Ebola: Difference between revisions
Replaced two slightly older references (2007) with newer brief literature review (2014) that also names these three bats as likely natural hosts. |
Added to pathophys section that EBOV is thought to infect humans via contact with mucosa membrane surfaces (e.g., eyes, inside of the nose, mouth, etc) and breaks in the skin. Supported by new review (will complete citation when details made available). |
||
| Line 92: | Line 92: | ||
Similar to other [[filoviridae]], EBOV replicates very efficiently in many [[List of distinct cell types in the adult human body|cells]], producing large amounts of virus in [[monocyte]]s, [[macrophage]]s, [[dendritic cell|dendritic cells]] and other cells. Replication of the virus in monocytes triggers the [[cytokine storm|release of high levels of inflammatory chemical signals]].<ref name="isbn0-7910-8505-8">{{cite book |author=Murray, Patrick R., Ken S. Rosenthal, and Michael A. Pfaller |title=Medical Microbiology, with STUDENT CONSULT Online Access, 7: Medical Microbiology |publisher=Elsevier Health Sciences |location= |year=2013 |pages=537-538 |isbn=0323086926 |oclc= |doi= |accessdate=}}</ref> |
Similar to other [[filoviridae]], EBOV replicates very efficiently in many [[List of distinct cell types in the adult human body|cells]], producing large amounts of virus in [[monocyte]]s, [[macrophage]]s, [[dendritic cell|dendritic cells]] and other cells. Replication of the virus in monocytes triggers the [[cytokine storm|release of high levels of inflammatory chemical signals]].<ref name="isbn0-7910-8505-8">{{cite book |author=Murray, Patrick R., Ken S. Rosenthal, and Michael A. Pfaller |title=Medical Microbiology, with STUDENT CONSULT Online Access, 7: Medical Microbiology |publisher=Elsevier Health Sciences |location= |year=2013 |pages=537-538 |isbn=0323086926 |oclc= |doi= |accessdate=}}</ref> |
||
[[ |
EBOV is thought to infect humans through contact with mucous membranes or through skin breaks.<ref name="Funk2014">{{cite journal|author=Funk DJ, Kumar A|title=Ebola virus disease: an update for anesthesiologists and intensivists|journal=Can J Anaesth|volume=|issue=|pages=|date=November 2014|pmid=25373801|doi=10.1007/s12630-014-0257-z}}</ref> Once infected, [[endothelial cells]] (cells lining the inside of blood vessels), [[Mononuclear phagocyte system|macrophages, monocytes]] and [[hepatocytes|liver cells]] are the main targets of infection. Macrophages are the first cells infected with the virus, and this infection results in [[Apoptosis|cellular death]].<ref name="Chippaux2014"/> Endothelial cells may be infected within 3 days after exposure to the virus.<ref name="Chippaux2014"/> The breakdown of endothelial cells leading to [[Blood vessel|vascular]] injury can be attributed to EBOV [[glycoprotein|glycoproteins]]. The widespread [[Bleeding|hemorrhage]] that occurs in affected people causes [[edema]] and [[Hypovolemia|hypovolemic]] shock.<ref name="isbn0-7910-8505-8"/> After infection, a secreted [[glycoprotein]], small soluble glycoprotein (sGP) (or Ebola virus glycoprotein [GP]), is synthesized. EBOV replication overwhelms protein synthesis of infected cells and the host immune defenses. The GP forms a [[Trimer (biochemistry)|trimeric complex]], which tethers the virus to the endothelial cells. The sGP forms a [[protein dimer|dimeric protein]] that interferes with the signaling of [[neutrophils]], a type of [[white blood cell]], which enables the virus to evade the immune system by inhibiting early steps of neutrophil activation. These white blood cells also transport the virus within the entire body to tissues and organs such as the [[lymph node]]s, liver, lungs and spleen.<ref name="isbn0-7910-8505-8">{{cite book |author=Smith, Tara |title=Ebola (Deadly Diseases and Epidemics) |publisher=Chelsea House Publications |location= |year=2005 |pages= |isbn=0-7910-8505-8 |oclc= |doi= |accessdate=}}</ref> |
||
The presence of viral particles and the cell damage resulting from viruses budding out of the cell causes the release of [[cytokines|chemical signals]] (such as [[Tumor necrosis factor-alpha|TNF-α]], [[Interleukin 6|IL-6]] and [[Interleukin 8|IL-8]]), which are molecular signals for fever and inflammation. The [[cytopathic effect|damage to human cells]], caused by infection of the endothelial cells, decreases the integrity of blood vessels. This loss of vascular integrity increases with the synthesis of GP, which reduces the availability of specific [[integrin]]s responsible for cell adhesion to the intercellular structure and causes damage to the liver, leading to [[coagulopathy|improper clotting]].<ref name="doi10.1128/JVI.77.18.9733-9737.2003">{{cite journal | author = Sullivan N, Yang ZY, Nabel GJ | title = Ebola Virus Pathogenesis: Implications for VacDendritic cellcines and Therapies | journal = Journal of Virology | volume = 77 | issue = 18 | pages = 9733–9737 |date=September 2003 | pmid = 12941881 | pmc = 224575 | doi = 10.1128/JVI.77.18.9733-9737.2003 | url = http://jvi.asm.org/content/77/18/9733.full.pdf | format = Free full text }}</ref> |
The presence of viral particles and the cell damage resulting from viruses budding out of the cell causes the release of [[cytokines|chemical signals]] (such as [[Tumor necrosis factor-alpha|TNF-α]], [[Interleukin 6|IL-6]] and [[Interleukin 8|IL-8]]), which are molecular signals for fever and inflammation. The [[cytopathic effect|damage to human cells]], caused by infection of the endothelial cells, decreases the integrity of blood vessels. This loss of vascular integrity increases with the synthesis of GP, which reduces the availability of specific [[integrin]]s responsible for cell adhesion to the intercellular structure and causes damage to the liver, leading to [[coagulopathy|improper clotting]].<ref name="doi10.1128/JVI.77.18.9733-9737.2003">{{cite journal | author = Sullivan N, Yang ZY, Nabel GJ | title = Ebola Virus Pathogenesis: Implications for VacDendritic cellcines and Therapies | journal = Journal of Virology | volume = 77 | issue = 18 | pages = 9733–9737 |date=September 2003 | pmid = 12941881 | pmc = 224575 | doi = 10.1128/JVI.77.18.9733-9737.2003 | url = http://jvi.asm.org/content/77/18/9733.full.pdf | format = Free full text }}</ref> |
||
Revision as of 05:53, 8 November 2014
| Ebola | |
|---|---|
| Specialty | Infectious diseases |
Ebola virus disease (EVD; also Ebola hemorrhagic fever, or EHF), or simply Ebola, is a disease of humans and other primates caused by ebolaviruses. Signs and symptoms typically start between two days and three weeks after contracting the virus as a fever, sore throat, muscle pain, and headaches. Then, vomiting, diarrhea and rash usually follow, along with decreased function of the liver and kidneys. At this time some people begin to bleed both internally and externally.[1] The disease has a high risk of death, killing between 25 percent and 90 percent of those infected with the virus, averaging out at 50 percent.[1] This is often due to low blood pressure from fluid loss, and typically follows six to sixteen days after symptoms appear.[2]
The virus spreads by direct contact with blood or other body fluids of an infected human or other animal.[1] Infection with the virus may also occur by direct contact with a recently contaminated item or surface.[1] Spread of the disease through the air has not been documented in the natural environment.[3] EBOV may be spread by semen or breast milk for several weeks to months after recovery.[1][4] African fruit bats are believed to be the normal carrier in nature, able to spread the virus without being affected by it. Humans become infected by contact with the bats or with a living or dead animal that has been infected by bats. After human infection occurs, the disease may also spread between people. Other diseases such as malaria, cholera, typhoid fever, meningitis and other viral hemorrhagic fevers may resemble EVD. Blood samples are tested for viral RNA, viral antibodies or for the virus itself to confirm the diagnosis.[1]
Control of outbreaks requires coordinated medical services, along with a certain level of community engagement. The medical services include: rapid detection of cases of disease, contact tracing of those who have come into contact with infected individuals, quick access to laboratory services, proper care and management of those who are infected and proper disposal of the dead through cremation or burial.[1][5] Prevention includes limiting the spread of disease from infected animals to humans.[1] This may be done by handling potentially infected bush meat only while wearing protective clothing and by thoroughly cooking it before consumption.[1] It also includes wearing proper protective clothing and washing hands when around a person with the disease.[1] Samples of body fluids and tissues from people with the disease should be handled with special caution.[1]
No specific treatment or vaccine for the virus is commercially available. Efforts to help those who are infected are supportive; they include either oral rehydration therapy (drinking slightly sweetened and salty water) or giving intravenous fluids as well as treating symptoms. This supportive care improves outcomes. EVD was first identified in 1976 in an area of Sudan (now part of South Sudan), and in Zaire (now the Democratic Republic of the Congo). The disease typically occurs in outbreaks in tropical regions of sub-Saharan Africa.[1] Through 2013, the World Health Organization reported a total of 1,716 cases in 24 outbreaks.[1][6] The largest outbreak to date is the ongoing epidemic in West Africa, which is centered in Guinea, Sierra Leone and Liberia.[7][8][9]
It caused significant mortality, with a considerable case fatality rate.[10][11][12][note 1] By the end of the epidemic, 28,616 people had been infected; of these, 11,310 had died, for a case-fatality rate of 40%.[13] As of 8 May 2016[update], this outbreak has 28,646 reported cases resulting in 11,323 deaths.[14]
Signs and symptoms

The length of time between exposure to the virus and the development of symptoms of the disease is usually 2 to 21 days.[1][15]
Symptoms usually begin with a sudden influenza-like stage characterized by feeling tired, fever, pain in the muscles and joints, headache, and sore throat.[1][16][17] The fever is usually higher than 38.3 °C (100.9 °F).[18] This is often followed by vomiting, diarrhea and abdominal pain.[17] Next, shortness of breath and chest pain may occur, along with swelling, headaches and confusion.[17] In about half of the cases, the skin may develop a maculopapular rash (a flat red area covered with small bumps).[18]
In some cases, internal and external bleeding may occur.[1] This typically begins five to seven days after the first symptoms.[19] All infected people show some decreased blood clotting.[18] Bleeding from mucous membranes or from sites of needle punctures has been reported in 40–50 percent of cases.[20] This may result in the vomiting of blood, coughing up of blood or blood in stool.[21] Bleeding into the skin may create petechiae, purpura, ecchymoses or hematomas (especially around needle injection sites).[22] Bleeding into the whites of the eyes may also occur. Heavy bleeding is uncommon, and if it occurs, it is usually located within the gastrointestinal tract.[18][23]
Recovery may begin between 7 and 14 days after the start of symptoms.[17] Death, if it occurs, follows typically 6 to 16 days from the start of symptoms and is often due to low blood pressure from fluid loss.[2] In general, bleeding often indicates a worse outcome, and this blood loss may result in death.[16] People are often in a coma near the end of life.[17] Those who survive often have ongoing muscle and joint pain, liver inflammation, and decreased hearing among other difficulties.[17]
Cause
EVD in humans is caused by four of five viruses of the genus Ebolavirus. The four are Bundibugyo virus (BDBV), Sudan virus (SUDV), Taï Forest virus (TAFV) and one simply called Ebola virus (EBOV, formerly Zaire Ebola virus).[24] EBOV is the only member of the Zaire ebolavirus species and the most dangerous of the known EVD-causing viruses, and is responsible for the largest number of outbreaks.[25] The fifth virus, Reston virus (RESTV), is not thought to cause disease in humans, but has caused disease in other primates.[26][27] All five viruses are closely related to marburgviruses.[24]
Transmission
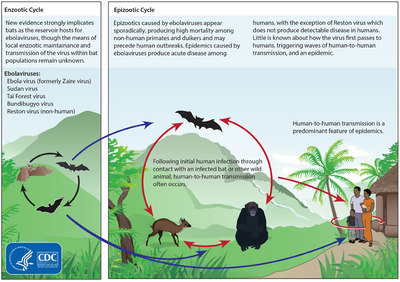
Between people, Ebola disease spreads only by direct contact with the blood or body fluids of a person who has developed symptoms of the disease.[28][29] Body fluids that may contain ebolaviruses include saliva, mucus, vomit, feces, sweat, tears, breast milk, urine and semen.[30] The WHO states that only people who are very sick are able to spread Ebola disease in saliva, and whole virus has not been reported to be transmitted through sweat. Most people spread the virus through blood, feces and vomit.[31] Entry points for the virus include the nose, mouth, eyes, open wounds, cuts and abrasions.[30] Ebola may be spread through large droplets; however, this is believed to occur only when a person is very sick.[32] This can happen if a person is splashed with droplets.[32] Contact with surfaces or objects contaminated by the virus, particularly needles and syringes, may also transmit the infection.[33][34] The virus is able to survive on objects for a few hours in a dried state and can survive for a few days within body fluids.[30]
The Ebola virus may be able to persist for up to 7 weeks in the semen of survivors after they recovered, which could lead to infections via sexual intercourse.[1] Ebola may also occur in the breast milk of women after recovery, and it is not known when it is safe to breastfeed again.[4] Otherwise, people who have recovered are not infectious.[33]
The potential for widespread infections in countries with medical systems capable of observing correct medical isolation procedures is considered low.[35] Usually when someone has symptoms of the disease, they are unable to travel without assistance.[36]
Dead bodies remain infectious; thus, people handling human remains in practices such as traditional burial rituals or more modern processes such as embalming are at risk.[35] Nearly two thirds of the cases of Ebola infections in Guinea during the 2014 outbreak are believed to have been contracted via unprotected (or unsuitably protected) contact with infected corpses during certain Guinean burial rituals.[37][38]
Health-care workers treating those who are infected are at greatest risk of getting infected themselves.[33] The risk increases when these workers do not have appropriate protective clothing such as masks, gowns, gloves and eye protection; do not wear it properly; or handle contaminated clothing incorrectly.[33] This risk is particularly common in parts of Africa where health systems function poorly and where the disease mostly occurs.[39] Hospital-acquired transmission has also occurred in some African countries resulting from the reuse of needles.[40][41] Some health-care centers caring for people with the disease do not have running water.[42] In the United States the spread to two medial workers treating an infected patients prompted criticism of inadequate training and procedures.[43]
Viral transmission through the air has not been reported to occur during EVD outbreaks[3] and has only been demonstrated in very strict laboratory conditions in rhesus monkeys.[34][44] The apparent lack of airborne transmission among humans may be due to levels of the virus in the lungs that are insufficient to cause new infections.[45] Spread of EBOV by water or food, other than bushmeat, has also not been observed.[33][34] No spread by mosquitos or other insects has been reported.[33]
