.) Carbohydrates are not technically [[hydrate]]s of carbon. Structurally it is more accurate to view them as [[Polyhydroxyaldehyde|polyhydroxy aldehydes]] and [[ketone]]s. |
||
The term is most common in [[biochemistry]], where it is a synonym of '''saccharide'''. The carbohydrates (saccharides) are divided into four chemical groupings: [[monosaccharide]]s, [[disaccharide]]s, [[oligosaccharide]]s, and [[polysaccharide]]s. In general, the monosaccharides and disaccharides, which are smaller (lower [[molecular weight]]) carbohydrates, are commonly referred to as [[sugar]]s.<ref>Flitsch, SL & Ulijn, RV (2003). [http://www.nature.com/nature/journal/v421/n6920/pdf/421219a.pdf "Sugars tied to the spot." ''Nature'' 421: 219–220].</ref> The word ''saccharide'' comes from the [[Greek language|Greek]] word ''σάκχαρον'' (''sákkharon''), meaning "[[sugar]]". While the scientific nomenclature of carbohydrates is complex, the names of the monosaccharides and disaccharides very often end in the suffix [[-ose]]. For example, [[blood sugar]] is the monosaccharide [[glucose]], table sugar is the disaccharide [[sucrose]], and milk sugar is the disaccharide [[lactose]] (see illustration). |
The term is most common in [[biochemistry]], where it is a synonym of '''saccharide'''. The carbohydrates (saccharides) are divided into four chemical groupings: [[monosaccharide]]s, [[disaccharide]]s, [[oligosaccharide]]s, and [[polysaccharide]]s. In general, the monosaccharides and disaccharides, which are smaller (lower [[molecular weight]]) carbohydrates, are commonly referred to as [[sugar]]s.<ref>Flitsch, SL & Ulijn, RV (2003). [http://www.nature.com/nature/journal/v421/n6920/pdf/421219a.pdf "Sugars tied to the spot." ''Nature'' 421: 219–220].</ref> The word ''saccharide'' comes from the [[Greek language|Greek]] word ''σάκχαρον'' (''sákkharon''), meaning "[[sugar]]". While the scientific nomenclature of carbohydrates is complex, the names of the monosaccharides and disaccharides very often end in the suffix [[-ose]]. For example, [[blood sugar]] is the monosaccharide [[glucose]], table sugar is the disaccharide [[sucrose]], and milk sugar is the disaccharide [[lactose]] (see illustration). |
||
| Line 62: | Line 62: | ||
* Their ring types: glucose is a [[pyranose]], and fructose is a [[furanose]] |
* Their ring types: glucose is a [[pyranose]], and fructose is a [[furanose]] |
||
* How they are linked together: the oxygen on carbon number 1 (C1) of α-D-glucose is linked to the C2 of D-fructose. |
* How they are linked together: the oxygen on carbon number 1 (C1) of α-D-glucose is linked to the C2 of D-fructose. |
||
* The ''-oside'' suffix indicates that the [[anomeric |
* The ''-oside'' suffix indicates that the [[anomeric carbono��U���G��T��g�|O5�rO���E,��#�!��ݓ��6@W�V��� |
||
���t�v��̚[bmC������&���%kb$1�߂��C/ |
|||
��O/�����M3l��h���O5�X��5�f��JV�(���19-�]�7k/�_�/�����z�8ftH�&v��� |
|||
o�]�P����~ ����C<���ԲSg�=xn��uIʤcurs naturally in mammalian [[milk]]. The [[systematic name]] for lactose is ''O''-β-D-galactopyranosyl-(1→4)-D-glucopyranose. Other notable disaccharides include [[maltose]] (two D-glucoses linked α-1,4) and cellulobiose (two D-glucoses linked β-1,4). |
|||
disaccharides can be classified into two types.They are reducing and non-reducing disaccahrides if the functional group is present in bonding with another sugar unit it is called a reducing disaccharide or biose. |
disaccharides can be classified into two types.They are reducing and non-reducing disaccahrides if the functional group is present in bonding with another sugar unit it is called a reducing disaccharide or biose. |
||
| Line 74: | Line 75: | ||
