Wikipedia:Reference desk/Science: Difference between revisions
→Scar formation: ~~~~ |
→Scar formation: Medical advice removed. |
||
| Line 381: | Line 381: | ||
I am wondering whether exposure of a wound to sunlight increases , decreases or has no effect on the development of a visible, permanent scar? [[Special:Contributions/67.253.78.55|67.253.78.55]] ([[User talk:67.253.78.55|talk]]) 00:25, 9 July 2020 (UTC) |
I am wondering whether exposure of a wound to sunlight increases , decreases or has no effect on the development of a visible, permanent scar? [[Special:Contributions/67.253.78.55|67.253.78.55]] ([[User talk:67.253.78.55|talk]]) 00:25, 9 July 2020 (UTC) |
||
: No medical questions here. <span style="font-family: Cambria;">[[User:Abductive|<span style="color: teal;">'''Abductive'''</span>]] ([[User talk:Abductive|reasoning]])</span> 01:59, 9 July 2020 (UTC) |
|||
: Seattle Children's Hospital says "Scars should be carefully protected from the sun for at least 1 year after surgery or injury. Sun exposure can darken scars permanently, making them more noticeable. After about 2 weeks of healing, you can start applying sunscreen over your child's scar. Apply sunscreen in every season, not just in the summer."[https://www.seattlechildrens.org/pdf/PE2043.pdf] --[[User:Guy Macon|Guy Macon]] ([[User talk:Guy Macon|talk]]) 01:25, 9 July 2020 (UTC) |
|||
Revision as of 01:59, 9 July 2020
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
July 2
Rechargeable lithium batteries: recharge-discharge-recharge-discharge-recharge-overheat-explode!
This question is mostly directed at our two resident experts on electrical engineering: Guy Macon and Nimur. Though of course, if others can contribute an answer, go for it!
See the story here. Apparently, the tenant, when evicted from the building, left some rechargeable torches (presumably with Lithium-ion or Lithium-polymer batteries) plugged in. After a few weeks, where the batteries were left to discharge and recharge continuously, a battery overheated and exploded - and the entire building went up in flames!
My question is this: When designing a product with rechargeable Lithium batteries (be it a torch, a cellphone, a notebook computer, etc), WHAT, exactly, should the electrical engineer do to stop exactly this from happening?
Obviously, ideally, a product with a lithium battery should never be left plugged in for more than a day or so (or whatever). But if YOU were the one designing the product, what are the measures that can be taken to mitigate or eliminate this particular scenario from occurring? Eliyohub (talk) 08:26, 2 July 2020 (UTC)
- Lithium batteries almost always have a charge controller built in. this will stop excessive current flowing in or out, over charging, and also stop discharge when the voltage level is too low. I don't know if they measure temperature. but presumably the battery developed an internal fault. Once it shorts internally the controller cannot do anything to stop that. Graeme Bartlett (talk) 11:35, 2 July 2020 (UTC)
- Temperature is commonly measured but not universally. Also there have been some very crappy lithium-ion cylindrical cell (18650 etc) chargers which are known to do some very silly things. Likewise hobbyist chargers often also allow a wide selection of settings meaning it's easy for someone careless to screw up charging. But anyway, the above source suggests it was batteries inside a device. Most of these tend to at least cut off charging, although whether they still do a proper CC-CV charging cycle or instead do things incorrectly may vary, and in addition their protection circuitry may be limited to preventing over charging and over discharging, with no real health monitoring. A bigger concern may be that the cheaper ones from generic brands out of China may use cylindrical cells of dubious repute, e.g. second hand or that weren't good enough for even a crappy laptop battery pack. But also since this was a torch, those can be treated poorly leading to damage. While it's probably not as bad as the RC/model vehicle situation where the batteries can get really dinged up so that potentially great care needs to be taken when charging them, it's still likely to be risky than a phone or even a laptop. BTW I'd note that source doesn't support Eliyohub's claim about the incident. There's no mention of a few weeks or charging and discharging continuously. Instead it simply says they were left charging overnight. I had a quick look but didn't find any sources suggesting they were charged for weeks. Nil Einne (talk) 16:47, 2 July 2020 (UTC)
- Let me just circle back to the original question:
- "WHAT, exactly, should the electrical engineer do to stop exactly this from happening"?
- Well, this is a loaded question. Which electrical engineer?
- And it's a doubly-loaded question: this is a question of safety. I am categorically not going to provide specific safety advice that meets your engineering safety requirements. I will provide some general, broad, non-specific guidance to help orient you in a direction that I believe is generally correct, and refer you to resources that I know and use; but the safety of your engineering project is a complicated and multi-faceted ask: I simply can't "sign off" on any method that "will work" for you. The core design for safety in an engineering product has ethical and legal consequences: the reference desk ain't the place for that kind of thing.
- Okay - fine-print out of the way...
- In a consumer product, a different engineer(-ing team) designs the battery, the power supply, and the "load". (In power engineering, everything else is a "load" - electrical load - whether that load is a CPU, a display, a radio, a motor, a complex system-on-chip, a fully-integrated device... )
- So, all of these teams need to work together cohesively to define the parameters that enable safe operation.
- I am a firm believer that the core of battery safety-engineering starts at the battery design itself: the cell designer is the primary responsible party for ensuring that runaway thermal events don't ever occur. The cell design team is only one sub-part of the entire battery engineering team; they're the ones who engineer the chemical and mechanical packing of the individual cells; they are part of the battery team who design the "pack" (or its equivalent). Once again, the pack should be mechanically and electrically designed to preclude thermal runaway events.
- A lot of the really really serious engineering work is in correctly specifying, and qualifying that specification is being met in mass-production. A well-designed battery should not undergo a thermal runaway event even if it is subjected to an abuse load. This thesis of mine - which is really a statement of political nature, not of engineering merit - ultimately means that the electrical engineering teams who design the power-supply and the load need to do nothing except to verify the specification is met.
- Now there are diverse opinions on this topic. The existence of a thing called a "battery management system" is the manifestation of the exact counterpoint to my personal opinion: others believe that the circuit designer should actively control and protect the battery. As far as it pertains to safety, I respectfully disagree: the use of a BMS to ensure safety is the shirking of responsibility, or the proverbial leaking of abstraction, of this task into the power-supply and ultimately into the "intelligent load" (e.g. the software or firmware of the device that's consuming energy). Broadly speaking, the objective for moving this kind of safety-logic out of cell and pack design, and into circuit- and software-, is motivated by a desire to make the cell and pack more energy-dense: more volt-amps per cubic meter, more volt-amps per kilogram. It is my opinion that this motivation yields a cell that is less safe; and that some engineers justify this by claiming that they have made a whole system that is equally safe. This is one element of the "political nature" of the problem: it really depends on how your engineering team delegates responsibility among its sub-teams. And, it is my opinion that a whole system cannot be made more safe than its individual sub-components: that's just not how I understand the concept of "safety." But... at least I am humble enough to admit that my opinion is a personal interpretation of a general trend in the industry, and not a fact in its own right. You may inform your own opinions by comparing factual data or case-studies on various failures, if you read far and wide, broad and deep, in the literature in this field.