Initial case

Although it is not entirely clear how Ebola initially spreads from animals to humans, the spread is believed to involve direct contact with an infected wild animal or fruit bat.[33] Besides bats, other wild animals sometimes infected with EBOV include several monkey species, chimpanzees, gorillas, baboons and duikers.[49]
Animals may become infected when they eat fruit partially eaten by bats carrying the virus.[50] Fruit production, animal behavior and other factors may trigger outbreaks among animal populations.[50]
Evidence indicates that both domestic dogs and pigs can also be infected with EBOV.[51] Dogs do not appear to develop symptoms when they carry the virus, and pigs appear to be able to transmit the virus to at least some primates.[51] Although some dogs in an area in which a human outbreak occurred had antibodies to EBOV, it is unclear whether they played a role in spreading the disease to people.[51]
Reservoir
The natural reservoir for Ebola has yet to be confirmed; however, bats are considered to be the most likely candidate species.[34] Three types of fruit bats (Hypsignathus monstrosus, Epomops franqueti and Myonycteris torquata) were found to possibly carry the virus without getting sick.[52] As of 2013, whether other animals are involved in its spread is not known.[51] Plants, arthropods and birds have also been considered possible viral reservoirs.[1]
Bats were known to roost in the cotton factory in which the first cases of the 1976 and 1979 outbreaks were observed, and they have also been implicated in Marburg virus infections in 1975 and 1980.[53] Of 24 plant and 19 vertebrate species experimentally inoculated with EBOV, only bats became infected.[54] The bats displayed no clinical signs of disease, which is considered evidence that these bats are a reservoir species of EBOV. In a 2002–2003 survey of 1,030 animals including 679 bats from Gabon and the Republic of the Congo, 13 fruit bats were found to contain EBOV RNA.[55] Antibodies against Zaire and Reston viruses have been found in fruit bats in Bangladesh, suggesting that these bats are also potential hosts of the virus and that the filoviruses are present in Asia.[56]
Between 1976 and 1998, in 30,000 mammals, birds, reptiles, amphibians and arthropods sampled from regions of EBOV outbreaks, no Ebola virus was detected apart from some genetic traces found in six rodents (belonging to the species Mus setulosus and Praomys) and one shrew (Sylvisorex ollula) collected from the Central African Republic.[53][57] However, further research efforts have not confirmed rodents as a reservoir.[58] Traces of EBOV were detected in the carcasses of gorillas and chimpanzees during outbreaks in 2001 and 2003, which later became the source of human infections. However, the high rates of death in these species resulting from EBOV infection make it unlikely that these species represent a natural reservoir for the virus.[53]
Virology

Ebolaviruses contain single-stranded, non-infectious RNA genomes.[59] Ebolavirus genomes are approximately 19 kilobase pairs long and contain seven genes in the order 3'-UTR-NP-VP35-VP40-GP-VP30-VP24-L-5'-UTR.[60] The genomes of the five different ebolaviruses (BDBV, EBOV, RESTV, SUDV and TAFV) differ in sequence and the number and location of gene overlaps. As all filoviruses, ebolavirions are filamentous particles that may appear in the shape of a shepherd's crook, of a "U" or of a "6," and they may be coiled, toroid or branched.[60] In general, ebolavirions are 80 nanometers (nm) in width and may be as long as 14,000 nm.[61]
Their life cycle begins with a virion attaching to specific cell-surface receptors, followed by fusion of the virion envelope with cellular membranes and the concomitant release of the virus nucleocapsid into the cytosol. Ebolavirus' structural glycoprotein (known as GP1,2) is responsible for the virus' ability to bind to and infect targeted cells.[62] The viral RNA polymerase, encoded by the L gene, partially uncoats the nucleocapsid and transcribes the genes into positive-strand mRNAs, which are then translated into structural and nonstructural proteins. The most abundant protein produced is the nucleoprotein, whose concentration in the host cell determines when L switches from gene transcription to genome replication. Replication of the viral genome results in full-length, positive-strand antigenomes that are, in turn, transcribed into genome copies of negative-strand virus progeny. Newly synthesized structural proteins and genomes self-assemble and accumulate near the inside of the cell membrane. Virions bud off from the cell, gaining their envelopes from the cellular membrane from which they bud from. The mature progeny particles then infect other cells to repeat the cycle. The genetics of the Ebola virus are difficult to study because of EBOV's virulent characteristics.[63]
Pathophysiology
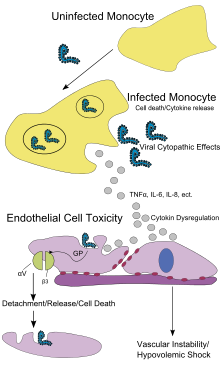
Similar to other filoviridae, EBOV replicates very efficiently in many cells, producing large amounts of virus in monocytes, macrophages, dendritic cells and other cells. Replication of the virus in monocytes triggers the release of high levels of inflammatory chemical signals.[64]
EBOV is thought to infect humans through contact with mucous membranes or through skin breaks.[65] Once infected, endothelial cells (cells lining the inside of blood vessels), macrophages, monocytes and liver cells are the main targets of infection. Macrophages are the first cells infected with the virus, and this infection results in cellular death.[61] Endothelial cells may be infected within 3 days after exposure to the virus.[61] The breakdown of endothelial cells leading to vascular injury can be attributed to EBOV glycoproteins. The widespread hemorrhage that occurs in affected people causes edema and hypovolemic shock.[64] After infection, a secreted glycoprotein, small soluble glycoprotein (sGP) (or Ebola virus glycoprotein [GP]), is synthesized. EBOV replication overwhelms protein synthesis of infected cells and the host immune defenses. The GP forms a trimeric complex, which tethers the virus to the endothelial cells. The sGP forms a dimeric protein that interferes with the signaling of neutrophils, a type of white blood cell, which enables the virus to evade the immune system by inhibiting early steps of neutrophil activation. These white blood cells also transport the virus within the entire body to tissues and organs such as the lymph nodes, liver, lungs and spleen.[64]
The presence of viral particles and the cell damage resulting from viruses budding out of the cell causes the release of chemical signals (such as TNF-α, IL-6 and IL-8), which are molecular signals for fever and inflammation. The damage to human cells, caused by infection of the endothelial cells, decreases the integrity of blood vessels. This loss of vascular integrity increases with the synthesis of GP, which reduces the availability of specific integrins responsible for cell adhesion to the intercellular structure and causes damage to the liver, leading to improper clotting.[66]
Filoviral infection also interferes with proper functioning of the innate immune system.[67] EBOV proteins blunt the human immune system's response to viral infections by interfering with the cells' ability to produce and respond to interferon proteins such as interferon-alpha, interferon-beta, and interferon gamma.[62][68]
The VP24 and VP35 structural proteins of EBOV play a key role in this interference. When a cell is infected with EBOV, receptors located in the cell's cytosol (such as RIG-I and MDA5) or outside of the cytosol (such as Toll-like receptor 3 (TLR3), TLR7, TLR8 and TLR9), recognize infectious molecules associated with the virus.[62] On TLR activation, proteins including interferon regulatory factor 3 and interferon regulatory factor 7 trigger a signaling cascade that leads to the expression of type 1 interferons.[62] The type 1 interferons are then released and bind to the IFNAR1 and IFNAR2 receptors expressed on the surface of a neighboring cell.[62] Once interferon has bound to its receptors on the neighboring cell, the signaling proteins STAT1 and STAT2 are activated and move to the cell's nucleus.[62] This triggers the expression of interferon-stimulated genes, which code for proteins with antiviral properties.[62] EBOV's V24 protein blocks the production of these antiviral proteins by preventing the STAT1 signaling protein in the neighboring cell from entering the nucleus.[62] The VP35 protein directly inhibits the production of interferon-beta.[68] By inhibiting these immune responses, EBOV may quickly spread throughout the body.[61]
Diagnosis
When EVD is suspected in a person, his or her travel and work history, along with an exposure to wildlife, are important factors to consider for possible further medical examination.
Nonspecific laboratory testing
Possible laboratory indicators of EVD include a low platelet count; an initially decreased white blood cell count followed by an increased white blood cell count; elevated levels of the liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST); and abnormalities in blood clotting often consistent with disseminated intravascular coagulation (DIC) such as a prolonged prothrombin time, partial thromboplastin time, and bleeding time.[69]
Specific laboratory testing
The diagnosis of EVD is confirmed by isolating the virus, detecting its RNA or proteins, or detecting antibodies against the virus in a person's blood. Isolating the virus by cell culture, detecting the viral RNA by polymerase chain reaction (PCR) and detecting proteins by enzyme-linked immunosorbent assay (ELISA) are methods best used in the early stages of the disease and also for detecting the virus in human remains. Detecting antibodies against the virus is most reliable in the later stages of the disease and in those who recover.[70]
During an outbreak, isolation of the virus via cell culture methods is often not feasible. In field or mobile hospitals, the most common and sensitive diagnostic methods are real-time PCR and ELISA.[71] In 2014, with new mobile testing facilities deployed in parts of Liberia, test results were obtained 3–5 hours after sample submission.[72]
Filovirions, such as EBOV, may be identified by their unique filamentous shapes in cell cultures examined with electron microscopy, but this method cannot distinguish the various filoviruses.[73]
Differential diagnosis
Early symptoms of EVD may be similar to those of other diseases common in Africa, including malaria and dengue fever.[16] The symptoms are also similar to those of Marburg virus disease and other viral hemorrhagic fevers.[74]
The complete differential diagnosis is extensive and requires consideration of many other infectious diseases such as typhoid fever, shigellosis, rickettsial diseases, cholera, sepsis, borreliosis, EHEC enteritis, leptospirosis, scrub typhus, plague, Q fever, candidiasis, histoplasmosis, trypanosomiasis, visceral leishmaniasis, measles and viral hepatitis among others.[75]
Non-infectious diseases that may result in symptoms similar to those of EVD include acute promyelocytic leukemia, hemolytic uremic syndrome, snake envenomation, clotting factor deficiencies/platelet disorders, thrombotic thrombocytopenic purpura, hereditary hemorrhagic telangiectasia, Kawasaki disease and warfarin poisoning.[71][76][77][78]
Prevention

Infection control
People who care for those infected with the Ebola virus should wear protective clothing including masks, gloves, gowns and goggles.[79] The US Centers for Disease Control (CDC) recommend that the protective gear leaves no skin exposed.[80] These measures are also recommended for those who may handle objects contaminated by an infected person's body fluids.[81] In 2014, the CDC began recommending that medical personnel receive training on the proper suit-up and removal of personal protective equipment (PPE); in addition, a designated person, appropriately trained in biosafety, should be watching each step of these procedures to ensure they are done correctly.[80] In Sierra Leone, the typical training period for the use of such safety equipment lasts approximately 12 days.[82]
The infected person should be in barrier-isolation from other people.[79] All equipment, medical waste, patient waste and surfaces that may have come into contact with body fluids need to be disinfected.[81] During the 2014 outbreak, kits were put together to help families treat Ebola disease in their homes, which include protective clothing as well as chlorine powder and other cleaning supplies.[83] Education of those who provide care in these techniques, and the provision of such barrier-separation supplies has been a priority of Doctors Without Borders.[84]
Ebolaviruses can be eliminated with heat (heating for 30 to 60 minutes at 60 °C or boiling for 5 minutes). To disinfect surfaces, some lipid solvents such as some alcohol-based products, detergents, sodium hypochlorite (bleach) or calcium hypochlorite (bleaching powder), and other suitable disinfectants may be used at appropriate concentrations.[49][85] Education of the general public about the risk factors for Ebola infection and of the protective measures individuals may take to prevent infection is recommended by the World Health Organization.[1] These measures include avoiding direct contact with infected people and regular hand washing using soap and water.[86]
Bushmeat, an important source of protein in the diet of some Africans, should be handled and prepared with appropriate protective clothing and thoroughly cooked before consumption.[1] Some research suggests that an outbreak of Ebola disease in the wild animals used for consumption may result in a corresponding human outbreak. Since 2003, such animal outbreaks have been monitored to predict and prevent Ebola outbreaks in humans.[87]
If a person with Ebola disease dies, direct contact with the body should be avoided.[79] Certain burial rituals, which may have included making various direct contacts with a dead body, require reformulation such that they consistently maintain a proper protective barrier between the dead body and the living.[88][89][90] Social anthropologists may help find alternatives to traditional rules for burials.[91]
Transportation crews are instructed to follow a certain isolation procedure should anyone exhibit symptoms resembling EVD.[92] As of August 2014, the WHO does not consider travel bans to be useful in decreasing spread of the disease.[36] In October 2014, the CDC defined four risk levels used to determine the level of 21-day monitoring for symptoms and restrictions on public activities.[93] In the United States, the CDC recommends that restrictions on public activity, including travel restrictions, are not required for the following defined risk levels:[93]
- having been in a country with widespread Ebola disease transmission and having no known exposure (low risk); or having been in that country more than 21 days ago (no risk)
- encounter with a person showing symptoms; but not within 3 feet of the person with Ebola without wearing PPE; and no direct contact of body fluids
- having had brief skin contact with a person showing symptoms of Ebola disease when the person was believed to be not very contagious (low risk)
- in countries without widespread Ebola disease transmission: direct contact with a person showing symptoms of the disease while wearing PPE (low risk)
- contact with a person with Ebola disease before the person was showing symptoms (no risk).