Carbohydrates are not necessary building blocks of other molecules, and the body can obtain all its energy from protein and fats.<ref>[http://www.ajcn.org/cgi/content/full/75/5/951-a Is dietary carbohydrate essential for human nutrition? - Westman 75 (5): 951 - American Journal of Clinical Nutrition<!-- Bot generated title -->]</ref><ref>[http://jn.nutrition.org/cgi/reprint/136/5/1256?ijkey=ebf0450b5cf21e8d83dd43f62b5559254694f65f A High-Protein, High-Fat, Carbohydrate-Free Diet Reduces Energy Intake, Hepatic Lipogenesis, and Adiposity in Rats - Pichon et al. 136 (5): 1256 - Journal of Nutrition<!-- Bot generated title -->]</ref> The brain and neurons generally cannot burn fat for energy, but use glucose or [[Ketone bodies|ketones]]. Humans can synthesize some glucose (in a set of processes known as [[gluconeogenesis]]) from specific amino acids, from the [[glycerol]] backbone in [[triglyceride]]s and in some cases from fatty acids. Carbohydrate and protein contain 4 kilocalories per [[gram]], while fats contain 9 kilocalories per gram. In the case of protein, this is somewhat misleading as only some amino acids are usable for fuel. |
Carbohydrates are not necessary building blocks of other molecules, and the body can obtain all its energy from protein and fats.<ref>[http://www.ajcn.org/cgi/content/full/75/5/951-a Is dietary carbohydrate essential for human nutrition? - Westman 75 (5): 951 - American Journal of Clinical Nutrition<!-- Bot generated title -->]</ref><ref>[http://jn.nutrition.org/cgi/reprint/136/5/1256?ijkey=ebf0450b5cf21e8d83dd43f62b5559254694f65f A High-Protein, High-Fat, Carbohydrate-Free Diet Reduces Energy Intake, Hepatic Lipogenesis, and Adiposity in Rats - Pichon et al. 136 (5): 1256 - Journal of Nutrition<!-- Bot generated title -->]</ref> The brain and neurons generally cannot burn fat for energy, but use glucose or [[Ketone bodies|ketones]]. Humans can synthesize some glucose (in a set of processes known as [[gluconeogenesis]]) from specific amino acids, from the [[glycerol]] backbone in [[triglyceride]]s and in some cases from fatty acids. Carbohydrate and protein contain 4 kilocalories per [[gram]], while fats contain 9 kilocalories per gram. In the case of protein, this is somewhat misleading as only some amino acids are usable for fuel. |
||
Organisms typically cannot metabolize all types of carbohydrate to yield energy. Glucose is a nearly universal and accessible source of calories. Many organisms also have the ability to metabolize other [[monosaccharide]]s and [[Disaccharide]]s, though glucose is preferred. In ''[[Escherichia coli]]'', for example, the ''lac'' operon will express enzymes for the digestion of lactose when it is present, but if both lactose and glucose are present the ''lac'' operon is repressed, resulting in the glucose being used first (see: [[Diauxie]]). [[ |
Organisms typically cannot metabolize all types of carbohydrate to yield energy. Glucose is a nearly universal and accessible source of calories. Many organisms also have the ability to metabolize other [[monosaccharide]]s and [[Disaccharide]]s, though glucose is preferred. In ''[[Escherichia coli]]'', for example, the ''lac'' operon will express enzymes for the digestion of lactose when it is present, but if both lactose and glucose are present the ''lac'' operon is repressed, resulting in the glucose being used first (see: [[Diauxie]]). [[Polysaccharid������JFIF��������������*����C��������������� |
||
� |
|||
���������������%���#��� , #&')*)��-0-(0%()(���C���� |
|||
� |
|||
� |
|||
�(���((((((((((((((((((((((((((((((((((((((((((((((((((������d�d��"������������in]] and [[arabinoxylans]]. These carbohydrates types can be metabolized by some bacteria and protists. [[Ruminant]]s and [[termite]]s, for example, use microorganisms to process [[cellulose]]. Even though these complex carbohydrates are not very digestible, they may comprise important dietary elements for humans. Called dietary fiber, these carbohydrates enhance digestion among other benefits.<ref>[http://archinte.ama-assn.org/cgi/content/short/archinternmed.2011.18 Dietary Fiber Intake and Mortality in the NIH-AARP Diet and Health Study - Part, et al. 171 (12): 1061 - Archives of Internal Medicine<!-- Bot generated title -->]</ref> |