- Now, whether an "intelligent" power-system is safety-critical or not, putting "intelligence" into the power system is a hot topic among power designers. Here are a few white-papers on it:
- ... and sure, you can find the same kind of white-paper at the website of your favorite power-supply vendor. This is a broad topic: but the general trend over the last, uh, ...decades, has been that the power supply should use "intelligence" (complicated digital circuitry and software) to protect the load from the supply, and to protect the supply from the load. This is of critical design importance when primary energy is wholly- or partially- sourced by a volatile electrochemical cell.
- The place to go is APEC (Applied Power Electronics Conference), where you can meet the people and teams who do this stuff.
- Ultimately, a safe product is one whose design is suitable for its actual use: that means taking into account normal and abnormal envelope of operational and environmental factors, plus reliability and robustness to a reasonable expectation of wear-and-tear as well as damage. There isn't one specific circuit or one specific software control algorithm, and there isn't even one particular cell mechanical structure electrochemistry recipe that makes the product safe. It's all of these things together. If you're an electrical engineer who cares: hang out at APEC and IEEE and other organizations; read their books and journals:
- IEEE Power and Energy Society publications
- APEC's list of useful publications
- EE Times is great light reading; and there are a lot more heavy-hitting books and journals listed there. Those are the places where you can see current case-studies for designs (like specific circuit topologies and specific part numbers, if you're a power EE; or specific methodologies for firmware and software designers if you're a "load" engineer).
- For example, just this week we had Buck-Boost Devices Extend Battery Life...; and while you're over there, you can read the two-part Perspectives piece on battery electric vehicles from last week and this week: Part 1 and Part 2, by Egil Juliussen, who is a very experienced engineer but is considerably more optimistic than I am.
- The OP asked:
- "what are the measures that can be taken to mitigate or eliminate this particular scenario"?
- So let me just close by hammering it in: safe design is the whole-system. Your product can not be made safe by adding the juice from a single circuit-topology, a specific magic part-number, a particular control-algorithm. Your product is made safe by robust, broad, deep, engineering design, validation, test, and verification.
- Nimur (talk) 18:04, 2 July 2020 (UTC)
July 3
Is it possible for human to have two foreskins (one on another)?
In case there are two penes, it's s called Diphallia. But my question is about foreskin if it's possible to be doubled? (not talking about the physiological double layer which everyone has, but about an anomaly. If it exists, what's its name? --ThePupil (talk) 00:52, 3 July 2020 (UTC)
- Apparently males in the genera Equus and Bos normally have a double foreskin.[1] There it is not an anomaly. I did not see evidence of the condition having been observed in other mammal genera. If it is, a possible choice of a medical Latin name for the anomaly will be diacrobystiosis.
- Thank you, but I'm interested to know about human, if it's possible to happen (with documentation). --ThePupil (talk) 09:55, 3 July 2020 (UTC)
- Pretty much any deformity is possible. You have already been told that there is no evidence of this in humans or any other mammal outside of the two mentioned above. Please accept the answers that are given to you. --Guy Macon (talk) 13:59, 3 July 2020 (UTC)
- I see, thank you.--ThePupil (talk) 14:27, 3 July 2020 (UTC)
- Pretty much any deformity is possible. You have already been told that there is no evidence of this in humans or any other mammal outside of the two mentioned above. Please accept the answers that are given to you. --Guy Macon (talk) 13:59, 3 July 2020 (UTC)
- @ThePupil: I somewhat doubt everyone has a foreskin, whether doubled or not...
 --CiaPan (talk) 15:02, 3 July 2020 (UTC)
--CiaPan (talk) 15:02, 3 July 2020 (UTC)
- Body modifications like circumcision are one thing, but, as with the penis and clitoris themselves, while it's not called "foreskin" the clitoral hood is essentially the same thing. --47.146.63.87 (talk) 16:39, 3 July 2020 (UTC)
- exactly! but very rarely people are born without foreskin. See Aposthia. --ThePupil (talk) 19:29, 3 July 2020 (UTC)
- Body modifications like circumcision are one thing, but, as with the penis and clitoris themselves, while it's not called "foreskin" the clitoral hood is essentially the same thing. --47.146.63.87 (talk) 16:39, 3 July 2020 (UTC)
Mistake in article
In the table: "cost per year: 2019"59.153.241.128 (talk) 05:31, 3 July 2020 (UTC)
- It was added on April 2,[2] and I'm not so sure it was a mistake, just peculiar wording. ←Baseball Bugs What's up, Doc? carrots→ 06:30, 3 July 2020 (UTC)
- Looks like an error in Template:Infobox rocket. The parameters cpl and cpl-year are documented as meaning "cost per launch" and "year of stated cost per launch" respectively, but they display as two rows in a table, labeled Cost per launch (in this case, "$2 million (anticipated)") and Cost per year (in this case "2019"). If it's really worthwhile having an entry for the date of this particular data item, then it obviously should display in the same row, which would appear as something like "Cost per launch: $2 million (anticipated) (2019 data)". I'll post a note on the talk page for that template. --76.71.5.208 (talk) 06:42, 3 July 2020 (UTC)
July 4
Tea bitterness
When you leave tea leaves in tea, tea quickly gets more bitter. I presume that's because of tannins from tea getting released into the water. However, if you remove the leaves and leave the tea alone for several hours, it also will have become quite a bit bitterer even though the source of the tannins has been taken out. How does this happen? — Preceding unsigned comment added by 93.136.4.100 (talk) 00:53, 4 July 2020 (UTC)
- Tannase. Abductive (reasoning) 07:47, 4 July 2020 (UTC)
- Care to elaborate? 93.136.4.100 (talk) 22:11, 4 July 2020 (UTC)
- ...and is left as an exercise for the reader... Abductive (reasoning) 22:39, 4 July 2020 (UTC)
- Care to elaborate? 93.136.4.100 (talk) 22:11, 4 July 2020 (UTC)
A hosepipe floating in water
Hi. For the purposes of a fictional world I want a situation where a typical hosepipe (like you'd use to water your garden with) drops into a large flowing body of water (river?) and is carried along. It struck me that this possibly isn't realistic because the pipe is hollow and it might immediately fill with water and sink. How can I make this work? If the pipe was jammed up with (say) dead leaves at both ends, would that allow it to float, since there would be inaccessible air in the middle? Or how else could I swing it? Thanks. Equinox ◑ 04:23, 4 July 2020 (UTC)
