Pyrantel
 | |
| Clinical data | |
|---|---|
| Trade names | Pin-X, Combantrin, others[1] |
| Routes of administration | by mouth |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.036.143 |
| Chemical and physical data | |
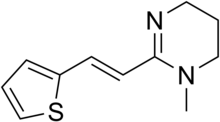
| Formula | C11H14N2S |
| Molar mass | 206.31 g/mol |
| 3D model (JSmol) | |
| |
| |
Pyrantel is a medication used to treat a number of parasitic worm infections.[2] This includes ascariasis, hookworm infections, enterobiasis (pinworm infection), trichostrongyliasis, and trichinellosis.[2] It is taken by mouth.[2]
Side effects include nausea, headache, dizziness, trouble sleeping, and rash.[2] A lower dose should be used in people with liver disease.[2] While it does not appear to be harmful during pregnancy, it has not been studied for this use.[3] It is unclear if it is safe for use during breastfeeding.[2] It is in the antihelmintic family of medications.[4] It works by paralyzing worms.[4]
Pyrantel was initially described in 1965.[5] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[6] Pyrantel is available as a generic medication.[4] It costs less than 25 USD per course of treatment in the United States.[1] It may also be used to treat worms in a number of other animals.[5]
Pregnancy and breastfeeding
Pyrantel pamoate is considered a Pregnancy category C drug for use during pregnancy for humans, but is in category A for canines and felines. Pyrantel is considered safe to use in nursing animals.[7]
Mechanism of action
Pyrantel pamoate acts as a depolarizing neuromuscular blocking agent, thereby causing sudden contraction, followed by paralysis, of the helminths. This has the result of causing the worm to "lose its grip" on the intestinal wall and be passed out of the system by natural process. Since Pyrantel is poorly absorbed by the host's intestine, the host is unaffected by the small dosage of medication used. Spastic (tetanic) paralyzing agents, in particular pyrantel pamoate, may induce complete intestinal obstruction in a heavy worm load.[8] This obstruction is usually in the form of a worm impaction and happens when a very small, but heavily parasitized animal is treated and tries to pass a large number of dislodged worms at once. Worms usually pass in normal stool or with diarrhea, straining, and occasional vomiting.
Names
There are a number of brands, including "Reese's Pinworm Medicine", "Pin-X", "Pin-Rid","PYRANTRIN" formally called "COMBANTRIN", "Anthel", "Helmintox", "Helmex", "Strongid" and Drontal Cat.
References
- ^ a b Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 54. ISBN 9781284057560.
- ^ a b c d e f WHO Model Formulary 2008 (PDF). World Health Organization. 2009. pp. 89, 608. ISBN 9789241547659. Archived from the original (PDF) on 13 December 2016. Retrieved 8 December 2016.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Pyrantel Use During Pregnancy | Drugs.com". www.drugs.com. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c "Pyrantel Pamoate". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Maddison, Jill E.; Page, Stephen W.; (BVSc.), David Church (2008). Small Animal Clinical Pharmacology. Elsevier Health Sciences. p. 209. ISBN 0702028584. Archived from the original on 2016-12-20.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived from the original (PDF) on 13 December 2016. Retrieved 8 December 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Plumb, D. C. (2005). Plumb's veterinary drug handbook. Stockholm, Wis: PharmaVet. ISBN 0-8138-0518-X.
- ^ Salman, A. B. (1997). "Management of intestinal obstruction caused by ascariasis". Journal of Pediatric Surgery. 32 (4): 585–587. doi:10.1016/S0022-3468(97)90712-0. PMID 9126759.