The CDC recommends monitoring for the symptoms of Ebola disease for those both at "low risk" and at higher risk.[93]
In laboratories where diagnostic testing is carried out, biosafety level 4-equivalent containment is required[citation needed], since ebolaviruses are WHO Risk Group 4 pathogens. Laboratory researchers must be properly trained in BSL-4 practices and wear proper PPE.[citation needed]
Putting on and removing protective equipment
Isolation
Isolation refers to separating those who are sick from those who are not. Quarantine refers to separating those who may have been exposed to a disease until they either show signs of the disease or are no longer at risk.[94] Quarantine, also known as enforced isolation, is usually effective in decreasing spread.[95][96] Governments often quarantine areas where the disease is occurring or individuals who may transmit the disease outside of an initial area.[97]
In the United States, the law allows quarantine of those infected with ebolaviruses.[98] During the 2014 Ebola disease outbreak, Liberia closed schools.[99]
Contact tracing
Contact tracing is considered important to contain an outbreak. It involves finding everyone who had close contact with infected individuals and watching for signs of illness for 21 days. If any of these contacts comes down with the disease, they should be isolated, tested and treated. Then the process is repeated by tracing the contacts' contacts.[100][101]
Treatment
Standard support

No specific treatment is currently approved.[102] However, survival is improved by early supportive care with rehydration and symptomatic treatment.[1] Treatment is primarily supportive in nature.[103] These measures may include management of pain, nausea, fever and anxiety, as well as rehydration via the oral or by intravenous route.[103] The World Health Organization recommends avoiding the use of aspirin or ibuprofen for pain due to the bleeding risk associated with use of these medications.[104] Blood products such as packed red blood cells, platelets or fresh frozen plasma may also be used.[103] Other regulators of coagulation have also been tried including heparin in an effort to prevent disseminated intravascular coagulation and clotting factors to decrease bleeding.[103] Antimalarial medications and antibiotics are often used before the diagnosis is confirmed,[103] though there is no evidence to suggest such treatment is in any way helpful. Interferon therapies have been tried as a form of treatment for EVD, but were found to be ineffective.[61]
If professional care is not possible, guidelines by WHO for care at home have been relatively successful. In such situations, recommendations include using towels soaked in bleach solutions when moving infected people or bodies and applying bleach on stains. It is also recommended that the caregivers wash hands with bleach solutions and cover their mouth and nose with a cloth.[105]
Intensive care
Intensive care is often used in the developed world.[22] This may include maintaining blood volume and electrolytes (salts) balance as well as treating any bacterial infections that may develop.[22] Dialysis may be needed for kidney failure, and extracorporeal membrane oxygenation may be used for lung dysfunction.[22]
Alternative medicine
The Food and Drug Administration (FDA) advises people to be careful of advertisements making unverified or fraudulent claims of benefits supposedly gained from various anti-Ebola products.[106] The FDA has already sent out at least one letter of warning to a seller of colloidal silver who made unverified claims of Ebola related benefits, supposedly derived from the use of their products.[107]
Prognosis
EVD has a high risk of death in those infected which varies between 25 percent and 90 percent of those infected.[1][108] As of September 2014[update], the average risk of death among those infected is 50 percent.[1] The risk of death was 90 percent in the 2002–2003 Republic of the Congo outbreak.[109]
Death, if it occurs, follows typically six to sixteen days after symptoms appear and is often due to low blood pressure from fluid loss.[2] Early supportive care to prevent dehydration may reduce the risk of death.[110]
If an infected person survives, recovery may be quick and complete. Prolonged cases are often complicated by the occurrence of long-term problems, such as inflammation of the testicles, joint pains, muscle pains, skin peeling, or hair loss. Eye symptoms, such as light sensitivity, excess tearing, iritis, iridocyclitis, choroiditis, and blindness have also been described.
Epidemiology


The disease typically occurs in outbreaks in tropical regions of Sub-Saharan Africa.[1] From 1976 (when it was first identified) through 2013, the World Health Organization reported 1,716 confirmed cases.[1][6] The largest outbreak to date is the ongoing 2014 West Africa Ebola virus outbreak, which is affecting Guinea, Sierra Leone, Liberia and Nigeria.[8][9]
It caused significant mortality, with a considerable case fatality rate.[10][11][12][note 1] By the end of the epidemic, 28,616 people had been infected; of these, 11,310 had died, for a case-fatality rate of 40%.[111] As of 8 May 2016[update], 28,646 suspected cases and 11,323 deaths had been reported.[112][113]
1976
Sudan outbreak
The first known outbreak of EVD was identified only after the fact, occurring between June and November 1976 in Nzara, South Sudan,[24][114] (then part of Sudan) and was caused by Sudan virus (SUDV). The Sudan outbreak infected 284 people and killed 151. The first identifiable case in Sudan occurred on 27 June in a storekeeper in a cotton factory in Nzara, who was hospitalized on 30 June and died on 6 July.[115][22] While the WHO medical staff involved in the Sudan outbreak were aware that they were dealing with a heretofore unknown disease, the actual "positive identification" process and the naming of the virus did not occur until some months later in the Democratic Republic of the Congo.[115]
Zaire outbreak
On 26 August 1976, a second outbreak of EVD began in Yambuku, a small rural village in Mongala District in northern Zaire (now known as the Democratic Republic of the Congo).[116][117] This outbreak was caused by EBOV, formerly designated Zaire ebolavirus, which is a different member of the genus Ebolavirus than in the first Sudan outbreak. The first person infected with the disease was village school headmaster Mabalo Lokela, who began displaying symptoms on 26 August 1976.[118] Lokela had returned from a trip to Northern Zaire near the Central African Republic border, having visited the Ebola River between 12 and 22 August. He was originally believed to have malaria and was given quinine. However, his symptoms continued to worsen, and he was admitted to Yambuku Mission Hospital on 5 September. Lokela died on 8 September, 14 days after he began displaying symptoms.[119][120][121]
Soon after Lokela's death, others who had been in contact with him also died, and people in the village of Yambuku began to panic. This led the country's Minister of Health along with Zaire President Mobutu Sese Seko to declare the entire region, including Yambuku and the country's capital, Kinshasa, a quarantine zone. No one was permitted to enter or leave the area, with roads, waterways, and airfields placed under martial law. Schools, businesses and social organizations were closed.[122] Researchers from the CDC, including Peter Piot, co-discoverer of Ebola, later arrived to assess the effects of the outbreak, observing that "the whole region was in panic."[123][124][125] Piot concluded that the Belgian nuns had inadvertently started the epidemic by giving unnecessary vitamin injections to pregnant women, without sterilizing the syringes and needles. The outbreak lasted 26 days, with the quarantine lasting 2 weeks. Among the reasons that researchers speculated caused the disease to disappear, were the precautions taken by locals, the quarantine of the area, and discontinuing the injections.[122]
During this outbreak, Dr. Ngoy Mushola recorded the first clinical description of EVD in Yambuku, where he wrote the following in his daily log: "The illness is characterized with a high temperature of about 39 °C (102 °F), hematemesis, diarrhea with blood, retrosternal abdominal pain, prostration with "heavy" articulations, and rapid evolution death after a mean of 3 days."[126]
The virus responsible for the initial outbreak, first thought to be Marburg virus, was later identified as a new type of virus related to marburgviruses. Virus strain samples isolated from both outbreaks were named as the "Ebola virus" after the Ebola River, located near the originally identified viral outbreak site in Zaire.[22] Reports conflict about who initially coined the name: either Karl Johnson of the American CDC team[127] or Belgian researchers.[128] Subsequently a number of other cases were reported, almost all centered on the Yambuku mission hospital or having close contact with another case.[118] 318 cases and 280 deaths (a 88 percent fatality rate) occurred in Zaire.[129] Although it was assumed that the two outbreaks were connected, scientists later realized that they were caused by two distinct ebolaviruses, SUDV and EBOV.[117] The Zaire outbreak was contained with the help of the World Health Organization and transport from the Congolese air force, by quarantining villagers, sterilizing medical equipment, and providing protective clothing.
1995 to 2012
The second major outbreak occurred in Zaire (now the Democratic Republic of the Congo) in 1995, affecting 315 and killing 254.[130][131] The next major outbreak occurred in Uganda in 2000, affecting 425 and killing 224; in this case the Sudan virus was found to be the ebolavirus species responsible for the outbreak. In 2003 there was an outbreak in the Republic of the Congo that affected 143 and killed 128, a death rate of 90 percent, the highest to date.[132]
In 2004 a Russian scientist died from Ebola after sticking herself with an infected needle.[133]
Between April and August 2007, a fever epidemic[130] in a four-village region[131] of the Democratic Republic of the Congo was confirmed in September to have cases of Ebolavirus.[134] Many people who attended the recent funeral of a local village chief died.[131] The 2007 outbreak eventually affected 264 individuals and resulted in the deaths of 187.[1]
On 30 November 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District in Western Uganda. After confirmation of samples tested by the United States National Reference Laboratories and the Centers for Disease Control, the World Health Organization confirmed the presence of a new species of Ebolavirus, which was tentatively named Bundibugyo.[135] The WHO reported 149 cases of this new strain and 37 of those led to deaths.[1]
The WHO confirmed two small outbreaks in Uganda in 2012. The first outbreak affected 7 people and resulted in the death of 4 and the second affected 24, resulting in the death of 17. The Sudan variant was responsible for both outbreaks.[1]
On 17 August 2012, the Ministry of Health of the Democratic Republic of the Congo reported an outbreak of the Ebola-Bundibugyo variant[136] in the eastern region.[137][138] Other than its discovery in 2007, this was the only time that this variant has been identified as the ebolavirus responsible for an outbreak. The WHO revealed that the virus had sickened 57 people and claimed 29 lives. The probable cause of the outbreak was tainted bush meat hunted by local villagers around the towns of Isiro and Viadana.[1][139]
2013 to 2014 West African outbreak
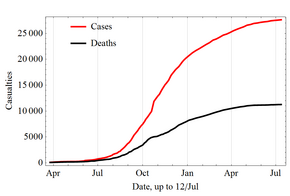
In March 2014, the World Health Organization (WHO) reported a major Ebola outbreak in Guinea, a western African nation.[140] Researchers traced the outbreak to a two-year old child who died December 2013.[141][142] The disease then rapidly spread to the neighboring countries of Liberia and Sierra Leone. It is the largest Ebola outbreak ever documented, and the first recorded in the region.[140]
On 8 August 2014, the WHO declared the epidemic to be an international public health emergency. Urging the world to offer aid to the affected regions, the Director-General said, "Countries affected to date simply do not have the capacity to manage an outbreak of this size and complexity on their own. I urge the international community to provide this support on the most urgent basis possible."[143] By mid-August 2014, Doctors Without Borders reported the situation in Liberia's capital Monrovia as "catastrophic" and "deteriorating daily". They reported that fears of Ebola among staff members and patients had shut down much of the city’s health system, leaving many people without treatment for other conditions.[144] By late August 2014, the disease had spread to Nigeria, and one case was reported in Senegal.[145][146] [147][148] On 30 September 2014, the first confirmed case of Ebola in the United States was diagnosed.[149] The patient died 8 days later.[150]
Aside from the human cost, the outbreak has severely eroded the economies of the affected countries. A Financial Times report suggested the economic impact of the outbreak could kill more people than the virus itself. As of 23 September, in the three hardest hit countries, Liberia, Sierra Leone and Guinea, only 893 treatment beds were available even though the current need was 2122 beds. In a 26 September statement, the WHO said, "The Ebola epidemic ravaging parts of West Africa is the most severe acute public health emergency seen in modern times. Never before in recorded history has a biosafety level four pathogen infected so many people so quickly, over such a broad geographical area, for so long."[151] The WHO reported that by 25 August more than 216 health-care workers were among the dead, partly due to the lack of equipment and long hours.[152] On 23 October, the Malian government confirmed its first case.[153] In response, UNMEER, in cooperation with the Logistics Cluster, air-lifted 1,050 kg of personal protective equipment (PPE) and body bags from Monrovia to Mali.[154]
It caused significant mortality, with a considerable case fatality rate.[10][11][12][note 1] By the end of the epidemic, 28,616 people had been infected; of these, 11,310 had died, for a case-fatality rate of 40%.[155] As of 8 May 2016[update], 28,646 suspected cases and 11,323 deaths had been reported;[14][113] however, the WHO has said that these numbers may be vastly underestimated.[154][156][157]
2014 DRC Congo outbreak
An outbreak in Boende District in Equatorial Province was stopped effectively with flexible organization and funding,[158] as well as social mobilization led by UNICEF advising action people could use.[159][160] The DRC outbreak was from a local Ebola strain and not the one from West Africa (WHO).[161]
2014 spread outside of Africa
As of 15 October 2014, there have been 17 cases of Ebola treated outside of Africa, four of whom have died.[162] In early October, Teresa Romero, a 44-year-old Spanish nurse, contracted Ebola after caring for a priest who had been repatriated from West Africa. This was the first transmission of the virus to occur outside of Africa.[163] On 20 October, it was announced that Teresa Romero had tested negative for the Ebola virus, suggesting that she may have recovered from Ebola infection.[164]
On 19 September, Eric Duncan flew from his native Liberia to Texas; 5 days later he began showing symptoms and visited a hospital, but was sent home. His condition worsened and he returned to the hospital on 28 September, where he died on 8 October.[165] Health officials confirmed a diagnosis of Ebola on 30 September—the first case in the United States.[43] On 12 October, the CDC confirmed that a nurse in Texas who had treated Duncan was found to be positive for the Ebola virus, the first known case of the disease to be contracted in the United States.[166] On 15 October, a second Texas health-care worker who had treated Duncan was confirmed to have the virus.[167] They have both recovered.