- It already works. Try throwing a garden hose in a swimming pool or bathtub. It will be very difficult trying to get the air out. You pretty much have to make every part of it "downhill" in the same direction. --Guy Macon (talk) 06:31, 4 July 2020 (UTC)
- Aren't any hosepipes made from a rubber, plastic, or other material that floats on water? If this isn't the case in the real world, could you not think of some justification for such a hosepipe in your fictional setting? {The poster formerly known as 87.81.230.195} 2.122.56.20 (talk) 15:31, 4 July 2020 (UTC)
- Just wet the end of the pipe and the palm of your hand (ensure a nice clean cut at the end of the hose), but it still wont solve the problem. It must be rigid, particularly at the length. ~ R.T.G 21:24, 4 July 2020 (UTC)
Pumping water upwards
Hi. Me and my fictional world again. Suppose we have got an old-skool hand pump for water (as described at hand pump, maybe the suction kind, or anything that will take up water from a nearby source underground). And suppose further that we attach a hosepipe to the pump, so that pumped water goes through the pipe. And that pipe is carried up to a vertical height, and fixed there. It's two floors/storeys of a house. Will this "work" in terms of pumping water up there by hand, using the hand-pump lever? Or are there reasons (maybe related to pressure, or the general weight of the water) that will prevent this pumping from happening? I would like the "pumper" to be able to get several litres up to that height. Thanks. Equinox ◑ 04:26, 4 July 2020 (UTC)
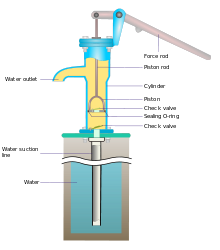
A hand pump - Typical hand pumps pump very well. The only problem is that they are slow. No matter how good the pump, you can only lift water about 10 meters if the pump is at the top. Any higher that that and you end up creating a vacuum. :In real life, the hose collapses long before that. Pumping from the bottom, no problem. at 10 meters you will only be adding 1 atmosphere to the hose. The normal range of water pressure in a house is between 2.5 and 5 atmospheres. --Guy Macon (talk) 06:44, 4 July 2020 (UTC)
- First, "the hose collapses" only if it is on the intake side of the pump. If the intake is a rigid pipe, how close to the 10-meter limit you can come depends on how good the pump is. Second, since you are designing a fictional world, you can adjust the surface gravity and the atmospheric pressure to be what you like. The limit of 10 meters for pumping from above is correct for Earth. --76.71.5.208 (talk) 09:02, 4 July 2020 (UTC)
- The person asking the original question specified a "hosepipe", which is UK for the US "garden hose". --Guy Macon (talk) 14:21, 4 July 2020 (UTC)
- It's still on the outlet not the inlet, so collapsing isn't the problem. DuncanHill (talk) 14:26, 4 July 2020 (UTC)
- I was careful to specify "if the pump is at the top". Please read the comments you are replying to before you start feverishly typing to tell experienced hydraulics engineers that they are wrong. --Guy Macon (talk) 16:47, 4 July 2020 (UTC)
- The OP clearly had the hosepipe going up from the pump. Try reading the question before feverishly trying to show off. DuncanHill (talk) 19:17, 4 July 2020 (UTC)
- ...Which is why I was careful to specify "pumping from the bottom" and "if the pump is at the top" in my answer, thus covering the configuration in the original question and the other commonly-used configuration. --Guy Macon (talk) 22:38, 4 July 2020 (UTC)
- The OP clearly had the hosepipe going up from the pump. Try reading the question before feverishly trying to show off. DuncanHill (talk) 19:17, 4 July 2020 (UTC)
- I was careful to specify "if the pump is at the top". Please read the comments you are replying to before you start feverishly typing to tell experienced hydraulics engineers that they are wrong. --Guy Macon (talk) 16:47, 4 July 2020 (UTC)
- It's still on the outlet not the inlet, so collapsing isn't the problem. DuncanHill (talk) 14:26, 4 July 2020 (UTC)
- The person asking the original question specified a "hosepipe", which is UK for the US "garden hose". --Guy Macon (talk) 14:21, 4 July 2020 (UTC)
- First, "the hose collapses" only if it is on the intake side of the pump. If the intake is a rigid pipe, how close to the 10-meter limit you can come depends on how good the pump is. Second, since you are designing a fictional world, you can adjust the surface gravity and the atmospheric pressure to be what you like. The limit of 10 meters for pumping from above is correct for Earth. --76.71.5.208 (talk) 09:02, 4 July 2020 (UTC)
- You need some additional force. If the pump normally pumps the water 3 metres up and you want to pump it an additional 6 metres, you need 3 times the force to operate the handle of the pump. That shouldn't be a problem usually. Then there is the question of the seal. In the pump in the picture, the water outlet is below the level where the piston rod enters the body of the pump. This means that a proper seal between the piston rod and the cap at the top of the pump is not required. When you add a hose to the water outlet to bring the water further up, it may leak out of the top of the pump. PiusImpavidus (talk) 08:55, 4 July 2020 (UTC)
- This sounds immediately wrong to my ears - to raise water higher, you need more energy - and you don't necessarily need more force to get more energy. You can also use a longer lever; or you can use the same lever and same force applied for a longer amount of time; and so on. You could apply more force, but if your hand-pump works in the "conventional" way, by drawing a piston, more force won't do very much of anything helpful: what you'd actually want to do is to to pump more cycles using the same force. Review our basic physics definitions at force, potential energy, ... and so on.
- If this were just a pedantic nitpick, I and all other physicists could let it slide... but it's not pedantic - it's a core concept of physics that relates applied force to the work done. There are a dozen variations on the basic formulae and you can find them in a good introductory book like Giancoli's Physics.
- Nimur (talk) 16:12, 4 July 2020 (UTC)
- This is one of those areas where practical engineering takes the results from physics and runs with it. A typical hand pump is basically a hydraulic cylinder and some valves, and we know a lot about designing hydraulic cylinders. Make the diameter the size of a soda straw (remember to subtract the area used up by the rod) and a baby can lift a very tall column of water with one hand. Make it the size of a 55 gallon drum and you will have to be The Incredible Hulk to operate it. And that's assuming a fairly short lever for the handle. With many pumps you move the handle a lot to get the cylinder to move a little, making it even easier to pump (but sacrificing how much you can pump in a given amount of time) --Guy Macon (talk) 16:41, 4 July 2020 (UTC)
- You don't have to tell me about physics. I'm a physicist too. Maybe you were thinking about a different kind of pump than the one I put in the picture. A bucket on a rope maybe? (BTW, to nitpick, if you apply the same force for a longer amount of time, you get more impulse, not necessarily more energy).