|||
Based on the effects on risk of heart disease and obesity,<ref>[http://www.ajcn.org/content/92/4/733.abstract?sid=f97a2937-5a29-4143-8f1a-676f100177ab Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized, controlled trial - Tighe, et al. 92 (4): 733 - American Journal of Clinical Nutrition<!-- Bot generated title -->]</ref> the [[Institute of Medicine]] recommends that American and Canadian adults get between 45–65% of [[food energy|dietary energy]] from carbohydrates.<ref>Food and Nutrition Board (2002/2005). ''[http://newton.nap.edu/books/0309085373/html Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids]''. Washington, D.C.: The [[National Academies Press]]. Page [http://newton.nap.edu/books/0309085373/html/769.html 769]. ISBN 0-309-08537-3.</ref> The [[Food and Agriculture Organization]] and [[World Health Organization]] jointly recommend that national dietary guidelines set a goal of 55–75% of total energy from carbohydrates, but only 10% directly from sugars (their term for simple carbohydrates).<ref>Joint WHO/FAO expert consultation (2003). ''[http://www.who.int/hpr/NPH/docs/who_fao_expert_report.pdf Diet, Nutrition and the Prevention of Chronic Diseases]'' ([[Portable Document Format|PDF]]). Geneva: [[World Health Organization]]. Pages 55–56. ISBN 92-4-120916-X.</ref> |
Based on the effects on risk of heart disease and obesity,<ref>[http://www.ajcn.org/content/92/4/733.abstract?sid=f97a2937-5a29-4143-8f1a-676f100177ab Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized, controlled trial - Tighe, et al. 92 (4): 733 - American Journal of Clinical Nutrition<!-- Bot generated title -->]</ref> the [[Institute of Medicine]] recommends that American and Canadian adults get between 45–65% of [[food energy|dietary energy]] from carbohydrates.<ref>Food and Nutrition Board (2002/2005). ''[http://newton.nap.edu/books/0309085373/html Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids]''. Washington, D.C.: The [[National Academies Press]]. Page [http://newton.nap.edu/books/0309085373/html/769.html 769]. ISBN 0-309-08537-3.</ref> The [[Food and Agriculture Organization]] and [[World Health Organization]] jointly recommend that national dietary guidelines set a goal of 55–75% of total energy from carbohydrates, but only 10% directly from sugars (their term for simple carbohydrates).<ref>Joint WHO/FAO expert consultation (2003). ''[http://www.who.int/hpr/NPH/docs/who_fao_expert_report.pdf Diet, Nutrition and the Prevention of Chronic Diseases]'' ([[Portable Document Format|PDF]]). Geneva: [[World Health Organization]]. Pages 55–56. ISBN 92-4-120916-X.</ref> |
||
| Line 232: | Line 241: | ||
[[tr:Karbonhidrat]] |
[[tr:Karbonhidrat]] |
||
[[uk:Вуглеводи]] |
[[uk:Вуглеводи]] |
||
[[ur:کاربو ہ���L��(�����VJ�O�Һ��:J�EE�E�)���JVJJ��������[vec:Carboidrati]] |
|||
[[ur:کاربو ہائیڈریٹ]] |
|||
[[za:Danqsuijvaqhozvuz]] |
|||
[[vec:Carboidrati]] |
|||
[[vi:Cacbohydrat]] |
[[vi:Cacbohydrat]] |
||
[[war:Karbohidrato]] |
[[war:Karbohidrato]] |
||
Revision as of 05:27, 29 August 2012
A carbohydrate is an inorganic compound that consists only of carbon, hydrogen, and oxygen, usually with a hydrogen:oxygen atom ratio of 2:1 (as in water); in other words, with the empirical formula Cm(H2O)n. (Some exceptions exist; for example, deoxyribose, a component of DNA, has the empirical formula C5H10O4.) Carbohydrates are not technically hydrates of carbon. Structurally it is more accurate to view them as polyhydroxy aldehydes and ketones.