On 23 October, a doctor in New York City, who returned to the United States from Guinea after working with Doctors Without Borders, tested positive for Ebola. His case is unrelated to the Texas cases.[168]
Society and culture
Weaponization
Ebolavirus is classified as a biosafety level 4 agent, as well as a Category A bioterrorism agent by the Centers for Disease Control and Prevention. It has the potential to be weaponized for use in biological warfare,[169][170] and was investigated by Biopreparat for such use, but might be difficult to prepare as a weapon of mass destruction because the virus becomes ineffective quickly in open air.[171] Fake emails pretending to be Ebola information from the WHO or the Mexican Government have in 2014 been misused to spread computer malware.[172]
Literature
Richard Preston's 1995 best-selling book, The Hot Zone, dramatized the Ebola outbreak in Reston, Virginia.[173]
William Close's 1995 Ebola: A Documentary Novel of Its First Explosion and 2002 Ebola: Through the Eyes of the People focused on individuals' reactions to the 1976 Ebola outbreak in Zaire.[174]
Tom Clancy's 1996 novel, Executive Orders, involves a Middle Eastern terrorist attack on the United States using an airborne form of a deadly Ebola virus strain named "Ebola Mayinga" (see Mayinga N'Seka).[175]
As the Ebola virus epidemic in West Africa developed in 2014, a number of popular self-published and well-reviewed books containing sensational and misleading information about the disease appeared in electronic and printed formats. The authors of some such books admitted that they lacked medical credentials and were not technically qualified to give medical advice. The World Health Organization and the United Nations stated that such misinformation had contributed to the spread of the disease.[176]
Other animals
Wild animals
Ebola has a high mortality among primates.[102] Frequent outbreaks of Ebola may have resulted in the deaths of 5,000 gorillas.[177] Outbreaks of Ebola may have been responsible for an 88 percent decline in tracking indices of observed chimpanzee populations in 420 square kilometer Lossi Sanctuary between 2002 and 2003.[178] Transmission among chimpanzees through meat consumption constitutes a significant risk factor, whereas contact between the animals, such as touching dead bodies and grooming, is not.[179]
Recovered carcasses from gorillas contain multiple Ebola virus strains, which suggest multiple introductions of the virus. Bodies decompose quickly and carcasses are not infectious after 3 to 4 days. Contact between gorilla groups is rare, suggesting transmission among gorilla groups is unlikely, and that outbreaks result from transmission between viral reservoir and animal populations.[178]
Domestic animals
In 2012 it was demonstrated that the virus can travel without contact from pigs to nonhuman primates, although the same study failed to achieve transmission in that manner between primates.[51][180]
Dogs may become infected with EBOV but not develop symptoms. Dogs in some parts of Africa scavenge for food, and they sometimes eat EBOV-infected animals and also the corpses of humans. A 2005 survey of dogs during an EBOV outbreak found that although they remain asymptomatic, about 32 percent of dogs closest to an outbreak showed a seroprevalence for EBOV versus 9 percent of those farther away.[181] The authors concluded that there were "potential implications for preventing and controlling human outbreaks."
Reston virus
In late 1989, Hazelton Research Products' Reston Quarantine Unit in Reston, Virginia, suffered an outbreak of fatal illness amongst certain lab monkeys. This lab outbreak was initially diagnosed as simian hemorrhagic fever virus (SHFV), and occurred amongst a shipment of crab-eating macaque monkeys imported from the Philippines. Hazelton's veterinary pathologist sent tissue samples from dead animals to the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) at Fort Detrick, Maryland, where an ELISA test indicated the antibodies present in the tissue were a response to Ebola virus and not SHFV.[182] An electron microscopist from USAMRIID discovered filoviruses similar in appearance to Ebola in the tissue samples sent from Hazelton Research Products' Reston Quarantine Unit.[183]
A US Army team headquartered at USAMRIID euthanized the surviving monkeys, and brought all the monkeys to Ft. Detrick for study by the Army's veterinary pathologists and virologists, and eventual disposal under safe conditions.[182] Blood samples were taken from 178 animal handlers during the incident.[184] Of those, six animal handlers eventually seroconverted, including one who had cut himself with a bloody scalpel.[64][185] Despite its status as a Level‑4 organism and its apparent pathogenicity in monkeys, when the handlers did not become ill, the CDC concluded that the virus had a very low pathogenicity to humans.[185][186]
The Philippines and the United States had no previous cases of Ebola infection, and upon further isolation, researchers concluded it was another strain of Ebola, or a new filovirus of Asian origin, which they named Reston ebolavirus (RESTV) after the location of the incident.[182] Reston virus (RESTV) can be transmitted to pigs.[51] Since the initial outbreak it has since been found in nonhuman primates in Pennsylvania, Texas, and Italy,[187] where the virus had infected pigs.[188] According to the WHO, routine cleaning and disinfection of pig (or monkey) farms with sodium hypochlorite or detergents should be effective in inactivating the Reston ebolavirus. Pigs that have been infected with RESTV tend to show symptoms of the disease.
Research
A number of experimental treatments are being studied.[189] In the United States, the Food and Drug Administration (FDA)'s animal efficacy rule is being used to demonstrate reasonable safety to obtain permission to treat people who are infected with Ebola. It is being used because the normal path for testing drugs is not possible for diseases caused by dangerous pathogens or toxins. Experimental drugs are made available for use with the approval of regulatory agencies under named patient programs, known in the US as "expanded access".[190] On 12 August 2014 the WHO released a statement that the use of not yet proven treatments is ethical in certain situations in an effort to treat or prevent the disease.[191]
Medications

Antivirals
A number of antiviral medications are being studied.
- Favipiravir, approved in Japan for stockpiling against influenza pandemics, appears to be useful in a mouse model of Ebola.[16][192] On 4 October 2014, it was reported that a French nun who contracted Ebola while volunteering in Liberia was cured with Favipiravir treatment.[193]
- BCX4430 is a broad-spectrum small molecule antiviral drug developed by BioCryst Pharmaceuticals and undergoing animal testing as a potential human treatment for Ebola by USAMRIID.[194] The drug has been approved to progress to Phase 1 trials, expected late in 2014.[195]
- Brincidofovir is a broad-spectrum antiviral drug. Its maker has been granted FDA approval to proceed with a trial to test its safety and efficacy in Ebola patients.[196] It has been used to treat the first patient diagnosed with Ebola in the USA, after he had recently returned from Liberia.[197][198]
- Lamivudine, usually used to treat HIV/AIDS, was reported in September 2014 to have been used successfully to treat 13 out of 15 Ebola-infected patients by a doctor in Liberia, as part of a combination therapy also involving intravenous fluids and antibiotics to combat opportunistic bacterial infection of Ebola-compromised internal organs.[199] Western virologists have however expressed caution about the results, due to the small number of patients treated and confounding factors present. Researchers at the NIH stated that lamivudine had so far failed to demonstrate anti-Ebola activity in preliminary in vitro tests, but that they would continue to test it under different conditions and would progress it to trials if even slight evidence for efficacy is found.[200]
- JK-05 is developed by the Chinese company Sihuan Pharmaceutical along with the Chinese Academy of Military Medical Sciences. It is reportedly being fast tracked through human trials for Ebola treatment after successful tests in mice.[201][202]
- Lack of available treatment options has spurred research into a number of other possible antivirals targeted against Ebola,[203][204] including natural products such as scytovirin and griffithsin,[205][206] as well as synthetic drugs including DZNep,[207] FGI-103, FGI-104, FGI-106, dUY11 and LJ-001,[208] and other newer agents.[209][210][211][212][213][214][215]
Antisense technology
Other promising treatments rely on antisense technology. Both small interfering RNAs (siRNAs) and phosphorodiamidate morpholino oligomers (PMOs) targeting EBOV RNA polymerase L protein may prevent disease in nonhuman primates.[216][217] TKM-Ebola is a small interfering RNA compound, currently being tested in a Phase I clinical trial in humans.[218][219] Sarepta Therapeutics has completed a Phase I clinical trial with its PMO protecting up to 80-100 percent of the nonhuman primates tested.[220]
Antibodies
ZMapp is a monoclonal antibody vaccine. The limited supply of the drug has been used to treat a small number of individuals infected with the Ebola virus. Although some individuals have recovered, the outcome is not considered statistically significant.[221] ZMapp has proved effective in a trial involving rhesus macaque monkeys.[218][222][223] The Bill & Melinda Gates Foundation has donated $150,000 to help Amgen increase its production, and the U.S. Department of Health and Human Services has asked a number of centers to also increase production.[224] There was no confirmation or proof that the ZMapp drug was a factor in the recovery of two American Ebola patients, however;[225] a Spanish priest with Ebola had taken ZMapp but died afterward.[226]
Researchers in Thailand claim to have developed an antibody-based treatment for Ebola using synthesized fragments of the virus. It has not been tested against Ebola itself. Scientists from the WHO and NIH have offered to test the treatment against live Ebola virus, but there is still a great deal of development needed before human trials.[227]
Other
Two selective estrogen receptor modulators usually used to treat infertility and breast cancer (clomiphene and toremifene) have been found to inhibit the progress of Ebola virus in vitro as well as in infected mice. Ninety percent of the mice treated with clomiphene and 50 percent of those treated with toremifene survived the tests.[228] The study authors conclude that given their oral availability and history of human use, these drugs would be candidates for treating Ebola virus infection in remote geographical locations, either on their own or together with other antiviral drugs.
A 2014 study found that three ion channel blockers used in the treatment of heart arrhythmias, amiodarone, dronedarone and verapamil, block the entry of Ebola virus into cells in vitro.[229]
Blood products
The WHO has stated that transfusion of whole blood or purified serum from Ebola survivors is the therapy with the greatest potential to be implemented immediately, although there is little information as to its efficacy.[230] In September 2014, WHO issued an interim guideline for this therapy.[231] The blood serum from those who have survived an infection is currently being studied to see if it is an effective treatment.[232] During a meeting arranged by WHO, this research was deemed to be a top priority.[232] Seven of eight people with Ebola survived after receiving a transfusion of blood donated by individuals who had previously survived the infection in an 1999 outbreak in the Democratic Republic of the Congo.[103][233] This treatment, however, was started late in the disease meaning they may have already been recovering on their own and the rest of their care was better than usual.[103] Thus this potential treatment remains controversial.[22] Intravenous antibodies appear to be protective in nonhuman primates who have been exposed to large doses of Ebola.[234] The WHO has approved the use of convalescent serum and whole blood products to treat people with Ebola.[235]
Vaccine
Many Ebola vaccine candidates had been developed in the decade prior to 2014,[236] but as of October 2014, none had yet been approved by the United States Food and Drug Administration (FDA) for clinical use in humans.[232][237][238] Several promising vaccine candidates have been shown to protect nonhuman primates (usually macaques) against lethal infection.[24][239] These include replication-deficient adenovirus vectors, replication-competent vesicular stomatitis (VSV) and human parainfluenza (HPIV-3) vectors, and virus-like particle preparations. Conventional trials to study efficacy by exposure of humans to the pathogen after immunization are obviously not feasible in this case. For such situations, the FDA has established the “animal rule” allowing licensure to be approved on the basis of animal model studies that replicate human disease, combined with evidence of safety and a potentially potent immune response (antibodies in the blood) from humans given the vaccine. Phase I clinical trials involve the administration of the vaccine to healthy human subjects to evaluate the immune response, identify any side effects and determine the appropriate dosage.
In September 2014, two Phase I clinical trials began for the vaccine cAd3-EBO Z, which is based on an attenuated version of a chimpanzee adenovirus (cAd3) that has been genetically altered so that it is unable to replicate in humans.[240] It was developed by NIAID in collaboration with Okairos, now a division of GlaxoSmithKline. For the trial designated VRC 20, 20 volunteers were recruited by the NIAID in Bethesda, Maryland, while three dose-specific groups of 20 volunteers each were recruited for trial EBL01 by University of Oxford, U.K.
A replication-competent vaccine based on the vesicular stomatitis virus, called VSV-EBOV, was developed by the Canadian National Microbiology Laboratory and licensed to the small company NewLink Genetics. With the strong support of the U.S. Defense Threat Reduction Agency, it started Phase I clinical trials on healthy human subjects on 13 October 2014 at the Walter Reed Army Institute of Research in Silver Spring, Md.[241][242][243] Also in October 2014, the U.S. National Institute of Allergy and Infectious Diseases (NIAID) was recruiting healthy human volunteers for a "Phase 1 Randomized, Double-Blind, Placebo Controlled, Dose-Escalation Study to Evaluate the Safety and Immunogenicity of Prime-Boost VSV Ebola Vaccine in Healthy Adults".[244] On 20 October, the Public Health Agency of Canada began air shipment of 800 doses of the VSV-EBOV vaccine to the WHO in Geneva.[245] This vaccine is intended to be used in Phase I clinical trials, to start in late October or early November. The WHO has recruited 250 volunteers ready to begin Phase I clinical trials in four locations: Switzerland, Germany, Gabon and Kenya. If the results of this and following trials show that the earlier results in nonhuman primates are replicable in humans, this vaccine could be deployed in areas such as West Africa and would be expected to require only a single dose. Also, its efficacy in protecting nonhuman primates when administered even after viral exposure has occurred may help protect health-care workers after a suspected exposure.