- If you attach a hose to the output side of an existing pump to move the water higher, you need more pressure. Using a particular piston, this means more force on the piston. With a particular lever to move that piston, that means more force to move that lever. Sure, there are alternative ways to do this. You could move the water up one floor at a time, temporarily storing it in a bucket and moving the pump up each time. That means more pumping cycles at the same force (it would be easier to carry the filled bucked up the stairs). Or you could replace the pump with a different one, or add additional levers or pulleys to operate it. But the question was about taking a pump and adding a hose to it to pump the water higher than what would normally be the case, using the lever of the pump. That means more pressure and for a positive displacement pump (like all handpumps I've ever seen) that means more force to operate it.
- Or if you want to talk about energy, to move the water higher, you need more energy, so that's more force times distance. With our pre-existing pump, the distance we move the handle is directly proportional to the volume of water we move. To move it higher, we have to increase the force. PiusImpavidus (talk) 11:24, 5 July 2020 (UTC)
Pascal's principle relates the hydrostatic pressure to fluid density and height of the column of water. In english units the rule-of-thumb is 1 psi for every two feet of lift. For your piston pump you should be able to easily calculate the force on the handle from the surface area of the piston, the ambient pressure at the outlet, and difference in height between the outlet and top of the piston. On the intake side there are theoretical and practical limits to the height of the pump above the water source. If you are also concerned with the rate water can be pumped, then messy hydrodynamics and friction loss come into play. fiveby(zero) 13:17, 4 July 2020 (UTC)
- These are quite popular where the water table isn't too deep: [ https://www.youtube.com/watch?&v=rLRm0D6RmEU ]. I have seen them fitted with a hose on the output pumping to an elevated tank so the user gets gravity feed instead of having to pump every time they need water. --Guy Macon (talk) 14:36, 4 July 2020 (UTC)
- Guy is correct about the vacuum. A hose is designed for water to be pressured into it, not sucked out. But I think you will find it will not suck up through the hose at all. First you'd have to fill the hose with water, just to have a chance to suck anything through it at all, and even then it might start to flatten immediately when you try. Think about it this way, you will not be pushing water into the bottom of the pipe to fill it with water, you'll be sucking air from the top of it, and the air will suck out faster than the water will suck in. It will take less force to flatten the pipe slightly, than to suck water without any real pressure on the walls of the pipe at all. Each suck of the pump will flatten it slightly more, with exponential effect, until you reach a tipping point and it just flattens without drawing water at all. You might get somewhere with PEX pipe, which is flexible, but much more rigid than a hosepipe, but similar in price range and versatility, but
ideallyyou are looking at slightly more expensive HDPE pipe or PVC. The rigid plastics are not going to be unreachably more expensive at 10 meters, just somewhat more. Of course, some hosepipes will be relatively rigid and reinforced, and may work for a while, but over 10 meters with such force through a small hole to fill a big pump... you are definitely going to flatten the hosepipe. Also, PEX is pretty tough, but if you are talking about a pump which sucks through a much wider hole than the pipe, you might need a wider pipe or a truly rigid one over time. ~ R.T.G 20:11, 4 July 2020 (UTC)- Edit: Ideally you are looking at a length of pressure hose, more expensive than garden hose and plumbing pipes but not astronomical. I can't remember the exact pricing but I believe you'd get ten meters under 100 euros (might need to shop around a little) ~ R.T.G 20:16, 4 July 2020 (UTC)
- Of course, looking at the question you posted above as well, you may think that filling the hose with water then putting your hand over one end will solve the problem with flattening. It won't. It will just slow it down a little. Each time the pump sucks water out of the hose, it does not suck against the hole in the water at the bottom. It sucks against every part of the inside of the hose equally. Every molecule which comes through the hole at the bottom does so by tugging on the inside walls of the hose. It will flatten. Only a rigid pipe can suffice for you, sorry. ~ R.T.G 21:17, 4 July 2020 (UTC)
- Edit: Ideally you are looking at a length of pressure hose, more expensive than garden hose and plumbing pipes but not astronomical. I can't remember the exact pricing but I believe you'd get ten meters under 100 euros (might need to shop around a little) ~ R.T.G 20:16, 4 July 2020 (UTC)
- Guy is correct about the vacuum. A hose is designed for water to be pressured into it, not sucked out. But I think you will find it will not suck up through the hose at all. First you'd have to fill the hose with water, just to have a chance to suck anything through it at all, and even then it might start to flatten immediately when you try. Think about it this way, you will not be pushing water into the bottom of the pipe to fill it with water, you'll be sucking air from the top of it, and the air will suck out faster than the water will suck in. It will take less force to flatten the pipe slightly, than to suck water without any real pressure on the walls of the pipe at all. Each suck of the pump will flatten it slightly more, with exponential effect, until you reach a tipping point and it just flattens without drawing water at all. You might get somewhere with PEX pipe, which is flexible, but much more rigid than a hosepipe, but similar in price range and versatility, but
Global warming potential
Hello, please help me understand something about climate science.
Not all greenhouse gases are the same. The difference is measured as global warming potential. Carbon dioxide has a GWP of 1 as the basic unit of measurement. The gases also dissipate at different rates, so timeframe is relevant. In short, CO2 has a GWP of 1 over 100 years. Methane has a GWP of roughly (average estimates) 30 over 100 years. Nitrous oxide has a GWP of roughly 300 over 100 years. This means that these gases cause 30x and 300x the greenhouse effect (over a 100 year period).
And so, according to sources Wikipedia is using, in 2018, 72% of greenhouse emissions was CO2, 19% was methane, and 6% was nitrous oxide. When I multiply these figures by their GWP I get CO" - 72%, methane - 570%, and nitrous - 1800%. To compare these as a multiple of CO2 in particular, rather than a portion of the total, we can divide by 72 and multiply by 100. This gives methane at 790x and nitrous at 2500x the amount of CO2 global warming output in 2018...
Yet, when reporting the effect of methane and nitrous, sources generally say the effects of these gasses are far lower than CO2, like under 15% combined. I haven't found a source saying "Here is why methane and nitrous have a massively huge GWP but do not cause more global warming than CO2." Nor have I found a source which says, "Nirtous oxide is causing thousands of times more global warming than co2, and methane 800 times" How can that make sense?
For perspective, apparently most methane and nitrous are released through animal farming industries. The Humane Society of the USA has an interest in reducing animal agriculture, so they are going to be biased as much as they can towards negativity for methane and nitrous, or at least, they are not going to be biased in the other direction. Yet, according to the HSUS[3], animal industry accounts for less than one fifth. The wildest claim I can find is from the Daily Mail, which claims that replacing all animal industries would account for a 50% reduction (not enough to be worth it, of course, according to the ever popular Daily Mail).