The term is most common in biochemistry, where it is a synonym of saccharide. The carbohydrates (saccharides) are divided into four chemical groupings: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. In general, the monosaccharides and disaccharides, which are smaller (lower molecular weight) carbohydrates, are commonly referred to as sugars.[1] The word saccharide comes from the Greek word σάκχαρον (sákkharon), meaning "sugar". While the scientific nomenclature of carbohydrates is complex, the names of the monosaccharides and disaccharides very often end in the suffix -ose. For example, blood sugar is the monosaccharide glucose, table sugar is the disaccharide sucrose, and milk sugar is the disaccharide lactose (see illustration).
Carbohydrates perform numerous roles in living organisms. Polysaccharides serve for the storage of energy (e.g., starch and glycogen), and as structural components (e.g., cellulose in plants and chitin in arthropods). The 5-carbon monosaccharide ribose is an important component of coenzymes (e.g., ATP, FAD, and NAD) and the backbone of the genetic molecule known as RNA. The related deoxyribose is a component of DNA. Saccharides and their derivatives include many other important biomolecules that play key roles in the immune system, fertilization, preventing pathogenesis, blood clotting, and development.[2]
In food science and in many informal contexts, the term carbohydrate often means any food that is particularly rich in the complex carbohydrate starch (such as cereals, bread, and pasta) or simple carbohydrates, such as sugar (found in candy, jams, and desserts).
Structure
Formerly the name "carbohydrate" was used in chemistry for any compound with the formula Cm (H2O) n. Following this definition, some chemists considered formaldehyde (CH2O) to be the simplest carbohydrate,[3] while others claimed that title for glycolaldehyde.[4] Today the term is generally understood in the biochemistry sense, which excludes compounds with only one or two carbons.
Natural saccharides are generally built of simple carbohydrates called monosaccharides with general formula (CH2O)n where n is three or more. A typical monosaccharide has the structure H-(CHOH)x(C=O)-(CHOH)y-H, that is, an aldehyde or ketone with many hydroxyl groups added, usually one on each carbon atom that is not part of the aldehyde or ketone functional group. Examples of monosaccharides are glucose, fructose, and glyceraldehydes. However, some biological substances commonly called "monosaccharides" do not conform to this formula (e.g., uronic acids and deoxy-sugars such as fucose), and there are many chemicals that do conform to this formula but are not considered to be monosaccharides (e.g., formaldehyde CH2O and inositol (CH2O)6).[5]
The open-chain form of a monosaccharide often coexists with a closed ring form where the aldehyde/ketone carbonyl group carbon (C=O) and hydroxyl group (-OH) react forming a hemiacetal with a new C-O-C bridge.
Monosaccharides can be linked together into what are called polysaccharides (or oligosaccharides) in a large variety of ways. Many carbohydrates contain one or more modified monosaccharide units that have had one or more groups replaced or removed. For example, deoxyribose, a component of DNA, is a modified version of ribose; chitin is composed of repeating units of N-acetyl glucosamine, a nitrogen-containing form of glucose.
Monosaccharides

Monosaccharides are the simplest carbohydrates in that they cannot be hydrolyzed to smaller carbohydrates. They are aldehydes or ketones with two or more hydroxyl groups. The general chemical formula of an unmodified monosaccharide is (C•H2O) n, literally a "carbon hydrate." Monosaccharides are important fuel molecules as well as building blocks for nucleic acids. The smallest monosaccharides, for which n = 3, are dihydroxyacetone and D- and L-glyceraldehydes.
Classification of monosaccharides
Monosaccharides are classified according to three different characteristics: the placement of its carbonyl group, the number of carbon atoms it contains, and its chiral handedness. If the carbonyl group is an aldehyde, the monosaccharide is an aldose; if the carbonyl group is a ketone, the monosaccharide is a ketose. Monosaccharides with three carbon atoms are called trioses, those with four are called tetroses, five are called pentoses, six are hexoses, and so on.[6] These two systems of classification are often combined. For example, glucose is an aldohexose (a six-carbon aldehyde), ribose is an aldopentose (a five-carbon aldehyde), and fructose is a ketohexose (a six-carbon ketone).
