User:Fvasconcellos/T-DM1
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Clinical data | |
| Trade names | Kadcyla |
| Routes of administration | Intravenous infusion |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | 93% (in vitro) |
| Metabolism | Hepatic (CYP3A4/3A5-mediated) |
| Elimination half-life | 4 days |
| Identifiers | |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C6448H9948N1720O2012S44·(C47H62ClN4O13S)n |
| Molar mass | 148.5 kDa |
| | |
Trastuzumab emtansine (INN;[1][2] in the United States, ado-trastuzumab emtansine, trade name Kadcyla) is an antibody-drug conjugate consisting of themonoclonal antibody trastuzumab (Herceptin) linked to the cytotoxic agent mertansine (DM1).[3][4][5][6] Trastuzumab alone kills cancer cells by binding to the HER2 receptor. The conjugate includes emtansine, which enters the cell and destroys the cell by binding to tubulin.[7] Because monoclonal antibodies target HER2, and HER2 is only expressed in breast cancer cells, the conjugate delivers the toxin emtansine only to breast cancer cells.
In the EMILIA clinical trial of women with advanced HER2 positive breast cancer who were already resistant to trastuzumab alone, it improved survival by 5.8 months compared to the combination of lapatinib and capecitabine.[8] Based on that trial, the U.S. FDA approved marketing on February 22, 2013.[9] [10][11]
Trastuzumab emtansine was developed by Genentech. The planned cost will be $9,800 a month, or $94,000 for a typical course of treatment.[10]
Uses
[edit]In the United States, trastuzumab emtansine was approved specifically for treatment of HER2-positive metastatic breast cancer (mBC) in patients who have been treated previously with trastuzumab and a taxane (paclitaxel or docetaxel), and who have already been treated for mBC or developed tumor recurrence within 6 months of adjuvant therapy.[12]
Approval was based on the EMILIA study, a phase III clinical trial that compared trastuzumab emtansine versus capecitabine (Xeloda) plus lapatinib (Tykerb) in 991 people with unresectable, locally advanced or metastatic HER2-positive breast cancer who had previously been treated with trastuzumab and taxane chemotherapy. This trial showed improved progression-free survival in patients treated with trastuzumab emtansine (median 9.6 vs. 6.4 months), along with improved overall survival (median 30.9 vs. 25.1 months) and safety.[8]
Investigational
[edit]As of 2013[update], several clinical studies of trastuzumab emtansine for other indications are planned or ongoing:
- the MARIANNE study[13] compares taxane (docetaxel or paclitaxel) plus trastuzumab vs T-DM1 vs T-DM1 plus pertuzumab as first-line treatment for people with HER2 positive unresectable locally advanced or metastatic breast cancer;
- the TH3RESA study is comparing T-DM1 vs treatment of physician's choice for people with HER2 positive metastatic breast cancer previously treated with trastuzumab and lapatinib.[14]
- a phase III trial for HER2+ gastric cancer compares T-DM1 to physician's choice of taxane (docetaxel or paclitaxel).[15]
Adverse effects
[edit]During clinical trials, the most common adverse effects of trastuzumab emtansine were fatigue, nausea, musculoskeletal pain, thrombocytopenia (low platelet counts), headache, increased liver enzyme levels, and constipation.[12]
Severe adverse events identified during the EMILIA trial included hepatotoxicity (liver damage), including rare cases of liver failure, hepatic encephalopathy, and nodular regenerative hyperplasia; heart damage (dysfunction of the left ventricle); interstitial lung disease, including acute interstitial pneumonitis; thrombocytopenia; and peripheral neuropathy.[12] Overall, trastuzumab emtansine was better tolerated than the control treatment, a combination of lapatinib (Tykerb) and capecitabine (Xeloda), with 43% of patients in the trastuzumab emtansine group experiencing severe toxic effects, versus 59% of those who received lapatinib/capecitabine; furthermore, fewer patients had to stop treatment due to adverse effects than with lapatinib or capecitabine.[12] Anemia, low platelet counts, and peripheral neuropathy were more common among patients who received trastuzumab emtansine, whereas heart damage and gastrointestinal effects, such as vomiting, diarrhea, and stomatitis, were more common with lapatinib/capecitabine.[12]
In the United States, Kadcyla carries black box warnings for liver toxicity, heart damage (reduction in left ventricular ejection fraction), and fetal harm if given to pregnant women.[12]
Nomenclature
[edit]In the United States, Kadcyla was approved with the generic name "ado-trastuzumab emtansine", rather than the original United States Adopted Name issued in 2009, "trastuzumab emtansine". The "ado-" prefix was added at the request of the FDA to help prevent dispensing errors.[16] During preclinical development and clinical trials, the drug was also known as trastuzumab-DM1 (after the codename for mertansine) or trastuzumab-MCC-DM1 (after the codename for emtansine), both abbreviated T-DM1, and by the codename PRO132365.[5]
Chemical properties
[edit]
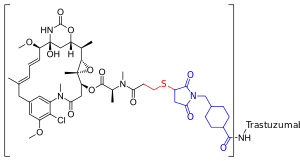
Trastuzumab emtansine is an antibody-drug conjugate (ADC), a combination between a monoclonal antibody and a small-molecule drug. Each molecule of trastuzumab emtansine consists of a single trastuzumab molecule bound to several molecules of mertansine, a cytotoxic maytansinoid containing a sulfhydryl group, through a crosslinking reagent known as SMCC.[17] SMCC, or succinimidyl trans-4-(maleimidylmethyl)cyclohexane-1-carboxylate, is a heterobifunctional crosslinker, a type of chemical reagent that contains two reactive functional groups, a succinimide ester and a maleimide. The succinimide group of SMCC reacts with the free amino group of a lysine residue in the trastuzumab molecule and the maleimide moiety of SMCC links to the free sulfhydryl group of mertansine, forming a covalent bond between the antibody and mertansine. Each trastuzumab molecule may be linked to zero to eight mertansine molecules (3.5 on average).[17][18]
Several other linker structures were tested during preclinical development, all containing disulfide bonds, which can undergo reduction within the body, separating the antibody from the maytansinoid and releasing the latter into the circulation.[18] The SMCC linker forms a thioether bond instead of a disulfide bond, and is thus nonreducible (cannot undergo cleavage by reduction within the cell). The fact that mertansine is only released after the antibody-drug conjugate has been taken up by a tumor cell reduces toxic effects while maintaining antitumor efficacy. Indeed, trastuzumab-MCC-DM1 was found to have better efficacy and pharmacokinetics and less toxicity than conjugates built with other linkers, and was thus selected for further development as trastuzumab emtansine.[18]
References
[edit]- ^ Niculescu-Duvaz I (June 2010). "Trastuzumab emtansine, an antibody-drug conjugate for the treatment of HER2+ metastatic breast cancer". Curr. Opin. Mol. Ther. 12 (3): 350–60. PMID 20521224.