So why is my direct application of GWP absolutely nothing like the figures the others turn out? What else is there in the calculation? ~ R.T.G 18:37, 4 July 2020 (UTC)
- There's a problem with your calculation. What it should give is 7.9x for methane and 25x for N2O. However I'm finding much smaller values for N2O concentration: [4]. Methane is similarly less than 1/200 as abundant as CO2 [5]. Where did you get the 72-19-6% numbers? 93.136.4.100 (talk) 18:53, 4 July 2020 (UTC)
- @93.136.4.100:Our global warming article references that to the IPCC as far as I recall... it is reliably sourced, there is no doubt, various figures are similar. As for the multiplication figure, what you have is after 500 years for methane, but for nitrous, after 500 years the GWP is still 150x. So I take it the 15% figures represent the total of the industrial era since the 1850s, in leave of the introduction and sustained increase of methane and nitrous production since the 1950s? ~ R.T.G 19:27, 4 July 2020 (UTC)
- It is referenced to a couple of books, 19% and 6%, but I have seen across various sources at similar or matching rates. ~ R.T.G 19:31, 4 July 2020 (UTC)
- I think the main issue here is the differing concentrations. GWP is the power per molecule. The concentrations and yearly emissions methane and N20 are significantly lower than CO2, see f.i.: https://www.epa.gov/ghgemissions/overview-greenhouse-gases. So even if they are stronger per molecule, they have an overall smaller effect. Femke Nijsse (talk) 19:52, 4 July 2020 (UTC)
- Also note, from the EPA source; Methane's lifetime in the atmosphere is much shorter than carbon dioxide [but it] is more efficient at trapping radiation. Think methane deteriorates leaving carbon dioxide, so in the longer run its pace of absorbing radiation steps down but keeps going at the carbon dioxide rate. . . dave souza, talk 20:28, 4 July 2020 (UTC)
- Sadly, if you account for GWP, the emissions of methane and nitrous are much greater, more than 34x the effect according to the figures in 2018, unless there is some further metric... Yes, Dave, see global warming potential. +If you look at the global warming article... the second image is a graph of temperature observations, which rises sharply in the 1950s, alarmingly more significant than the trends relevant to industrial advancement... but correlative to when methane and nitrous emissions became significant... ~ R.T.G 21:10, 4 July 2020 (UTC)
- I've found the source of the 72-19-6-3% distribution claim [6]. Unfortunately the table heading says "share gas in GHG" which is useless by itself as it doesn't say what has been divided by what. Looking at methane, N2O and flouride emission graphs on pp.22-24 we get figures of rougly 9.8, 2.7 and 1.7 Gt CO2 equivalent. The total in Gt CO2 equivalent in the graph on p.15 adds up to roughly 51 Gt. Taking percentages we get 19%, 5% and 3%, and the remainder is 73% for CO2. I think it's then reasonable to conclude that the emission numbers you gave have already been adjusted for GWP. 93.136.4.100 (talk) 22:11, 4 July 2020 (UTC)
- Aha! The IPCC full reports do indeed note when they are using co2 equivalents[7], while groups involved with education and awareness (Wikipedia for instance), generally do not. It's so much easier when you know straight out, what you are looking for. And so much more difficult, when you receive a skewed end unnecessarily. All the same, denying that continuing 1950s spike is like denying global warming itself. And that's kind of funny.. ~ R.T.G 00:08, 5 July 2020 (UTC)
- Thank you, IP person. o/ ~ R.T.G 00:10, 5 July 2020 (UTC)
- Aha! The IPCC full reports do indeed note when they are using co2 equivalents[7], while groups involved with education and awareness (Wikipedia for instance), generally do not. It's so much easier when you know straight out, what you are looking for. And so much more difficult, when you receive a skewed end unnecessarily. All the same, denying that continuing 1950s spike is like denying global warming itself. And that's kind of funny.. ~ R.T.G 00:08, 5 July 2020 (UTC)
- I've found the source of the 72-19-6-3% distribution claim [6]. Unfortunately the table heading says "share gas in GHG" which is useless by itself as it doesn't say what has been divided by what. Looking at methane, N2O and flouride emission graphs on pp.22-24 we get figures of rougly 9.8, 2.7 and 1.7 Gt CO2 equivalent. The total in Gt CO2 equivalent in the graph on p.15 adds up to roughly 51 Gt. Taking percentages we get 19%, 5% and 3%, and the remainder is 73% for CO2. I think it's then reasonable to conclude that the emission numbers you gave have already been adjusted for GWP. 93.136.4.100 (talk) 22:11, 4 July 2020 (UTC)
- Sadly, if you account for GWP, the emissions of methane and nitrous are much greater, more than 34x the effect according to the figures in 2018, unless there is some further metric... Yes, Dave, see global warming potential. +If you look at the global warming article... the second image is a graph of temperature observations, which rises sharply in the 1950s, alarmingly more significant than the trends relevant to industrial advancement... but correlative to when methane and nitrous emissions became significant... ~ R.T.G 21:10, 4 July 2020 (UTC)
- It is referenced to a couple of books, 19% and 6%, but I have seen across various sources at similar or matching rates. ~ R.T.G 19:31, 4 July 2020 (UTC)
- @93.136.4.100:Our global warming article references that to the IPCC as far as I recall... it is reliably sourced, there is no doubt, various figures are similar. As for the multiplication figure, what you have is after 500 years for methane, but for nitrous, after 500 years the GWP is still 150x. So I take it the 15% figures represent the total of the industrial era since the 1850s, in leave of the introduction and sustained increase of methane and nitrous production since the 1950s? ~ R.T.G 19:27, 4 July 2020 (UTC)
Is there still an open question, or can this be closed? NewsAndEventsGuy (talk) 21:50, 4 July 2020 (UTC)
Oxygen saturation in blood
If each haemoglobin may bind 4 oxygen molecules at the most, how could it be that oxygen saturation in the blood can be measured in values of 78% 92% etc. while considering on the fact that the haemoglobin can bind 4 molecules of O2, it should be measured in one of these four values only: 25% (1 molecule), 50% (2 molecules), 75% (3 molecules) and 100% 4 molecules. So where the other numbers may come from? (I thought maybe it's a combination of two parameters: 1. how many haemoglobins bind to oxygen. 2. how many molecules of O2 in each haemoglobin (not all haemoglobin have the same number of bound O2 molecules. Some may have more and some less, and the saturation is the mean and combination of all of these. Am I right?). --ThePupil (talk) 18:53, 4 July 2020 (UTC)
- How would you indicate blood where 99% of the haemoglobin has 4 oxygen molecules and 1% has 0? --Guy Macon (talk) 19:13, 4 July 2020 (UTC)
- Maybe by average as I said (in this case it's 99% saturation). Isn't it? --ThePupil (talk) 19:27, 4 July 2020 (UTC)
- Although Guy Macon answer is probably good enough, did you read Oxygen saturation (medicine)? It seems to describe the situation well enough. Nil Einne (talk) 05:28, 5 July 2020 (UTC)
- Did you not notice I mentioned this article in my question?:) --ThePupil (talk) 11:43, 5 July 2020 (UTC)
- I don't see where you mentioned the article. You mentioned the oxygen saturation article which is a different article. Also, a lot of people mention link to articles but either don't seem to have read them or don't seem to have understood them. It seems possible one of these apply here since as I said, the article seems to deal with the situation raised in your initial question. Indeed your clarification seems to be a further example of why it's uncertain. The medicine article is linked in the general article, but you linked to the general article even though it barely discusses the specifics of your question. Nil Einne (talk) 13:02, 5 July 2020 (UTC)
