Magnesium carbonate
| |

| |
| Names | |
|---|---|
| Other names | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.106 |
| E number | E504(i) (acidity regulators, ...) |
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
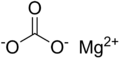
| MgCO3 | |
| Molar mass | 84.3139 g/mol (anhydrous) |
| Appearance | white solid hygroscopic |
| Odor | odorless |
| Density | 2.958 g/cm3 (anhydrous) 2.825 g/cm3 (dihydrate) 1.837 g/cm3 (trihydrate) 1.73 g/cm3 (pentahydrate) |
| Melting point | 350 °C (662 °F; 623 K) decomposes (anydrous) 165 °C (329 °F; 438 K) (trihydrate) |
| anhydrous: 0.0106 g/100ml (25 °C) 0.0063 g/100ml (100 °C)[1] | |
Solubility product (Ksp)
|
10−7.8[2] |
| Solubility | soluble in acid, aqueous CO2 insoluble in acetone, ammonia |
Refractive index (nD)
|
1.717 (anhydrous) 1.458 (dihydrate) 1.412 (trihydrate) |
| Structure | |
| Trigonal | |
| Thermochemistry | |
Heat capacity (C)
|
75.6 J/mol·K[1] |
Std molar
entropy (S⦵298) |
65.7 J/mol·K[1][3] |
Std enthalpy of
formation (ΔfH⦵298) |
-1113 kJ/mol[3] |
Gibbs free energy (ΔfG⦵)
|
-1029.3 kJ/mol[1] |
| Pharmacology | |
| A02AA01 (WHO) A06AD01 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[4] |
| Safety data sheet (SDS) | ICSC 0969 |
| Related compounds | |
Other anions
|
Magnesium bicarbonate |
Other cations
|
Beryllium carbonate Calcium carbonate Strontium carbonate Barium carbonate |
Related compounds
|
Artinite Hydromagnesite Dypingite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Magnesium carbonate, MgCO3 (archaic name magnesia alba), is an inorganic salt that is a white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.
Forms
The most common magnesium carbonate forms are the anhydrous salt called magnesite (MgCO3) and the di, tri, and pentahydrates known as barringtonite (MgCO3·2 H2O), nesquehonite (MgCO3·3 H2O), and lansfordite (MgCO3·5 H2O), respectively.[5] Some basic forms such as artinite (MgCO3·Mg(OH)2·3 H2O), hydromagnesite (4 MgCO3·Mg(OH)2·4 H2O), and dypingite (4 MgCO3· Mg(OH)2·5 H2O) also occur as minerals.
Magnesite consists of white trigonal crystals. The anhydrous salt is practically insoluble in water, acetone, and ammonia. All forms of magnesium carbonate react in acids. Magnesium carbonate crystallizes in the calcite structure where in Mg2+ is surrounded by six oxygen atoms. The dihydrate one has a triclinic structure, while the trihydrate has a monoclinic structure.
References to 'light' and 'heavy' magnesium carbonates actually refer to the magnesium hydroxy carbonates hydromagnesite and dypingite (respectively).[6]
Preparation
- Magnesium carbonate is ordinarily obtained by mining the mineral magnesite.
- Magnesium carbonate can be prepared in laboratory by reaction between any soluble magnesium salt and sodium bicarbonate:
- MgCl2(aq) + 2NaHCO3(aq) → MgCO3(s) + 2NaCl(aq) + H2O(l) + CO2(g)
- Note that when the solution of magnesium chloride (or sulfate) is treated with aqueous sodium carbonate, a precipitate of basic magnesium carbonate is formed:
- 5MgCl2(aq) + 5Na2CO3(aq) + 5H2O(l) → Mg(OH)2·3MgCO3·3H2O(s) + Mg(HCO3)2(aq) + 10NaCl(aq)
- High purity industrial routes include a path through magnesium bicarbonate: combining magnesium hydroxide and carbon dioxide.[5] A slurry of magnesium hydroxide is treated with 3.5 to 5 atm of carbon dioxide below 50 °C, giving the soluble bicarbonate, then vacuum drying the filtrate, which returns half of the carbon dioxide as well as water.