The Health Ministry of Russia also claims to have developed a vaccine called Triazoverin, which is said to be effective against both Ebola and Marburg filoviruses, and might be available for clinical trials in West Africa as soon as the start of 2015.[246][247][248]
At the 8th Vaccine and ISV Conference in Philadelphia on 27−28 October 2014, Novavax Inc. reported the development in a "few weeks" of a glycoprotein (GP) nanoparticle Ebola virus (EBOV GP) vaccine using their proprietary recombinant technology.[249] A recombinant protein is a protein whose code is carried by recombinant DNA. The vaccine is based on the newly published genetic sequence[250] of the 2014 Guinea Ebola strain that is responsible for the current Ebola disease epidemic in West Africa. In "preclinical models", a useful immune response was induced, and was found to be enhanced ten to a hundred-fold by the company's "Matrix-M" immunologic adjuvant. A study of the response of non-human primate to the vaccine had been initiated. Attractive features of such a vaccine could be no need for frozen storage, and the possibility of rapid scaling to manufacture of large dose quantities.[citation needed]
See also
References
Notes The article uses public domain text from the CDC as cited.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac "Ebola virus disease Fact sheet No. 103". World Health Organization. September 2014.
- ^ a b c Ruzek, edited by Sunit K. Singh, Daniel (2014). Viral hemorrhagic fevers. Boca Raton: CRC Press, Taylor & Francis Group. p. 444. ISBN 9781439884294.
{{cite book}}:|first1=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ a b "2014 Ebola Virus Disease (EVD) outbreak in West Africa". WHO. 21 April 2014. Retrieved 3 August 2014.
- ^ a b "Recommendations for Breastfeeding/Infant Feeding in the Context of Ebola". cdc.gov. 19 September 2014. Retrieved 26 October 2014.
- ^ "Guidance for Safe Handling of Human Remains of Ebola Patients in U. S. Hospitals and Mortuaries". Retrieved 10 October 2014.
- ^ a b "Ebola Viral Disease Outbreak — West Africa, 2014". CDC. 27 June 2014. Retrieved 26 June 2014.
- ^ "Ebola Response Roadmap Situation Report Update" (PDF). World Health Organization. 25 October 2014. Retrieved 26 October 2014.
- ^ a b "CDC urges all US residents to avoid nonessential travel to Liberia, Guinea and Sierra Leone because of an unprecedented outbreak of Ebola". CDC. 31 July 2014. Retrieved 2 August 2014.
- ^ a b "Outbreak of Ebola in Guinea, Liberia and Sierra Leone". CDC. 4 August 2014. Retrieved 5 August 2014.
- ^ a b c WHO Ebola Response Team (23 September 2014). "Ebola virus disease in West Africa – the first 9 months of the epidemic and forward projections". New England Journal of Medicine. 371 (16): 1481–1495. doi:10.1056/NEJMoa1411100. PMC 4235004. PMID 25244186.
... we estimate that the case fatality rate is 70.8% (95% confidence interval [CI], 69 to 73) among persons with known clinical outcome of infection.
- ^ a b c Cite error: The named reference
WHO2014_12_28was invoked but never defined (see the help page). - ^ a b c d e f "Case Fatality Rate for ebolavirus". University of Edinburgh. 2015. Archived from the original on 29 August 2014. Retrieved 28 January 2015.
- ^ Wappes J. "US health worker monitored as DRC Ebola nears 600 cases". CIDRAP. Retrieved 23 January 2019.
- ^ a b Ebola virus disease (Report). World Health Organization. Retrieved 6 June 2019.
- ^ a b "Ebola Hemorrhagic Fever Signs and Symptoms". CDC. 28 January 2014. Retrieved 2 August 2014.
- ^ a b c d Gatherer D (August 2014). "The 2014 Ebola virus disease outbreak in West Africa". J. Gen. Virol. 95 (Pt 8): 1619–1624. doi:10.1099/vir.0.067199-0. PMID 24795448.
- ^ a b c d e f Magill, Alan (2013). Hunter's tropical medicine and emerging infectious diseases (9th ed. ed.). New York: Saunders. p. 332. ISBN 9781416043904.
{{cite book}}:|edition=has extra text (help) - ^ a b c d Hoenen T, Groseth A, Falzarano D, Feldmann H (May 2006). "Ebola virus: unravelling pathogenesis to combat a deadly disease". Trends in Molecular Medicine. 12 (5): 206–215. doi:10.1016/j.molmed.2006.03.006. PMID 16616875.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Simpson DIH (1977). "Marburg and Ebola virus infections: a guide for their diagnosis, management, and control" (PDF). WHO Offset Publication No. 36. p. 10f.
- ^ "Ebola Virus, Clinical Presentation". Medscape. Retrieved 30 July 2012.
- ^ "Appendix A: Disease-Specific Chapters" (PDF). Chapter: Hemorrhagic fevers caused by: i) Ebola virus and ii) Marburg virus and iii) Other viral causes including bunyaviruses, arenaviruses, and flaviviruses. Ministry of Health and Long-Term Care. Retrieved 9 October 2014.
- ^ a b c d e f g Feldmann H, Geisbert TW (March 2011). "Ebola haemorrhagic fever". Lancet. 377 (9768): 849–62. doi:10.1016/S0140-6736(10)60667-8. PMC 3406178. PMID 21084112.
- ^ Fisher-Hoch SP, Platt GS, Neild GH, Southee T, Baskerville A, Raymond RT, Lloyd G, Simpson DI (November 1985). "Pathophysiology of shock and hemorrhage in a fulminating viral infection (Ebola)". J. Infect. Dis. 152 (5): 887–894. doi:10.1093/infdis/152.5.887. PMID 4045253.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Hoenen T, Groseth A, Feldmann H (July 2012). "Current Ebola vaccines". Expert Opin Biol Ther. 12 (7): 859–72. doi:10.1517/14712598.2012.685152. PMC 3422127. PMID 22559078.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kuhn JH, Becker S, Ebihara H, Geisbert TW, Johnson KM, Kawaoka Y, Lipkin WI, Negredo AI, Netesov SV, Nichol ST, Palacios G, Peters CJ, Tenorio A, Volchkov VE, Jahrling PB (December 2010). "Proposal for a revised taxonomy of the family Filoviridae: Classification, names of taxa and viruses, and virus abbreviations". Archives of Virology. 155 (12): 2083–103. doi:10.1007/s00705-010-0814-x. PMC 3074192. PMID 21046175.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Spickler, Anna. "Ebolavirus and Marburgvirus Infections" (PDF).
- ^ "About Ebola Virus Disease". CDC. Retrieved 18 October 2014.
- ^ "Ebola (Ebola Virus Disease) Transmission". CDC. 5 November 2014. Retrieved 7 November 2014.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 25347231, please use {{cite journal}} with
|pmid=25347231instead. - ^ a b c "Q&A on Transmission, Ebola". CDC. September 2014. Retrieved 3 October 2014.
- ^ Donald G. McNeil Jr. (3 October 2014). "Ask Well: How Does Ebola Spread? How Long Can the Virus Survive?". The New York Times. Retrieved 24 October 2014.
- ^ a b "How Ebola Is Spread" (PDF). Centers for Disease Control and Prevention (CDC). 1 November 2014.
- ^ a b c d e f g "Transmission". CDC. 17 October 2014. Retrieved 18 October 2014.
- ^ a b c d Chowell G, Nishiura H (October 2014). "Transmission dynamics and control of Ebola virus disease (EVD): a review". BMC Med. 12 (1): 196. doi:10.1186/s12916-014-0196-0. PMC 4207625. PMID 25300956.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b "CDC Telebriefing on Ebola outbreak in West Africa". CDC. 28 July 2014. Retrieved 3 August 2014.
- ^ a b "Air travel is low-risk for Ebola transmission". WHO. 14 August 2014.
- ^ Chan M (September 2014). "Ebola virus disease in West Africa—no early end to the outbreak". N Engl J Med. 371 (13): 1183–5. doi:10.1056/NEJMp1409859. PMID 25140856.
- ^ "Sierra Leone: a traditional healer and a funeral". World Health Organization. Retrieved 6 October 2014.
- ^ Tiaji Salaam-Blyther (26 August 2014). "The 2014 Ebola Outbreak: International and U.S. Responses" (pdf). Retrieved 9 September 2014.
- ^ Lashley, Felissa R.; Durham, Jerry D., eds. (2007). Emerging infectious diseases trends and issues (2nd ed.). New York: Springer. p. 141. ISBN 9780826103505.
- ^ Hunter's tropical medicine and emerging infectious disease (9th ed.). London, New York: Elsevier. 2013. pp. 170–172. OCLC 822525408.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ "Questions and Answers on Ebola | Ebola Hemorrhagic Fever | CDC". CDC.
- ^ a b "Ebola in Texas: Second Health Care Worker Tests Positive". 15 October 2014. Cite error: The named reference "NBC-ebolaTX" was defined multiple times with different content (see the help page).
- ^ Johnson E, Jaax N, White J, Jahrling P (August 1995). "Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus". International journal of experimental pathology. 76 (4): 227–236. ISSN 0959-9673. PMC 1997182. PMID 7547435.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Irving WL (August 1995). "Ebola virus transmission". International Journal of Experimental Pathology. 76 (4): 225–6. PMC 1997188. PMID 7547434.
- ^ "Risk of Exposure". CDC. 12 October 2014. Retrieved 18 October 2014.
- ^ "FAO warns of fruit bat risk in West African Ebola epidemic". fao.org. 21 July 2014. Retrieved 22 October 2014.
- ^ Williams E. "African monkey meat that could be behind the next HIV". Health News – Health & Families. The Independent.
25 people in Bakaklion, Cameroon killed due to eating of ape
- ^ a b "Ebolavirus – Pathogen Safety Data Sheets". Public Health Agency of Canada. Retrieved 22 August 2014. Cite error: The named reference "urlEbolavirus – Pathogen Safety Data Sheets" was defined multiple times with different content (see the help page).
- ^ a b Gonzalez JP, Pourrut X, Leroy E (2007). "Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission". Current Topics in Microbiology and Immunology. Ebolavirus and other filoviruses. 315: 363–387. doi:10.1007/978-3-540-70962-6_15. ISBN 978-3-540-70961-9. PMID 17848072.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Weingartl HM, Nfon C, Kobinger G (May 2013). "Review of Ebola virus infections in domestic animals". Dev Biol (Basel). 135: 211–8. doi:10.1159/000178495. PMID 23689899.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "Weingartl_2013" was defined multiple times with different content (see the help page). - ^ Laupland KB, Valiquette L (May 2014). "Ebola virus disease". Can J Infect Dis Med Microbiol. 25 (3): 128–9. PMC 4173971. PMID 25285105.
- ^ a b c Pourrut X, Kumulungui B, Wittmann T, Moussavou G, Délicat A, Yaba P, Nkoghe D, Gonzalez JP, Leroy EM (June 2005). "The natural history of Ebola virus in Africa". Microbes Infect. 7 (7–8): 1005–14. doi:10.1016/j.micinf.2005.04.006. PMID 16002313.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Swanepoel R, Leman PA, Burt FJ, Zachariades NA, Braack LE, Ksiazek TG, Rollin PE, Zaki SR, Peters CJ (October 1996). "Experimental inoculation of plants and animals with Ebola virus". Emerging Infectious Diseases. 2 (4): 321–325. doi:10.3201/eid0204.960407. ISSN 1080-6040. PMC 2639914. PMID 8969248.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Délicat A, Paweska JT, Gonzalez JP, Swanepoel R (December 2005). "Fruit bats as reservoirs of Ebola virus". Nature. 438 (7068): 575–576. Bibcode:2005Natur.438..575L. doi:10.1038/438575a. PMID 16319873.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Olival KJ, Islam A, Yu M, Anthony SJ, Epstein JH, Khan SA, Khan SU, Crameri G, Wang LF, Lipkin WI, Luby SP, Daszak P (February 2013). "Ebola virus antibodies in fruit bats, Bangladesh". Emerging Infect Dis. 19 (2): 270–3. doi:10.3201/eid1902.120524. PMC 3559038. PMID 23343532.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Morvan JM, Deubel V, Gounon P, Nakouné E, Barrière P, Murri S, Perpète O, Selekon B, Coudrier D, Gautier-Hion A, Colyn M, Volehkov V (December 1999). "Identification of Ebola virus sequences present as RNA or DNA in organs of terrestrial small mammals of the Central African Republic". Microbes and Infection. 1 (14): 1193–1201. doi:10.1016/S1286-4579(99)00242-7. PMID 10580275.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Groseth A, Feldmann H, Strong JE (September 2007). "The ecology of Ebola virus". Trends Microbiol. 15 (9): 408–16. doi:10.1016/j.tim.2007.08.001. PMID 17698361.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pringle, C. R. (2005). "Order Mononegavirales". In Fauquet, C. M.; Mayo, M. A.; Maniloff, J.; Desselberger, U.; Ball, L. A. (eds.). Virus Taxonomy – Eighth Report of the International Committee on Taxonomy of Viruses. San Diego, US: Elsevier/Academic Press. pp. 609–614. ISBN 0-12-370200-3.
- ^ a b Kiley MP, Bowen ET, Eddy GA, Isaäcson M, Johnson KM, McCormick JB, Murphy FA, Pattyn SR, Peters D, Prozesky OW, Regnery RL, Simpson DI, Slenczka W, Sureau P, van der Groen G, Webb PA, Wulff H (1982). "Filoviridae: A taxonomic home for Marburg and Ebola viruses?". Intervirology. 18 (1–2): 24–32. doi:10.1159/000149300. PMID 7118520.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Chippaux JP (October 2014). "Outbreaks of Ebola virus disease in Africa: the beginnings of a tragic saga". J Venom Anim Toxins Incl Trop Dis. 20 (1): 44. doi:10.1186/1678-9199-20-44. PMC 4197285. PMID 25320574.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e f g h Kühl A, Pöhlmann S (September 2012). "How Ebola virus counters the interferon system". Zoonoses Public Health. 59 (Supplement 2): 116–31. doi:10.1111/j.1863-2378.2012.01454.x. PMID 22958256.