- Well, I see what happened. Before I asked my question, I read the same article your linked to, but what happened, probably when I linked it, I linked to something different with a similar name according to the suggestion of Wikipedia algorithms (What's funny, I've just opened what I linked and it was strange to me since I didn't read it at all, but only the one you linked to, and since I didn't find an answer I came to ask here my question). --ThePupil (talk) 13:59, 5 July 2020 (UTC)
- I don't see where you mentioned the article. You mentioned the oxygen saturation article which is a different article. Also, a lot of people mention link to articles but either don't seem to have read them or don't seem to have understood them. It seems possible one of these apply here since as I said, the article seems to deal with the situation raised in your initial question. Indeed your clarification seems to be a further example of why it's uncertain. The medicine article is linked in the general article, but you linked to the general article even though it barely discusses the specifics of your question. Nil Einne (talk) 13:02, 5 July 2020 (UTC)
- Did you not notice I mentioned this article in my question?:) --ThePupil (talk) 11:43, 5 July 2020 (UTC)
- Although Guy Macon answer is probably good enough, did you read Oxygen saturation (medicine)? It seems to describe the situation well enough. Nil Einne (talk) 05:28, 5 July 2020 (UTC)
- Maybe by average as I said (in this case it's 99% saturation). Isn't it? --ThePupil (talk) 19:27, 4 July 2020 (UTC)
Can hemoglobin be empty of oxygen?
Usually, each haemoglobin can be up to four O2 molecules. Can haemoglobin be empty of oxygen? or it must carry at least one molecule of oxygen? --ThePupil (talk) 18:55, 4 July 2020 (UTC)
- Yes, it can be empty. Dragons flight (talk) 00:30, 5 July 2020 (UTC)
- Thank you! --ThePupil (talk) 02:33, 5 July 2020 (UTC)
- Or indeed, it can carry other things, though it's not a good thing when that happens (carbon monoxide, cyanide etc). Fgf10 (talk) 10:55, 5 July 2020 (UTC)
- Thank you for your important note.--ThePupil (talk) 11:44, 5 July 2020 (UTC)
July 5
Organ harvesting
Over at our Falun Gong article, there is an editor who made the following claim:
- "We know that this group has been subject to forced organ harvesting, which in the decision of the China Tribunal on Forced Organ Harvesting, has been taking place against a large number of member of this group for a substantial period of time."[8]
There is an (obviously unreliable) Falun Gong source that makes the same claim: [ https://faluninfo.net/forced-organ-harvesting-in-china-falun-gong/ ]
And it has made it into these Wikipedia articles:
- Organ transplantation in China#Allegations of organ harvesting from Falun Gong practitioners
- Persecution of Falun Gong#Organ harvesting
- Organ harvesting from Falun Gong practitioners in China
- Falun Gong#Organ harvesting
There are some obvious problems with these claims, such as using [ http://organharvestinvestigation.net/] and The Weekly Standard as sources, but is there any credible evidence that these accusations against the Chinese government are true? --Guy Macon (talk) 11:11, 5 July 2020 (UTC)
- From Reuters: China is harvesting organs from Falun Gong members, finds expert panel.
- From The Daily Telegraph: British government 'ignored' Chinese organ harvesting, Tribunal rules.
- From The Guardian: China is harvesting organs from detainees, tribunal concludes.
- All of the above are quoting the China Tribunal (article needs some work), which is chaired by Sir Geoffrey Nice QC and advised by Martin Elliott (surgeon) who sound like reliable chaps. Alansplodge (talk) 19:07, 5 July 2020 (UTC)
- And the British Medical Journal: China’s forced organ harvesting constitutes crimes against humanity, informal London tribunal finds (July 2019) and Chinese doctors admitted in undercover calls that harvested organs were available, informal tribunal finds (March 2020). Alansplodge (talk) 19:09, 5 July 2020 (UTC)
- Utility monster in practice... 93.136.52.139 (talk) 00:31, 6 July 2020 (UTC)
- I don't see that this is a case of a utility monster. The Chinese state is not justifying this practice on the grounds that favored citizens get more utility from the organs than disfavored one. Insofar as they are justifying it at all in a utilitarian sense, it is that the state gets more utility from compliant or otherwise favored citizens, than it does from ones it considers criminals. Of course, that depends on accepting the state definition of who is a criminal vs a valuable citizen. Mostly the state does not appear to justify this at all, it simply does it. DES (talk)DESiegel Contribs 20:38, 6 July 2020 (UTC)
- Utility monster in practice... 93.136.52.139 (talk) 00:31, 6 July 2020 (UTC)
- And the British Medical Journal: China’s forced organ harvesting constitutes crimes against humanity, informal London tribunal finds (July 2019) and Chinese doctors admitted in undercover calls that harvested organs were available, informal tribunal finds (March 2020). Alansplodge (talk) 19:09, 5 July 2020 (UTC)
Hue ring
In a RGB color picker, hue can be displayed on a color ring: R, R+G, G, G+B, B, R+B. This corresponds to colors of the rainbow: red, yellow, green, aqua, blue, purple. However the transition from R+B to R is also smooth, despite connnecting the top and bottom of the visible spectrum. How come? 31.45.224.2 (talk) 18:30, 5 July 2020 (UTC)
- The answer is already hidden in your question: you describe purple as R+B, which is essentially how the cone cells in the human eye work; they detect optical stimuli that (roughly) correspond to red, green and blue – not the actual wavelength of the light. This is also why you can see the full rainbow on your display, even though no purple or yellow light at all actually reaches your eyes. In an actual rainbow, purple is of course not created additively, it is the real thing. Cheers ⌘ hugarheimur 18:52, 5 July 2020 (UTC)
- I guess that means that our RGB screens and CMYK printouts don't really resemble reality to other lifeforms. Interesting, seems that we've adapted to perceive a linear combination of R and G or G and B as interim colors on the spectrum, but R and B without green in-between produce a sort of impossible color... 95.168.120.46 (talk) 20:11, 5 July 2020 (UTC)
- I think it's even worse than that. How do you define real colors without reference to the observer? How can I know that your brain processes the stimulus of a particular color of light the same way mine does? We could both state that something was red but still be experiencing red differently.--Khajidha (talk) 22:29, 5 July 2020 (UTC)
- Indeed: see Qualia. {The poster formerly known as 87.81.230.195} 2.122.56.20 (talk) —Preceding undated comment added 10:17, 6 July 2020 (UTC)
- Many people in my experience seem ok with the idea that "some people experience cilantro tasting like soap" but have trouble believing "some people see red as grey". Rmhermen (talk) 18:28, 6 July 2020 (UTC)
- Indeed: see Qualia. {The poster formerly known as 87.81.230.195} 2.122.56.20 (talk) —Preceding undated comment added 10:17, 6 July 2020 (UTC)
- I think it's even worse than that. How do you define real colors without reference to the observer? How can I know that your brain processes the stimulus of a particular color of light the same way mine does? We could both state that something was red but still be experiencing red differently.--Khajidha (talk) 22:29, 5 July 2020 (UTC)
- I guess that means that our RGB screens and CMYK printouts don't really resemble reality to other lifeforms. Interesting, seems that we've adapted to perceive a linear combination of R and G or G and B as interim colors on the spectrum, but R and B without green in-between produce a sort of impossible color... 95.168.120.46 (talk) 20:11, 5 July 2020 (UTC)
July 6
What's the relationship bewtween cyanosis and reality?