- Mg(OH)2 + 2 CO2 → Mg(HCO3)2
- Mg(HCO3)2 → MgCO3 + CO2 + H2O
Reactions
With acids
Like many common group 2 metal carbonates, magnesium carbonate reacts with aqueous acids to release carbon dioxide and water:
- MgCO3 + 2 HCl → MgCl2 + CO2 + H2O
- MgCO3 + H2SO4 → MgSO4 + CO2 + H2O
Decomposition
At high temperatures MgCO3 decomposes to magnesium oxide and carbon dioxide. This process is important in the production of magnesium oxide.[5] This process is called calcining:
- MgCO3 → MgO + CO2 (ΔH = +118 kJ/mol)
The decomposition temperature is given as 350 °C (662 °F).[7][8] However, calcination to the oxide is generally not considered complete below 900 °C due to interfering readsorption of liberated carbon dioxide.
It is also interesting to note that the hydrates of the salts lose water at different temperatures during decomposition.[9] For example in the trihydrate, which molecular formula may be written as Mg(HCO3)(OH)•2(H2O), the dehydration steps occur at 157 °C and 179 °C as follows:[10]
- Mg(HCO3)(OH)•2(H2O) → Mg(HCO3)(OH)•(H2O) + H2O at 157 °C
- Mg(HCO3)(OH)•(H2O) → Mg(HCO3)(OH) + H2O at 179 °C
Uses
The primary use of magnesium carbonate is the production of magnesium oxide by calcining. Magnesite and dolomite minerals are used to produce refractory bricks.[5] MgCO3 is also used in flooring, fireproofing, fire extinguishing compositions, cosmetics, dusting powder, and toothpaste. Other applications are as filler material, smoke suppressant in plastics, a reinforcing agent in neoprene rubber, a drying agent, a laxative to loosen the bowels, and color retention in foods. In addition, high purity magnesium carbonate is used as antacid and as an additive in table salt to keep it free flowing.
Because of its water-insoluble, hygroscopic properties MgCO3 was first added to salt in 1911 to make the salt flow more freely. The Morton Salt company adopted the slogan "When it rains it pours" in reference to the fact that its MgCO3-containing salt would not stick together in humid weather.[11] Magnesium carbonate, most often referred to as 'chalk', is used as a drying agent for hands in rock climbing, gymnastics, and weight lifting.
As a food additive magnesium carbonate is known as E504, for which the only known side effect is that it may work as a laxative in high concentrations.[12]
Magnesium carbonate is also used in taxidermy for whitening skulls. It can be mixed with hydrogen peroxide to create a paste, which is then spread on the skull to give it a white finish.
As a matte white coating for projection screens.[13]
Safety
Magnesium carbonate is non-toxic.
Compendial status
See also
- Calcium acetate/magnesium carbonate
- Upsalite, a reported amorphous form of magnesium carbonate
Notes and references
- ^ a b c d http://chemister.ru/Database/properties-en.php?dbid=1&id=634
- ^ Bénézeth, Pascale, et al. "Experimental determination of the solubility product of magnesite at 50 to 200 C." Chemical Geology 286.1 (2011): 21-31.
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 0-618-94690-X.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0373". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c d Margarete Seeger; Walter Otto; Wilhelm Flick; Friedrich Bickelhaupt; Otto S. Akkerman. "Magnesium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_595.pub2. ISBN 978-3527306732.
- ^ Botha, A.; Strydom, C.A. (2001). "Preparation of a magnesium hydroxy carbonate from magnesium hydroxide". Hydrometallurgy. 62 (3): 175. doi:10.1016/S0304-386X(01)00197-9.
- ^ "IAState MSDS".
- ^ Weast, Robert C.; et al. (1978). CRC Handbook of Chemistry and Physics (59th ed.). West Palm Beach, FL: CRC Press. p. B-133. ISBN 0-8493-0549-8.
- ^ "Conventional and Controlled Rate Thermal analysis of nesquehonite Mg(HCO3)(OH)·2(H2O)" (PDF).
- ^ "Conventional and Controlled Rate Thermal analysis of nesquehonite Mg(HCO3)(OH)•2(H2O)" (PDF).
- ^ "Morton Salt FAQ". Retrieved 2007-05-14.
- ^ "Food-Info.net : E-numbers : E504: Magnesium carbonates". 080419 food-info.net
- ^ Noronha, Shonan (2015). Certified Technology Specialist-Installation. McGraw Hill Education. p. 256. ISBN 978-0071835657.
- ^ British Pharmacopoeia Commission Secretariat (2009). "Index, BP 2009" (PDF). Retrieved 31 January 2010.
- ^ "Japanese Pharmacopoeia, Fifteenth Edition" (PDF). 2006. Retrieved 31 January 2010.