Each carbon atom bearing a hydroxyl group (-OH), with the exception of the first and last carbons, are asymmetric, making them stereo centers with two possible configurations each (R or S). Because of this asymmetry, a number of isomers may exist for any given monosaccharide formula. The aldohexose D-glucose, for example, has the formula (C·H2O) 6, of which all but two of its six carbons atoms are stereogenic, making D-glucose one of 24 = 16 possible stereoisomers. In the case of glyceraldehydes, an aldotriose, there is one pair of possible stereoisomers, which are enantiomers and epimers. 1, 3-dihydroxyacetone, the ketose corresponding to the aldose glyceraldehydes, is a symmetric molecule with no stereo centers). The assignment of D or L is made according to the orientation of the asymmetric carbon furthest from the carbonyl group: in a standard Fischer projection if the hydroxyl group is on the right the molecule is a D sugar, otherwise it is an L sugar. The "D-" and "L-" prefixes should not be confused with "d-" or "l-", which indicate the direction that the sugar rotates plane polarized light. This usage of "d-" and "l-" is no longer followed in carbohydrate chemistry.[7]
Ring-straight chain isomerism

The aldehyde or ketone group of a straight-chain monosaccharide will react reversibly with a hydroxyl group on a different carbon atom to form a hemiacetal or hemiketal, forming a heterocyclic ring with an oxygen bridge between two carbon atoms. Rings with five and six atoms are called furanose and pyranose forms, respectively, and exist in equilibrium with the straight-chain form.[8]
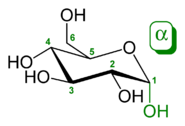
During the conversion from straight-chain form to the cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a stereogenic center with two possible configurations: The oxygen atom may take a position either above or below the plane of the ring. The resulting possible pair of stereoisomers is called anomers. In the α anomer, the -OH substituent on the anomeric carbon rests on the opposite side (trans) of the ring from the CH2OH side branch. The alternative form, in which the CH2OH substituent and the anomeric hydroxyl are on the same side (cis) of the plane of the ring, is called the β anomer.
Use in living organisms
Monosaccharides are the major source of fuel for metabolism, being used both as an energy source (glucose being the most important in nature) and in biosynthesis. When monosaccharides are not immediately needed by many cells they are often converted to more space-efficient forms, often polysaccharides. In many animals, including humans, this storage form is glycogen, especially in liver and muscle cells. In plants, starch is used for the same purpose.
Disaccharides

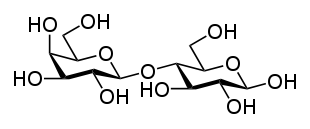
Two joined monosaccharides are called a disaccharide and these are the simplest polysaccharides. Examples include sucrose and lactose. They are composed of two monosaccharide units bound together by a covalent bond known as a glycosidic linkage formed via a dehydration reaction, resulting in the loss of a hydrogen atom from one monosaccharide and a hydroxyl group from the other. The formula of unmodified disaccharides is C12H22O11. Although there are numerous kinds of disaccharides, a handful of disaccharides are particularly notable.
Sucrose, pictured to the right, is the most abundant disaccharide, and the main form in which carbohydrates are transported in plants. It is composed of one D-glucose molecule and one D-fructose molecule. The systematic name for sucrose, O-α-D-glucopyranosyl-(1→2)-D-fructofuranoside, indicates four things:
- Its monosaccharides: glucose and fructose
- Their ring types: glucose is a pyranose, and fructose is a furanose
- How they are linked together: the oxygen on carbon number 1 (C1) of α-D-glucose is linked to the C2 of D-fructose.
- The -oside suffix indicates that the [[anomeric carbono��U���G��T��g�|O5�rO���E,��#�!��ݓ��6@W�V���
���t�v��̚[bmC������&���%kb$1�߂��C/ ��O/�����M3l��h���O5�X��5�f��JV�(���19-�]�7k/�_�/�����z�8ftH�&v��� o�]�P����~ ?����C<���ԲSg�=xn��uIʤcurs naturally in mammalian milk. The systematic name for lactose is O-β-D-galactopyranosyl-(1→4)-D-glucopyranose. Other notable disaccharides include maltose (two D-glucoses linked α-1,4) and cellulobiose (two D-glucoses linked β-1,4). disaccharides can be classified into two types.They are reducing and non-reducing disaccahrides if the functional group is present in bonding with another sugar unit it is called a reducing disaccharide or biose.