I have read the article cyanosis but still don't understand the way we see a bluish person while he doesn't have any kind of blue substant. The colour of unoxygenated blood isn't blue. (See here). It's written in the article: "The blood reaching the extremities is not oxygen-rich and when viewed through the skin a combination of factors can lead to the appearance of a blue color". What are these factors? --ThePupil (talk) 01:51, 6 July 2020 (UTC)
- Skin absorbs red light more than blue. --Khajidha (talk) 02:16, 6 July 2020 (UTC)
- Is that another way of saying that skin is blue? -- ToE 11:58, 6 July 2020 (UTC)
- Did Hemoglobin#Deoxygenated_hemoglobin (linked to from cyanosis) fail to answer your question? --Guy Macon (talk) 12:48, 6 July 2020 (UTC)
- No. See https://www.livescience.com/32212-if-blood-is-red-why-are-veins-blue.html --Khajidha (talk) 14:13, 6 July 2020 (UTC)
- Also, see the above discussion about color and perception. Color doesn't really exist in the world, it is created in your perceptions. What exists outside of you is simply electromagnetic waves. --Khajidha (talk) 14:42, 6 July 2020 (UTC)
- Is that another way of saying that skin is blue? -- ToE 11:58, 6 July 2020 (UTC)
- Why do veins appear blue? A new look at an old question[ResearchGate], Lothar Lilge, et al., Applied Optics, March 1996. -- ToE 21:47, 6 July 2020 (UTC)
- There's a relationship between veins appearance to the skin appearance in case of cyanosis? (cyanosis isn't limited to the veins but it seems on the skin. Also veins in healthy people looks bluish while cyanosis is a pathological sign) --ThePupil (talk) 00:01, 7 July 2020 (UTC)
- As an aside it is interesting just how small a range of the Electromagnetic spectrum is visible to the human eye - to me anyway. MarnetteD|Talk 00:06, 7 July 2020 (UTC)
- There's a relationship between veins appearance to the skin appearance in case of cyanosis? (cyanosis isn't limited to the veins but it seems on the skin. Also veins in healthy people looks bluish while cyanosis is a pathological sign) --ThePupil (talk) 00:01, 7 July 2020 (UTC)
Wearing masks and CO2
Does wearing a mask for a while capture CO2 in the mask? After a while of wearing a mask, I start to feel like I'm not getting enough oxygen. Bubba73 You talkin' to me? 03:36, 6 July 2020 (UTC)
- There's nothing to capture any appreciable amount of CO2. See here, for example. --174.89.49.204 (talk) 04:06, 6 July 2020 (UTC)
- What's more likely happening is that moisture in your exhaled breath is condensing in the mask, reducing the amount of air a normal breath delivers to your lungs. This moisture can also create a lovely breeding ground for all sorts of nasties.HiLo48 (talk) 04:17, 6 July 2020 (UTC)
- At most, that would be normal in terms of effort (feels harder to take in the breath). But the same size breath is still the same amount of air. The humidity might be higher as well. It feels to me more like breathing on a hot humid day because, well, I'm breathing through a warm humidifier. There's a TikTok or similar video circulating of someone using a pulse oximeter to demonstrate that maskless and a variety of mask types do not alter her 99% reading. DMacks (talk) 05:49, 6 July 2020 (UTC)
- It's pretty obvious that any mask retains a small amount of warm air that is heavy in CO2 and moisture, but the volume of air under the mask is small compared to the volume of your lungs. You are already re-breathing CO2 and moisture from your windpipe and from the air sacks not emptying completely. None of this is at all dangerous. The only effect is to make wearing a mask is slightly annoying. (editorializing) You know what else is annoying? Having grandma or some fellow who recently experienced cardiac arrest die because you weren't willing to put up with a slight annoyance. --Guy Macon (talk) 12:26, 6 July 2020 (UTC)
- At most, that would be normal in terms of effort (feels harder to take in the breath). But the same size breath is still the same amount of air. The humidity might be higher as well. It feels to me more like breathing on a hot humid day because, well, I'm breathing through a warm humidifier. There's a TikTok or similar video circulating of someone using a pulse oximeter to demonstrate that maskless and a variety of mask types do not alter her 99% reading. DMacks (talk) 05:49, 6 July 2020 (UTC)
- What's more likely happening is that moisture in your exhaled breath is condensing in the mask, reducing the amount of air a normal breath delivers to your lungs. This moisture can also create a lovely breeding ground for all sorts of nasties.HiLo48 (talk) 04:17, 6 July 2020 (UTC)
- 'The WHO says: "The prolonged use of medical masks when properly worn, does not cause CO2 intoxication nor oxygen deficiency. While wearing a medical mask, make sure it fits properly and that it is tight enough to allow you to breathe normally. Do not re-use a disposable mask and always change it as soon as it gets damp."' From: BBC - Coronavirus: 'Deadly masks' claims debunked. Alansplodge (talk) 15:57, 7 July 2020 (UTC)
Thanks. Bubba73 You talkin' to me? 02:26, 8 July 2020 (UTC)
Flu & Covid
How can someone get the flu if they have been following all of the covid guidelines? Facemask, hand washing etc. Is it the case that they could just as easily have contracted Covid in this situation but they were "lucky" enough to just get Influenza, or are these two viruses dissimilar and contracted through different methods? Thanks — Preceding unsigned comment added by 86.162.76.127 (talk) 10:07, 6 July 2020 (UTC)
- The two are not exactly the same, so one may be more infectious or able to survive outside the body better, but in general they spread the same way, so the same methods - masks, handwashing, not touching the eyes or mouth, cleaning surfaces, social distancing, isolating yourself when you are sick -- will reduce both kinds of infections.