- ^ Feldmann, H.; Geisbert, T. W.; Jahrling, P. B.; Klenk, H.-D.; Netesov, S. V.; Peters, C. J.; Sanchez, A.; Swanepoel, R.; Volchkov, V. E. (2005). "Family Filoviridae". In Fauquet, C. M.; Mayo, M. A.; Maniloff, J.; Desselberger, U.; Ball, L. A. (eds.). Virus Taxonomy – Eighth Report of the International Committee on Taxonomy of Viruses. San Diego, US: Elsevier/Academic Press. pp. 645–653. ISBN 0-12-370200-3.
{{cite book}}: Invalid|display-authors=9(help) - ^ a b c d Murray, Patrick R., Ken S. Rosenthal, and Michael A. Pfaller (2013). Medical Microbiology, with STUDENT CONSULT Online Access, 7: Medical Microbiology. Elsevier Health Sciences. pp. 537–538. ISBN 0323086926.
{{cite book}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "isbn0-7910-8505-8" was defined multiple times with different content (see the help page). - ^ Funk DJ, Kumar A (November 2014). "Ebola virus disease: an update for anesthesiologists and intensivists". Can J Anaesth. doi:10.1007/s12630-014-0257-z. PMID 25373801.
- ^ Sullivan N, Yang ZY, Nabel GJ (September 2003). "Ebola Virus Pathogenesis: Implications for VacDendritic cellcines and Therapies" (Free full text). Journal of Virology. 77 (18): 9733–9737. doi:10.1128/JVI.77.18.9733-9737.2003. PMC 224575. PMID 12941881.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Olejnik J, Ryabchikova E, Corley RB, Mühlberger E (August 2011). "Intracellular events and cell fate in filovirus infection". Viruses. 3 (8): 1501–31. doi:10.3390/v3081501. PMC 3172725. PMID 21927676.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Ramanan P, Shabman RS, Brown CS, Amarasinghe GK, Basler CF, Leung DW (September 2011). "Filoviral immune evasion mechanisms". Viruses. 3 (9): 1634–49. doi:10.3390/v3091634. PMC 3187693. PMID 21994800.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Kortepeter MG, Bausch DG, Bray M (November 2011). "Basic clinical and laboratory features of filoviral hemorrhagic fever". J Infect Dis. 204 (Supplement 3): S810–6. doi:10.1093/infdis/jir299. PMID 21987756.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Ebola Hemorrhagic Fever Diagnosis". CDC. 28 January 2014. Retrieved 3 August 2014.
- ^ a b Grolla A, Lucht A, Dick D, Strong JE, Feldmann H (September 2005). "Laboratory diagnosis of Ebola and Marburg hemorrhagic fever". Bull Soc Pathol Exot. 98 (3): 205–9. PMID 16267962.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ WHO 2014: Liberia: New Ebola mobile lab speeds up diagnosis and improves care,. "Liberia: New Ebola mobile lab speeds up diagnosis and improves care,". WHO.int. W.H.O. October 2014. Retrieved 26 October 2014.
- ^ Geisbert TW, Jahrling PB (December 1995). "Differentiation of filoviruses by electron microscopy". Virus Res. 39 (2–3): 129–50. doi:10.1016/0168-1702(95)00080-1. PMID 8837880.
- ^ Longo, DL; Kasper, DL; Jameson, JL; Fauci, AS; Hauser, SL; Loscalzo, J, eds. (2012). "Chapter 197". Harrison's Principles of Internal Medicine (18th ed.). McGraw-Hill. ISBN 0-07-174889-X.
- ^ "Viral Hemorrhagic Fever". San Francisco Department of Public Health. Communicable Disease Control and Prevention. Retrieved 17 August 2014.
- ^ Gear JH (May–June 1989). "Clinical aspects of African viral hemorrhagic fevers". Rev Infect Dis. 11 (Supplement 4): S777–82. doi:10.1093/clinids/11.supplement_4.s777. PMID 2665013.
- ^ Gear JH, Ryan J, Rossouw E (February 1978). "A consideration of the diagnosis of dangerous infectious fevers in South Africa". South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 53 (7): 235–237. PMID 565951.
{{cite journal}}: Missing pipe in:|journal=(help)CS1 maint: multiple names: authors list (link) - ^ Bogomolov BP (1998). "Differential diagnosis of infectious diseases with hemorrhagic syndrome". Terapevticheskii arkhiv. 70 (4): 63–68. PMID 9612907.
- ^ a b c "Ebola Hemorrhagic Fever Prevention". CDC. 31 July 2014. Retrieved 2 August 2014.
- ^ a b "Guidance on Personal Protective Equipment To Be Used by Healthcare Workers During Management of Patients with Ebola Virus Disease in U.S. Hospitals, Including Procedures for Putting On (Donning) and Removing (Doffing)". cdc.gov. 20 October 2014. Retrieved 22 October 2014.
- ^ a b C. J. Peters (December 1998). Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting (PDF). Centers for Disease Control and Prevention.
- ^ Ebola medics 'better trained in Sierra Leone than Spain' The Telegraph, by Fiona Govan, 11 October 2014
- ^ This Is How Ebola Patients Are Equipping Their Homes Time magazine, by Alexandra Sifferlin, 9 October 2014
- ^ Nossiter and Kanter (10 October 2014). "Doctors Without Borders Evolves as It Forms the Vanguard in Ebola Fight". The New York Times. Retrieved 12 October 2014.
- ^ "Infection Prevention and Control Guidance for Care of Patients with Suspected or Confirmed Filovirus Haemorrhagic Fever in Health-care Settings with Focus on Ebola" (PDF). Infection Prevention and Control Guidance for Care of Patients with Suspected or Confirmed Filovirus Haemorrhagic Fever in Health-care Settings with Focus on Ebola. WHO. August 2014. Retrieved 21 August 2014.
- ^ "Ebola – 5 tips to avoid the deadly disease". Plan International. 6 September 2014.
- ^ Rouquet P, Froment JM, Bermejo M, Kilbourn A, Karesh W, Reed P, Kumulungui B, Yaba P, Délicat A, Rollin PE, Leroy EM (February 2005). "Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001–2003" (Free full text). Emerging Infectious Diseases. 11 (2): 283–290. doi:10.3201/eid1102.040533. ISSN 1080-6040. PMC 3320460. PMID 15752448.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Centers for Disease Control and Prevention and World Health Organization (1998). Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting (PDF). Atlanta, Georgia, US: Centers for Disease Control and Prevention. Retrieved 8 February 2013.
- ^ "Section 7: Use Safe Burial Practices" (PDF). Information resources on Ebola virus disease. World Health Organization. 1 June 2014.
- ^ Blaine Harden (24 December 2000). "Ebola's Shadow". The New York Times. Retrieved 12 October 2014.
- ^ Faye SL (September 2014). "How anthropologists help medics fight Ebola in Guinea". SciDev.Net. Retrieved 3 October 2014.
- ^ "West Africa – Ebola virus disease Update: Travel and transport". International travel and health. World Health Organization.
- ^ a b c "Monitoring Symptoms and Controlling Movement to Stop Spread of Ebola". cdc.gov. 27 October 2014.
- ^ "About Quarantine and Isolation". cdc.gov. 28 August 2014. Retrieved 26 October 2014.
- ^ Sompayrac, Lauren (2002). How pathogenic viruses work (3. print. ed.). Boston: Jones and Bartlett Publishers. p. 87. ISBN 9780763720827.
- ^ Alazard-Dany N, Ottmann Terrangle M, Volchkov V (April 2006). "[Ebola and Marburg viruses: the humans strike back]". Med Sci (Paris) (in French). 22 (4): 405–10. doi:10.1051/medsci/2006224405. PMID 16597410.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Ebola virus disease update – west Africa". who.int. 19 August 2014. Retrieved 26 October 2014.
- ^ Schultz, edited by Kristi Koenig, Carl (2009). Koenig and Schultz's disaster medicine : comprehensive principles and practices. Cambridge: Cambridge University Press. p. 209. ISBN 9780521873673.
{{cite book}}:|first1=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Lewis, David (30 July 2014). "Liberia shuts schools, considers quarantine to curb Ebola". Reuters. Retrieved 3 August 2014.
- ^ "Ebola 2014 — New Challenges, New Global Response and Responsibility". NEJM. New England Journal of Medicine. Retrieved 15 September 2014.
- ^ "What is Contact Tracing?" (PDF). CDC. Centers for Disease Control. Retrieved 15 September 2014.
- ^ a b Choi JH, Croyle MA (December 2013). "Emerging targets and novel approaches to Ebola virus prophylaxis and treatment". BioDrugs. 27 (6): 565–83. doi:10.1007/s40259-013-0046-1. PMC 3833964. PMID 23813435.
- ^ a b c d e f g Clark DV, Jahrling PB, Lawler JV (September 2012). "Clinical management of filovirus-infected patients". Viruses. 4 (9): 1668–86. doi:10.3390/v4091668. PMC 3499825. PMID 23170178.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ "Ebola messages for the general public". World Health Organization. Retrieved 26 October 2014.
- ^ "Annex 18. Transmission risk reduction of filoviruses in home-care settings" (PDF). Ebola and Marburg virus disease epidemics: preparedness, alert, control, and evaluation Interim manual version 1.2 CHAPTER 7 ANNEXES. Retrieved 26 October 2014.
- ^ "FDA warns consumers about fraudulent Ebola treatment products". Retrieved 20 August 2014.
- ^ "Inspections, Compliance, Enforcement, and Criminal Investigations". FDA. Retrieved 9 October 2014.
- ^ C.M. Fauquet (2005). Virus taxonomy classification and nomenclature of viruses; 8th report of the International Committee on Taxonomy of Viruses. Oxford: Elsevier/Academic Press. p. 648. ISBN 9780080575483.
- ^ "More or Less behind the stats Ebola". www.bbc.co.uk. BBC World Service. Retrieved 8 October 2014.
- ^ "Who, What, Why: How many people infected with ebola die?". BBC News. 9 August 2014.
- ^ Wappes J. "US health worker monitored as DRC Ebola nears 600 cases". CIDRAP. Retrieved 23 January 2019.
- ^ WHO (22 October 2014). "Ebola Response Roadmap Situation Report" (PDF). who.int. Retrieved 22 October 2014.
- ^ a b "2014 Ebola Outbreak in West Africa – Case Counts". 2014 Ebola Outbreak in West Africa. CDC (Centers for Disease Control and Prevention). 2014.
- ^ Peterson AT, Bauer JT, Mills JN (2004). "Ecologic and Geographic Distribution of Filovirus Disease". Emerging Infectious Diseases. 10 (1): 40–47. doi:10.3201/eid1001.030125. PMC 3322747. PMID 15078595.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "Ebola haemorrhagic fever in Sudan, 1976" (pdf).
- ^ Hewlett, Barry; Hewlett, Bonnie (2007). Ebola, Culture and Politics: The Anthropology of an Emerging Disease. Cengage Learning. p. 103. Retrieved 31 July 2014.
- ^ a b Feldmann H, Jones S, Klenk HD, Schnittler HJ (August 2003). "Ebola virus: from discovery to vaccine". Nature Reviews. Immunology. 3 (8): 677–85. doi:10.1038/nri1154. PMID 12974482.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "Ebola haemorrhagic fever in Zaire, 1976" (PDF). Bull. World Health Organ. 56 (2): 271–93. 1978. PMC 2395567. PMID 307456.
- ^ "Outbreak of Ebola Viral Hemorrhagic Fever – Zaire, 1995". Morbidity and Mortality Weekly Report. 44 (19): 381–2. 1995.
- ^ "Ebola". Academic Kids.
- ^ Elezra M. "Ebola: The Truth Behind The Outbreak (Video) l". Mabalo Lokela Archives – Political Mol.
- ^ a b Ebola (1st ed.). New York: Rosen Pub. 2011. pp. 31, 52. ISBN 978-1435894334.
- ^ Piot P, Marshall R (2012). No time to lose: a life in pursuit of deadly viruses (1st ed.). New York: W.W. Norton & Co. pp. 30, 90. ISBN 978-0393063165.
- ^ Peter Piot (11 August 2014). "Part one: A virologist's tale of Africa's first encounter with Ebola". ScienceInsider.Free access
- ^ Peter Piot (13 August 2014). "Part two: A virologist's tale of Africa's first encounter with Ebola". ScienceInsider.Free access
- ^ Bardi, Jason Socrates. "Death Called a River". The Scripps Research Institute. Retrieved 9 October 2014.
- ^ Preston, Richard (20 July 1995). The Hot Zone. Anchor Books (Random House). p. 117.
Karl Johnson named it Ebola
- ^ Bredow, Rafaela von; Hackenbroch, Veronika (4 October 2014). "'In 1976 I Discovered Ebola – Now I Fear an Unimaginable Tragedy'". The Observer. Guardian Media Group.
- ^ King JW (2 April 2008). "Ebola Virus". eMedicine. WebMD. Retrieved 6 October 2008.
- ^ a b "Ebola outbreak in Congo". CBC.ca. CBC/Radio-Canada. 12 September 2007.
- ^ a b c "Mystery DR Congo fever kills 100". BBC News. 31 August 2007.
- ^ Formenty P, Libama F, Epelboin A, Allarangar Y, Leroy E, Moudzeo H, Tarangonia P, Molamou A, Lenzi M, Ait-Ikhlef K, Hewlett B, Roth C, Grein T (2003). "[Outbreak of Ebola hemorrhagic fever in the Republic of the Congo, 2003: a new strategy?]". Med Trop (Mars) (in French). 63 (3): 291–5. PMID 14579469.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Russian Scientist Dies in Ebola Accident at Former Weapons Lab". The New York Times. Retrieved 12 October 2014.
- ^ "Ebola Outbreak Confirmed in Congo". NewScientist.com. 11 September 2007.
- ^ "Uganda: Deadly Ebola Outbreak Confirmed – UN". UN News Service. 30 November 2007. Retrieved 25 February 2008.
- ^ "DRC Confirms Ebola Outbreak". Voanews.com. Retrieved 15 April 2013.
- ^ "WHO | Ebola outbreak in Democratic Republic of Congo". Who.int. 17 August 2012. Retrieved 15 April 2013.