Nutrition

Foods high in carbohydrate include fruits, sweets, soft drinks, breads, pastas, beans, potatoes, bran, rice, and cereals. Carbohydrates are a common source of energy in living organisms; however, no carbohydrate is an essential nutrient in humans.[9]
Carbohydrates are not necessary building blocks of other molecules, and the body can obtain all its energy from protein and fats.[10][11] The brain and neurons generally cannot burn fat for energy, but use glucose or ketones. Humans can synthesize some glucose (in a set of processes known as gluconeogenesis) from specific amino acids, from the glycerol backbone in triglycerides and in some cases from fatty acids. Carbohydrate and protein contain 4 kilocalories per gram, while fats contain 9 kilocalories per gram. In the case of protein, this is somewhat misleading as only some amino acids are usable for fuel.
Organisms typically cannot metabolize all types of carbohydrate to yield energy. Glucose is a nearly universal and accessible source of calories. Many organisms also have the ability to metabolize other monosaccharides and Disaccharides, though glucose is preferred. In Escherichia coli, for example, the lac operon will express enzymes for the digestion of lactose when it is present, but if both lactose and glucose are present the lac operon is repressed, resulting in the glucose being used first (see: Diauxie). [[Polysaccharid������JFIF��������������*����C��������������� �
���������������%���#��� , #&')*)��-0-(0%()(���C����
�
�
�(���((((((((((((((((((((((((((((((((((((((((((((((((((������d�d��"������������in]] and arabinoxylans. These carbohydrates types can be metabolized by some bacteria and protists. Ruminants and termites, for example, use microorganisms to process cellulose. Even though these complex carbohydrates are not very digestible, they may comprise important dietary elements for humans. Called dietary fiber, these carbohydrates enhance digestion among other benefits.[12]
Based on the effects on risk of heart disease and obesity,[13] the Institute of Medicine recommends that American and Canadian adults get between 45–65% of dietary energy from carbohydrates.[14] The Food and Agriculture Organization and World Health Organization jointly recommend that national dietary guidelines set a goal of 55–75% of total energy from carbohydrates, but only 10% directly from sugars (their term for simple carbohydrates).[15]
Classification
Historically nutritionists have classified carbohydrates as either simple or complex. However, the exact delineation of these categories is ambiguous. Today, simple carbohydrate typically refers to monosaccharides and disaccharides and complex carbohydrate means polysaccharides (and oligosaccharides). However, the term complex carbohydrate was first used in slightly different context in the U.S. Senate Select Committee on Nutrition and Human Needs publication Dietary Goals for the United States (1977). In this work, complex carbohydrate were defined as "fruit, vegetables and whole-grains".[16] Some nutritionists use complex carbohydrate to refer to any sort of digestible saccharide present in a whole food, where fiber, vitamins and minerals are also found (as opposed to processed carbohydrates, which provide calories but few other nutrients).
Some simple carbohydrates(e.g. fructose) are digested very slowly, while some complex carbohydrates (starches), especially if processed, raise blood sugar rapidly. The speed of digestion is determined by a variety of factors including which other nutrients are consumed with the carbohydrate, how the food is prepared, individual differences in metabolism, and the chemistry of the carbohydrate.[citation needed]
The USDA's Dietary Guidelines for Americans 2010 call for moderate- to high-carbohydrate consumption from a balanced diet that includes six one-ounce servings of grain foods each day, at least half from whole grain sources and the rest from enriched.[17]
The glycemic index (GI) and glycemic load concepts have been developed to characterize food behavior during human digestion. They rank carbohydrate-rich foods based on the rapidity and magnitude of their effect on blood glucose levels. Glycemic index is a measure of how quickly food glucose is absorbed, while glycemic load is a measure of the total absorbable glucose in foods. The insulin index is a similar, more recent classification method that ranks foods based on their effects on blood insulin levels, which are caused by glucose (or starch) and some amino acids in food.
Metabolism
This section needs expansion. You can help by adding to it. (June 2008) |
Catabolism
Catabolism is the metabolic reaction in which cells undergo to extract energy. There are two major metabolic pathways of monosaccharide catabolism: glycolysis and the citric acid cycle.