{{cite journal}}: CS1 maint: date and year (link) - ^ USAN Council (2009). "STATEMENT ON A NONPROPRIETARY NAME ADOPTED BY THE USAN COUNCIL: TRASTUZUMAB EMTANSINE" (PDF). American Medical Association. Retrieved 2013-02-22.
- ^ LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX (October 2011). "Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer". Clin. Cancer Res. 17 (20): 6437–47. doi:10.1158/1078-0432.CCR-11-0762. PMID 22003071.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Poon, Kirsten Achilles (2010-05-06). Safety Assessment of Antibody Drug Conjugates (PDF). Drug Development: From Small Molecules to Biologics. NorCal Society of Toxicology 2010 Spring Meeting. Retrieved 2013-02-23.
- ^ a b [No authors listed]. "Trastuzumab emtansine". NCI Drug Dictionary. U.S. National Cancer Institute. Retrieved 2013-02-23.
- ^ [No authors listed] (2010-08-27). "FDA denies accelerated approval of Genentech's trastuzumab-DM1 (T-DM1) BLA for metastatic breast cancer". News-Medical.net. Retrieved 2013-02-23.
- ^ Teicher BA, Doroshow JH (November 2012). "The promise of antibody-drug conjugates". N. Engl. J. Med. 367 (19): 1847–8. doi:10.1056/NEJMe1211736. PMID 23134386.
{{cite journal}}: CS1 maint: date and year (link) - ^ a b Verma S, Miles D, Gianni L; et al. (November 2012). "Trastuzumab emtansine for HER2-positive advanced breast cancer". N. Engl. J. Med. 367 (19): 1783–91. doi:10.1056/NEJMoa1209124. PMID 23020162.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ "New data from Phase III EMILIA study showed Roche's trastuzumab emtansine (T-DM1) significantly improved survival of people with HER2-positive metastatic breast cancer" (Press release). Hoffmann-La Roche. 2012-08-27. Retrieved 2013-02-23.
- ^ a b Pollack A (2013-02-22). "F.D.A. Approves Breast Cancer Drug". The New York Times. Retrieved 2013-02-22.
- ^ "FDA approves new treatment for late stage breast cancer" (Press release). U.S. Food and Drug Administration. 2013-02-22. Retrieved 2013-02-22.
- ^ a b c d e f U.S. FDA. "Full Prescribing Information for KADCYLA" (PDF). (556 kB). Retrieved 2012-02-23.
- ^ Clinical trial number NCT01120184 for "A Study of Trastuzumab Emtansine (T-DM1) Plus Pertuzumab/Pertuzumab Placebo Versus Trastuzumab (Herceptin} Plus a Taxane in Patients With Metastatic Breast Cancer (MARIANNE)" at ClinicalTrials.gov. Retrieved 2013-02-23.
- ^ Clinical trial number NCT01419197 for "A Study of Trastuzumab Emtansine in Comparison With Treatment of Physician's Choice in Patients With HER2-Positive Breast Cancer Who Have Received at Least Two Prior Regimens of HER2-Directed Therapy (TH3RESA)" at ClinicalTrials.gov. Retrieved 2013-02-23.
- ^ Clinical trial number NCT01641939 for "A Study of Trastuzumab Emtansine Versus Taxane in Patients With Advanced Gastric Cancer" at ClinicalTrials.gov. Retrieved 2013-02-23.
- ^ Kim TE, Pazdur R (2013). Summary Review for Regulatory Action (PDF) (Technical report). U.S. Food and Drug Administration. p. 8. Retrieved 2013-02-22.
- ^ a b Girish S, Gupta M, Wang B; et al. (May 2012). "Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer". Cancer Chemother. Pharmacol. 69 (5): 1229–40. doi:10.1007/s00280-011-1817-3. PMC 3337408. PMID 22271209.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ a b c Lewis Phillips GD, Li G, Dugger DL; et al. (November 2008). "Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate". Cancer Res. 68 (22): 9280–90. doi:10.1158/0008-5472.CAN-08-1776. PMID 19010901.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link)