- ^ "WHO | Ebola outbreak in Democratic Republic of Congo – update". Who.int. 21 August 2012. Retrieved 15 April 2013.
- ^ Castillo M (2012). "Ebola virus claims 31 lives in Democratic Republic of the Congo". United States: CBS News. Retrieved 14 September 2012.
{{cite journal}}: Cite journal requires|journal=(help); Invalid|ref=harv(help) - ^ a b "Guidelines for Evaluation of US Patients Suspected of Having Ebola Virus Disease". CDC. 1 August 2014. Retrieved 5 August 2014.
- ^ Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, Soropogui B, Sow MS, Keïta S, De Clerck H, Tiffany A, Dominguez G, Loua M, Traoré A, Kolié M, Malano ER, Heleze E, Bocquin A, Mély S, Raoul H, Caro V, Cadar D, Gabriel M, Pahlmann M, Tappe D, Schmidt-Chanasit J, Impouma B, Diallo AK, Formenty P, Van Herp M, Günther S (October 2014). "Emergence of Zaire Ebola Virus Disease in Guinea". New England Journal of Medicine. 371 (15): 1418–25. doi:10.1056/NEJMoa1404505. PMID 24738640.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "The first cases of this Ebola outbreak traced by WHO" (png). who.int. WHO. 2014.
- ^ "WHO raises global alarm over Ebola outbreak". CBS. Retrieved 2 August 2014.
- ^ "In Liberia's Ebola-Stricken Villages, Residents Face 'Stark' Choices". n Liberia's Ebola-Stricken Villages, Residents Face 'Stark' Choices. Common Dreams. 18 August 2014. Retrieved 20 August 2014.
- ^ "Disease outbreak news – Ebola virus disease, West Africa – update 28 August 2014". Epidemic & Pandemic Alert and Response (EPR) – Outbreak News. WHO. Retrieved 28 August 2014.
- ^ "Ebola virus disease update, West Africa – update 28 August 2014". Epidemic & Pandemic Alert and Response (EPR) – Outbreak News. WHO. Retrieved 28 August 2014.
- ^ "Senegalese minister of health confirms 1st case of Ebola virus in the West African country". Washington Post. 29 August 2014. Retrieved 29 August 2014.
- ^ "First case of Ebola virus is confirmed in Senegal". ITV. 29 August 2014. Retrieved 29 August 2014.
- ^ Staff (30 September 2014). "CDC and Texas Health Department Confirm First Ebola Case Diagnosed in the U.S." CDC. Retrieved 30 September 2014.
- ^ Bustillo, Miguel; Campoy, Ann; Hinshaw, Drew. "Dallas Ebola Patient Dies". The Wall Street Journal Online. Retrieved 8 October 2014.
- ^ Media centre (26 September 2014). "Experimental therapies: growing interest in the use of whole blood or plasma from recovered Ebola patients (convalescent therapies)". World Health Organization. Retrieved 28 September 2014.
- ^ "Unprecedented number of medical staff infected with Ebola". WHO. 25 August 2014. Retrieved 29 August 2014.
- ^ "Press Releases". THE MINISTRY OF HEALTH AND PUBLIC HEALTH (in French). Government of Mali. Retrieved 23 October 2014.
- ^ a b UN Ebola Crisis Desk (28 October 2014). "External Situation Report of UN Mission for Ebola Emergency Response" (PDF). United Nations. Retrieved 4 November 2014. Cite error: The named reference "UNMEER External Situation Report" was defined multiple times with different content (see the help page).
- ^ Wappes J. "US health worker monitored as DRC Ebola nears 600 cases". CIDRAP. Retrieved 23 January 2019.
- ^ "WHO: EBOLA RESPONSE ROADMAP UPDATE-8" (PDF). World Health Organization. 8 September 2014.
- ^ "Ebola Response Roadmap Situation Report" (PDF). World Health Organization. 1 October 2014.
- ^ Mounet, Joustine. "DRC: 21 days later, out of the Ebola outbreak". Retrieved 30 October 2014.
- ^ Amboko, Modestine. "Combatting Myths and Misconceptions to Defeat Ebola". http://ponabana.com/combatting-myths-and-misconceptions-to-defeat-ebola/?lang=en.
{{cite web}}:|access-date=requires|url=(help); External link in|website=|url=(help) - ^ Mukwampa, Félix K N. "How to prevent and fight against an outbreak of the Ebola virus in the DRC". http://ponabana.com/how-to-prevent-and-fight-against-an-outbreak-of-the-ebola-virus-in-the-drc/?lang=en.
{{cite web}}:|access-date=requires|url=(help); External link in|website=|url=(help) - ^ Yenko, Athena. "New String Of Ebola In The Democratic Republic Of The Congo; DFAT Advises Against Travel". Retrieved 30 October 2014.
- ^ "Ebola Facts: How Many Ebola Patients Are Being Treated Outside of West Africa?".
- ^ "Second US Ebola diagnosis 'deeply concerning', admits CDC chief".
- ^ "Ebola crisis: Tests show Spanish nurse Teresa Romero no longer has the virus". ABC News. 20 October 2014.
- ^ "Thomas Eric Duncan: First Ebola death in U.S."
- ^ Fernandez, Manny (12 October 2014). "Texas Health Worker Tests Positive for Ebola". New York Times. Retrieved 12 October 2014.
- ^ "Ebola in Texas: Second Health Care Worker Tests Positive".
- ^ Sanchez, Ray; Prokupecz, Shimon (23 October 2014). "N.Y. doctor positive for Ebola had no symptoms until Thursday, officials say". CNN. Retrieved 23 October 2014.
- ^ Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, Ksiazek T, Johnson KM, Meyerhoff A, O'Toole T, Ascher MS, Bartlett J, Breman JG, Eitzen EM, Hamburg M, Hauer J, Henderson DA, Johnson RT, Kwik G, Layton M, Lillibridge S, Nabel GJ, Osterholm MT, Perl TM, Russell P, Tonat K (May 2002). "Hemorrhagic fever viruses as biological weapons: medical and public health management". Journal of the American Medical Association. 287 (18): 2391–405. doi:10.1001/jama.287.18.2391. PMID 11988060.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Salvaggio MR, Baddley JW (July 2004). "Other viral bioweapons: Ebola and Marburg hemorrhagic fever". Dermatologic clinics. 22 (3): 291–302, vi. doi:10.1016/j.det.2004.03.003. PMID 15207310.
- ^ Zubray, Geoffrey (2013). Agents of Bioterrorism: Pathogens and Their Weaponization. New York, NY, USA: Columbia University Press. pp. 73–74. ISBN 9780231518130.
- ^ "Malicious Ebola-Themed Emails Are on the Rise". NYTimes.com. N.Y.Times. 24 October 2014. Retrieved 26 October 2014.
- ^ (1) Preston, Richard (1995). The Hot Zone, A Terrifying True Story. Anchor Books. ISBN 0-385-47956-5. OCLC 32052009. At Google Books.
(2) "Best Sellers: June 4, 1995". The New York Times Book Review. New York: The New York Times. 4 June 1995. Retrieved 10 September 2014.
(3) "About The Hot Zone". Random House. Retrieved 10 September 2014. - ^ (1) Close, William T. (1995). Ebola: A Documentary Novel of Its First Explosion. New York: Ivy Books. ISBN 0804114323. OCLC 32753758. At Google Books.
(2) Grove, Ryan (2 June 2006). "More about the people than the virus". Review of Close, William T., Ebola: A Documentary Novel of Its First Explosion. Amazon.com. Retrieved 17 September 2014.{{cite web}}: Italic or bold markup not allowed in:|work=(help)
(3) Close, William T. (2002). Ebola: Through the Eyes of the People. Marbleton, Wyoming: Meadowlark Springs Productions. ISBN 0970337116. OCLC 49193962. At Google Books.
(4) Pink, Brenda (24 June 2008). "A fascinating perspective". Review of Close, William T., Ebola: Through the Eyes of the People. Amazon.com. Retrieved 17 September 2014.{{cite web}}: Italic or bold markup not allowed in:|work=(help) - ^ (1) Clancy, Tom (1996). Executive Orders. New York: Putnam. ISBN 0399142185. OCLC 34878804. At Google Books.
(2) Line, Matt; Jeremy; Dan. "Executive Orders book reviews". AllReaders.com. Archived from the original on 1 August 2014. Retrieved 10 September 2014.{{cite web}}: External link in|publisher=
(3) Stone, Oliver (2 September 1996). "Who's That in the Oval Office?". Books News & Reviews. The New York Times Company. Archived from the original on 10 April 2009. Retrieved 10 September 2014. - ^ Dewey, Caitlin (2 October 2014). "Popular on Amazon: Wildly misleading self-published books about Ebola, by random people without medical degrees". The Washington Post. Retrieved 27 October 2014.
- ^ Ebola 'kills over 5,000 gorillas'. BBC. 8 December 2006. Retrieved 31 May 2009.
- ^ a b Leroy EM, Rouquet P, Formenty P, Souquière S, Kilbourne A, Froment JM, Bermejo M, Smit S, Karesh W, Swanepoel R, Zaki SR, Rollin PE (January 2004). "Multiple Ebola virus transmission events and rapid decline of central African wildlife". Science. 303 (5656): 387–390. Bibcode:2004Sci...303..387L. doi:10.1126/science.1092528. PMID 14726594.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Formenty P, Boesch C, Wyers M, Steiner C, Donati F, Dind F, Walker F, Le Guenno B (February 1999). "Ebola virus outbreak among wild chimpanzees living in a rain forest of Côte d'Ivoire". The Journal of infectious diseases. 179. Suppl 1 (s1): S120–S126. doi:10.1086/514296. PMID 9988175.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Weingartl HM, Embury-Hyatt C, Nfon C, Leung A, Smith G, Kobinger G (November 2012). "Transmission of Ebola virus from pigs to non-human primates". Sci Rep. 2: 811. doi:10.1038/srep00811. PMC 3498927. PMID 23155478.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Allela L, Boury O, Pouillot R, Délicat A, Yaba P, Kumulungui B, Rouquet P, Gonzalez JP, Leroy EM (March 2005). "Ebola virus antibody prevalence in dogs and human risk". Emerging Infect. Dis. 11 (3): 385–90. doi:10.3201/eid1103.040981. PMC 3298261. PMID 15757552.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Preston, Richard (1994). The Hot Zone. New York: Random House. p. 300. ISBN 978-0679437840.
- ^ McCormick & Fisher-Hoch 1999, pp. 277–279
- ^ Waterman, Tara (1999). Ebola Reston Outbreaks. Stanford University. Retrieved 2 August 2008.
- ^ a b McCormick & Fisher-Hoch 1999, pp. 298–299
- ^ McCormick & Fisher-Hoch 1999, p. 300
- ^ "Outbreaks Chronology: Ebola Virus Disease". cdc.gov. Retrieved 26 October 2014.
- ^ McNeil Jr, Donald G. (24 January 2009). "Pig-to-Human Ebola Case Suspected in Philippines". New York Times. Retrieved 26 January 2009.
- ^ Briggs H. "BBC News – Ebola: Experimental drugs and vaccines". BBC News. Retrieved 8 August 2014.
- ^ Gaffney A (7 August 2014). "Regulatory Explainer: What You Need to Know About the Regulation of Ebola Treatments". Regulatory Affairs Professionals Society (RAPS).
- ^ "Ethical considerations for use of unregistered interventions for Ebola virus disease (EVD)". WHO. Retrieved 20 August 2014.
- ^ Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S (May 2014). "Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model". Antiviral Res. 105: 17–21. doi:10.1016/j.antiviral.2014.02.014. PMID 24583123.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "French nurse cured of Ebola contracted in Liberia". MSN. Retrieved 7 October 2014.
- ^ "BioCryst to Launch NHP Ebola Drug Safety, Efficacy Studies 'Within Weeks'. David Kroll, Forbes Magazine. 29 August 2014". Forbes. 29 August 2014. Retrieved 5 October 2014.
- ^ "DURHAM: BioCryst receives additional funding for Ebola drug – WNCN: News, Weather". WNCN. 18 September 2014. Retrieved 5 October 2014.
- ^ "Chimerix gets FDA approval to test drug for Ebola". Associated Press. 17 October 2014.
- ^ "Chimerix Announces Emergency Investigational New Drug Applications for Brincidofovir Authorized by FDA for Patients With Ebola Virus Disease". Retrieved 7 October 2014.
- ^ Perrone, Matthew (6 October 2014). "Experimental Drug Provided to Dallas Ebola Patient". ABC. AP. Retrieved 6 October 2014.
- ^ "Doctor treats Ebola with HIV drug in Liberia – seemingly successfully. Elizabeth Cohen, CNN news. 29 September 2014". CNN. 27 September 2014. Retrieved 7 October 2014.
- ^ "A Liberian doctor is using HIV drugs to treat Ebola victims. The NIH is intrigued. Elahe Izadi, Washington Post. 2 October 2014". Washington Post. Retrieved 7 October 2014.
- ^ Ebola virus drug approved on mainland for emergency use only. Zhuang Pinghui, South China Morning Post, 01 September 2014
- ^ China military-linked firm eyes quick approval of drug to cure Ebola. Adam Jourdan, Yahoo News, 14 October 2014
- ^ Bray M, Paragas J (2002). "Experimental therapy of filovirus infections". Antiviral Res. 54 (1): 1–17. doi:10.1016/S0166-3542(02)00005-0. PMID 11888653.
- ^ Bray M (2003). "Defense against filoviruses used as biological weapons". Antiviral Res. 57 (1–2): 53–60. doi:10.1016/S0166-3542(02)00200-0. PMID 12615303.