In glycolysis, oligo/polysaccharides are cleaved first to smaller monosaccharides by enzymes called glycoside hydrolases. The monosaccharide units can then enter into monosaccharide catabolism. In some cases, as with humans, not all carbohydrate types are usable as the digestive and metabolic enzymes necessary are not present.
Carbohydrate chemistry
Carbohydrate chemistry is a large and economically important branch of organic chemistry. Some of the main organic reactions that involve carbohydrates are:
- Carbohydrate acetalisation
- Cyanohydrin reaction
- Lobry-de Bruyn-van Ekenstein transformation
- Amadori rearrangement
- Nef reaction
- Wohl degradation
- Koenigs–Knorr reaction
See also
References
- ^ Flitsch, SL & Ulijn, RV (2003). "Sugars tied to the spot." Nature 421: 219–220.
- ^ Maton, Anthea (1993). Human Biology and Health. Englewood Cliffs, New Jersey, USA: Prentice Hall. pp. 52–59. ISBN 0-13-981176-1.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ John Merle Coulter, Charler Reid Barnes, Henry Chandler Cowles (1930), A Textbook of Botany for Colleges and Universities"
- ^ Carl A. Burtis, Edward R. Ashwood, Norbert W. Tietz (2000), Tietz fundamentals of clinical chemistry
- ^ Matthews, C. E.; K. E. Van Holde; K. G. Ahern (1999) Biochemistry. 3rd edition. Benjamin Cummings. ISBN 0-8053-3066-6
- ^ Campbell, Neil A. (2006). Biology: Exploring Life. Boston, Massachusetts: Pearson Prentice Hall. ISBN 0-13-250882-6.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Pigman, Ward (1972). "Chapter 1: Stereochemistry of the Monosaccharides". In Pigman and Horton (ed.). The Carbohydrates: Chemistry and Biochemistry Vol 1A (2nd ed.). San Diego: Academic Press. pp. 1–67.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Pigman, Ward (1972). "Chapter 4: Mutarotations and Actions of Acids and Bases". In Pigman and Horton (ed.). The Carbohydrates: Chemistry and Biochemistry Vol 1A (2nd ed.). San Diego: Academic Press. pp. 165–194.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ http://www.ajcn.org/content/75/5/951.2.full
- ^ Is dietary carbohydrate essential for human nutrition? - Westman 75 (5): 951 - American Journal of Clinical Nutrition
- ^ A High-Protein, High-Fat, Carbohydrate-Free Diet Reduces Energy Intake, Hepatic Lipogenesis, and Adiposity in Rats - Pichon et al. 136 (5): 1256 - Journal of Nutrition
- ^ Dietary Fiber Intake and Mortality in the NIH-AARP Diet and Health Study - Part, et al. 171 (12): 1061 - Archives of Internal Medicine
- ^ Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized, controlled trial - Tighe, et al. 92 (4): 733 - American Journal of Clinical Nutrition
- ^ Food and Nutrition Board (2002/2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, D.C.: The National Academies Press. Page 769. ISBN 0-309-08537-3.
- ^ Joint WHO/FAO expert consultation (2003). Diet, Nutrition and the Prevention of Chronic Diseases (PDF). Geneva: World Health Organization. Pages 55–56. ISBN 92-4-120916-X.
- ^ Joint WHO/FAO expert consultation (1998), Carbohydrates in human nutrition, chapter 1. ISBN 92-5-104114-8.
- ^ DHHS and USDA, Dietary Guidelines for Americans 2010, [1]
External links
- Carbohydrates, including interactive models and animations (Requires MDL Chime)
- IUPAC-IUBMB Joint Commission on Biochemical Nomenclature (JCBN): Carbohydrate Nomenclature
- Carbohydrates detailed
- Complex And Simple Carbohydrates Explanation of the differences
- Carbohydrates and Glycosylation - The Virtual Library of Biochemistry and Cell Biology
- Functional Glycomics Gateway, a collaboration between the Consortium for Functional Glycomics and Nature Publishing Group
- Wine Carbohydrates
Template:Link GA [[ur:کاربو ہ���L��(�����VJ�O�Һ��:J�EE�E�)���JVJJ��������[vec:Carboidrati]]