- ^ Garrison AR, Giomarelli BG, Lear-Rooney CM, Saucedo CJ, Yellayi S, Krumpe LR, Rose M, Paragas J, Bray M, Olinger GG, McMahon JB, Huggins J, O'Keefe BR (2014). "The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus". Antiviral Research. 112C: 1–7. doi:10.1016/j.antiviral.2014.09.012. PMID 25265598.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Barton C, Kouokam JC, Lasnik AB, Foreman O, Cambon A, Brock G, Montefiori DC, Vojdani F, McCormick AA, O'Keefe BR, Palmer KE (2014). "Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models". Antimicrobial Agents and Chemotherapy. 58 (1): 120–7. doi:10.1128/AAC.01407-13. PMC 3910741. PMID 24145548.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Huggins J, Zhang ZX, Bray M (1999). "Antiviral drug therapy of filovirus infections: S-adenosylhomocysteine hydrolase inhibitors inhibit Ebola virus in vitro and in a lethal mouse model". J. Infect. Dis. 179 Suppl 1: S240–7. doi:10.1086/514316. PMID 9988190.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ De Clercq E (2013). "A Cutting-Edge View on the Current State of Antiviral Drug Development". Medicinal Research Reviews: n/a. doi:10.1002/med.21281. PMID 23495004.
- ^ Kolokoltsov A et al. (2012) Inhibition of Lassa virus and Ebola virus infection in host cells treated with the kinase inhibitors genistein and tyrphostin. Archives of Virology 157(1): 121-127. doi: 10.1007/s00705-011-1115-8
- ^ Panchal RG, Reid SP, Tran JP, Bergeron AA, Wells J, Kota KP, Aman J, Bavari S (January 2012). "Identification of an antioxidant small-molecule with broad-spectrum antiviral activity". Antiviral Res. 93 (1): 23–9. doi:10.1016/j.antiviral.2011.10.011. PMID 22027648.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Qiu X, Wong G, Fernando L, Ennis J, Turner JD, Alimonti JB, Yao X, Kobinger GP (July 2013). "Monoclonal antibodies combined with adenovirus-vectored interferon significantly extend the treatment window in Ebola virus-infected guinea pigs". J. Virol. 87 (13): 7754–7. doi:10.1128/JVI.00173-13. PMC 3700280. PMID 23616649.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Elshabrawy HA, Fan J, Haddad CS, Ratia K, Broder CC, Caffrey M, Prabhakar BS (April 2014). "Identification of a broad-spectrum antiviral small molecule against severe acute respiratory syndrome coronavirus and Ebola, Hendra, and Nipah viruses by using a novel high-throughput screening assay". Journal of Virology. 88 (8): 4353–65. doi:10.1128/JVI.03050-13. PMC 3993759. PMID 24501399.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mudhasani R, Kota KP, Retterer C, Tran JP, Whitehouse CA, Bavari S (August 2014). "High content image-based screening of a protease inhibitor library reveals compounds broadly active against Rift Valley fever virus and other highly pathogenic RNA viruses". PLoS Neglected Tropical Diseases. 8 (8): e3095. doi:10.1371/journal.pntd.0003095. PMC 4140764. PMID 25144302.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Johnson JC, Martinez O, Honko AN, Hensley LE, Olinger GG, Basler CF (June 2014). "Pyridinyl imidazole inhibitors of p38 MAP kinase impair viral entry and reduce cytokine induction by Zaire ebolavirus in human dendritic cells". Antiviral Research. 107: 102–9. doi:10.1016/j.antiviral.2014.04.014. PMC 4103912. PMID 24815087.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Madrid, PB; Chopra, S; Manger, ID; Gilfillan, L; Keepers, TR; Shurtleff, AC; Green, CE; Iyer, LV; Dilks, HH; Davey, RA; Kolokoltsov, AA; Carrion R, Jr; Patterson, JL; Bavari, S; Panchal, RG; Warren, TK; Wells, JB; Moos, WH; Burke, RL; Tanga, MJ (2013). "A systematic screen of FDA-approved drugs for inhibitors of biological threat agents". PloS one. 8 (4): e60579. PMID 23577127.
- ^ Geisbert TW, Lee AC, Robbins M, Geisbert JB, Honko AN, Sood V, Johnson JC, de Jong S, Tavakoli I, Judge A, Hensley LE, Maclachlan I (29 May 2010). "Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: A proof-of-concept study". The Lancet. 375 (9729): 1896–1905. doi:10.1016/S0140-6736(10)60357-1. PMID 20511019.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Warren TK, Warfield KL, Wells J, Swenson DL, Donner KS, Van Tongeren SA, Garza NL, Dong L, Mourich DV, Crumley S, Nichols DK, Iversen PL, Bavari S (September 2010). "Advanced antisense therapies for postexposure protection against lethal filovirus infections". Nature Medicine. 16 (9): 991–994. doi:10.1038/nm.2202. PMID 20729866.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Pollack, Andrew (7 August 2014). "Second drug is allowed for treatment of Ebola". The New York Times. Retrieved 8 August 2014.
- ^ Helen Branswell (3 August 2014). "Nancy Writebol, U.S. missionary, didn't get TKM-Ebola drug, Tekmira says". CBC News. Canadian Press.
- ^ Heald AE, Iversen PL, Saoud JB, Sazani P, Charleston JS, Axtelle T, Wong M, Smith WB, Vutikullird A, Kaye E (25 August 2014). "Safety and pharmacokinetic profiles of phosphorodiamidate morpholino oligomers with activity against Ebola virus and Marburg virus: results of two single ascending dose studies". Antimicrobial Agents and Chemotherapy. 58 (11): 6639–47. doi:10.1128/AAC.03442-14. PMID 25155593.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kroll, David (26 August 2014). "How will we know if the Ebola drugs worked?". Forbes. Retrieved 1 October 2014.
- ^ Nathan Seppa (29 August 2014). "ZMapp drug fully protects monkeys against Ebola virus". Science News. Society for Science & the Public. Retrieved 1 October 2014.
- ^ "12 Answers To Ebola’s Hard Questions", Time magazine, Oct. 23, 2014
- ^ "Amgen works with Gates Foundation to ramp up Ebola drug ZMapp production", Puget Sound Business Journal, October 20, 2014.
- ^ "What Cured Ebola Patients Kent Brantly and Nancy Writebol?", NBC News, August 21, 2014
- ^ Moffett, Matt (13 August 2014). "Ebola Virus: Infected Priest Has Died in Spain". Wall Street Journal. Retrieved 26 October 2014.
- ^ Thai researchers claim breakthrough in Ebola treatment. Ron Corben, Voice of America News, 3 October 2014
- ^ Johansen LM, Brannan JM, Delos SE, Shoemaker CJ, Stossel A, Lear C, Hoffstrom BG, Dewald LE, Schornberg KL, Scully C, Lehár J, Hensley LE, White JM, Olinger GG (June 2013). "FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection". Sci Transl Med. 5 (190): 190ra79. doi:10.1126/scitranslmed.3005471. PMC 3955358. PMID 23785035.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Gehring G, Rohrmann K, Atenchong N, Mittler E, Becker S, Dahlmann F, Pöhlmann S, Vondran FW, David S, Manns MP, Ciesek S, von Hahn T (2014). "The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry". J. Antimicrob. Chemother. 69 (8): 2123–31. doi:10.1093/jac/dku091. PMID 24710028.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Blood transfusion named as priority treatment for Ebola". Nature. Retrieved 11 September 2014.
- ^ "Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease Empirical treatment during outbreaks". WHO. Retrieved 4 October 2014.
- ^ a b c "Statement on the WHO Consultation on potential Ebola therapies and vaccines". WHO. 5 September 2014. Retrieved 1 October 2014.
- ^ Mupapa K, Massamba M, Kibadi K, Kuvula K, Bwaka A, Kipasa M, Colebunders R, Muyembe-Tamfum JJ (February 1999). "Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee" (PDF). J Infect Dis. 179 (Supplement 1): S18–23. doi:10.1086/514298. PMID 9988160.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Saphire EO (November 2013). "An update on the use of antibodies against the filoviruses". Immunotherapy. 5 (11): 1221–33. doi:10.2217/imt.13.124. PMID 24188676.
- ^ Gulland A (8 September 2014). "First Ebola treatment is approved by WHO". BMJ. 349 (sep08 7): g5539–g5539. doi:10.1136/bmj.g5539. PMID 25200068.
- ^ Richardson JS, Dekker JD, Croyle MA, Kobinger GP (June 2010). "Recent advances in Ebolavirus vaccine development". Human Vaccines (open access). 6 (6): 439–49. doi:10.4161/hv.6.6.11097. PMID 20671437.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "2014 Ebola Outbreak in West Africa". Retrieved Oct 2014.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Alison P. Galvani with three others (21 August 2014). "Ebola Vaccination: If Not Now, When?". Annals of Internal Medicine. doi:10.7326/M14-1904.
- ^ Fausther-Bovendo H, Mulangu S, Sullivan NJ (June 2012). "Ebolavirus vaccines for humans and apes". Curr Opin Virol. 2 (3): 324–29. doi:10.1016/j.coviro.2012.04.003. PMC 3397659. PMID 22560007.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vaccine Research Center, NIAID (29 September 2014). "Ebola Vaccine Clinical Development Overview (presentation)" (PDF). Retrieved 21 October 2014.
- ^ "Canadian-made Ebola vaccine to start clinical trials in healthy humans". Globe and Mail. 13 October 2014. Retrieved 13 October 2014.
- ^ "Ebola outbreak: 1st human trials of Canadian vaccine start in U.S." CBC News. 13 October 2014.
- ^ "Canadian Ebola vaccine license holder moving ahead with safety trials". Canadian Press. 8 October 2014. Retrieved 13 October 2014.
- ^ "A Phase 1 Randomized, Double-Blind, Placebo Controlled, Dose-Escalation Study to Evaluate the Safety and Immunogenicity of Prime-Boost VSV Ebola Vaccine in Healthy Adults". US NIAID. 9 October 2014. Retrieved 13 October 2014.
- ^ Hannah Thibedeau (19 October 2014). "Ebola vaccine to be sent to WHO on Monday for clinical trials". CBC News. Retrieved 21 October 2014.
- ^ Ebola crisis: Vaccine 'too late' for outbreak. BBC News, 17 October 2014
- ^ 70–90% efficiency: Russia to send Ebola vaccine to W. Africa in 2 months. RT.com 13 October 2014
- ^ Russian Scientists Develop New Vaccine to Fight Ebola Virus. The Moscow Times, 27 August 2014
- ^ Novavax (27 October 2014). "Novavax Announces Ebola Vaccine Development Program at the 8th Vaccine and ISV Conference in Philadelphia" (PDF). Retrieved 28 October 2014.
- ^ Gire SK, Goba A, Andersen KG, Sealfon RS, Park DJ, Kanneh L, Jalloh S, Momoh M, Fullah M, Dudas G, Wohl S, Moses LM, Yozwiak NL, Winnicki S, Matranga CB, Malboeuf CM, Qu J, Gladden AD, Schaffner SF, Yang X, Jiang PP, Nekoui M, Colubri A, Coomber MR, Fonnie M, Moigboi A, Gbakie M, Kamara FK, Tucker V, Konuwa E, Saffa S, Sellu J, Jalloh AA, Kovoma A, Koninga J, Mustapha I, Kargbo K, Foday M, Yillah M, Kanneh F, Robert W, Massally JL, Chapman SB, Bochicchio J, Murphy C, Nusbaum C, Young S, Birren BW, Grant DS, Scheiffelin JS, Lander ES, Happi C, Gevao SM, Gnirke A, Rambaut A, Garry RF, Khan SH, Sabeti PC (September 2014). "Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak". Science. 345 (6202): 1369–72. doi:10.1126/science.1259657. PMID 25214632.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Bibliography
- Klenk, Hans-Dieter (January 1999). Marburg and Ebola Viruses (Current Topics in Microbiology and Immunology). Berlin: Springer-Verlag Telos. ISBN 978-3-540-64729-4.
{{cite book}}: CS1 maint: ref duplicates default (link) - Klenk, Hans-Dieter; Feldmann, Heinz (2004). Ebola and Marburg viruses: molecular and cellular biology (Limited preview). Wymondham, Norfolk, UK: Horizon Bioscience. ISBN 978-0-9545232-3-7.
{{cite book}}: CS1 maint: ref duplicates default (link) - Kuhn, Jens H. (2008). Filoviruses: A Compendium of 40 Years of Epidemiological, Clinical, and Laboratory Studies. Archives of Virology Supplement, vol. 20 (Limited preview). Vienna: SpringerWienNewYork. ISBN 978-3-211-20670-6.
{{cite book}}: CS1 maint: ref duplicates default (link) - McCormick, Joseph; Fisher-Hoch, Susan (June 1999) [1996]. Level 4: Virus Hunters of the CDC (Limited preview). Horvitz, Leslie Alan (Updated [3rd] ed.). Barnes & Noble. ISBN 978-0-7607-1208-5.
{{cite book}}: CS1 maint: ref duplicates default (link) - Pattyn, S. R. (1978). Ebola Virus Haemorrhagic Fever (Full free text) (1st ed.). Amsterdam: Elsevier/North-Holland Biomedical Press. ISBN 0-444-80060-3.
{{cite book}}: CS1 maint: ref duplicates default (link) - Ryabchikova, Elena I.; Price, Barbara B. (2004). Ebola and Marburg Viruses: A View of Infection Using Electron Microscopy. Columbus, Ohio: Battelle Press. ISBN 978-1-57477-131-2.
{{cite book}}: CS1 maint: ref duplicates default (link)
External links
- Ebola at Curlie
- CDC: Ebola hemorrhagic fever – Centers for Disease Control and Prevention, Special Pathogens Branch
- WHO: Ebola haemorrhagic fever – World Health Organization, Global Alert and Response
- Google Map of Ebola Outbreaks
- [1] – World Health Organisation, Fact Sheet 103 Ebola Virus Disease, Updated September 2013
- [2] WHO Ebola videos
Cite error: There are <ref group=note> tags on this page, but the references will not show without a {{reflist|group=note}} template (see the help page).