--Guy Macon (talk) 12:38, 6 July 2020 (UTC)
- Also, the only thing that 100% prevents you from contracting any virus is to not encounter it. Precautions reduce risk, but do not absolutely prevent infection. The hypothetical person could have contracted the flu, covid, a cold, or any of a number of other respiratory viruses despite precautions. They just happened to encounter and contract the flu. --Khajidha (talk) 14:22, 6 July 2020 (UTC)
- Pedantry: if a virus can't infect humans you of course can't get infected with it. You've got tons of bacteriophages in your gut and on your skin/other "outside" surfaces, but they're not a problem for you; in fact they're often beneficial by helping to keep bacterial populations in check. --47.146.63.87 (talk) 19:42, 6 July 2020 (UTC)
- Unless it mutates to become infectious to humans. At which point things can get really ugly. See current events.--Khajidha (talk) 12:56, 7 July 2020 (UTC)
- Yes, viruses can mutate to infect new hosts. These are usually similar species; zoonotic diseases that have "jumped" to humans have generally done so from other mammals or birds. A bacterial virus is very unlikely to "jump" to eukaryotes. --47.146.63.87 (talk) 16:14, 7 July 2020 (UTC)
- Unless it mutates to become infectious to humans. At which point things can get really ugly. See current events.--Khajidha (talk) 12:56, 7 July 2020 (UTC)
- Pedantry: if a virus can't infect humans you of course can't get infected with it. You've got tons of bacteriophages in your gut and on your skin/other "outside" surfaces, but they're not a problem for you; in fact they're often beneficial by helping to keep bacterial populations in check. --47.146.63.87 (talk) 19:42, 6 July 2020 (UTC)
Are there non-human mammals where the typical female does not have two X chromosomes?
I know birds and some other non-mammals use a system where the female has two unalike chromosomes (ZW vs ZZ). Are there non-human mammals where the typical female (barring intersex conditions, etc) has something other than XX chromosomes? I ask because XY sex-determination system says (emphasis added) "The XY sex-determination system is [...] found in humans, most other mammals, [...] In humans, most mammals, and some other species, two of the chromosomes, called the X chromosome and Y chromosome, code for sex." Male says: "Most male mammals, including male humans, have a Y chromosome". Female until recently said "most female mammals, including female humans, have two X chromosomes", but the lead (but not body) was changed to drop "most", and I'm trying to figure out if that change was correct or not. The fact that none of these articles cites a source for its statements on this matter is less than helpful. -sche (talk) 17:02, 6 July 2020 (UTC)
- Some human females are XO. Or XXX. Or XY with non-functional SR-Y alleles. I assume the same conditions occur in other mammals. And monotremes get REALLY weird. --Khajidha (talk) 18:20, 6 July 2020 (UTC)
- Echoing the previous reply. XY sex-determination system § Other animals: Monotremes have all kinds of wild stuff going on, because they're believed to be the most basal living mammals. Therians are more like what we're "familiar" with because we are therians, although at a quick glance I can't find an explicit statement that all marsupials use the XY system. --47.146.63.87 (talk) 19:37, 6 July 2020 (UTC)
- Thank you both! :) (I now notice that the body of the Female article indeed mentions platypuses; I didn't spot it / recognize it earlier because it doesn't actually say what they use instead, which is apparently a system of ten sex chromosomes.) -sche (talk) 08:06, 7 July 2020 (UTC)
worldwide lockdowns MAP
I can´t find worlwide map of countries who applied lockdowns and where not. it was avalaible a few weeks ago. ¿Where is it? — Preceding unsigned comment added by 190.163.188.62 (talk) 18:10, 6 July 2020 (UTC)
- See Category:World maps about the COVID-19 pandemic. Probably one of those. If not, it's been deleted. Alansplodge (talk) 15:50, 7 July 2020 (UTC)
Is there any successful treatment and/or cure for presbyesophagus for people aged 110+?
If a person aged 110+ has presbyesophagus, is there any successful treatment and/or cure for this for this person? I am especially thinking of cases where presbyesophagus threatens this person's life by preventing them from getting adequate nutrition and nourishment. Futurist110 (talk) 20:23, 6 July 2020 (UTC)
- If you want general info about the topic we have an article on Presbyphagia, but it really does look like you are looking for medical advice. Here is my medical advice: Accompany the person as the see an M.D. and ask her what can be done. --Guy Macon (talk) 22:15, 6 July 2020 (UTC)
- The person in question was Walter Breuning, who died several years ago, age 114. ←Baseball Bugs What's up, Doc? carrots→ 00:59, 7 July 2020 (UTC)
- I see no evidence that Walter Breuning had presbyesophagus, much less died of it. He died in a hospital, so I assume that they did what they could to keep him alive. --Guy Macon (talk) 04:13, 7 July 2020 (UTC)
- See Futurist110's thread in the Humanities page. ←Baseball Bugs What's up, Doc? carrots→ 06:46, 7 July 2020 (UTC)
- I see no evidence that Walter Breuning had presbyesophagus, much less died of it. He died in a hospital, so I assume that they did what they could to keep him alive. --Guy Macon (talk) 04:13, 7 July 2020 (UTC)
- The person in question was Walter Breuning, who died several years ago, age 114. ←Baseball Bugs What's up, Doc? carrots→ 00:59, 7 July 2020 (UTC)
It would have been nice to have been alerted to the previous discussions.
- Wikipedia:Reference desk/Humanities#If someone has esophagus problems and they proceed to die from starvation, is the likely explanation for their death that they starved themselves on purpose?
- Wikipedia:Reference desk/Humanities#Is Walter Breuning's death record available on Ancestry.com?
I'm just saying. --Guy Macon (talk) 14:43, 7 July 2020 (UTC)
- Well, now you know. Anyway, after doing some research and discussions on this topic, one doesn't actually have to die from presbyesophagus, correct? Rather, one can be kept alive by being fed through a feeding tube, no? If so, this raises the question of why this was not done in Walter Breuning's case. Did Walter Breuning make a conscious decision to die as opposed to continue living but be fed through a feeding tube? Futurist110 (talk) 16:06, 7 July 2020 (UTC)
- This is what can happen when you spread a discussion across 2 or more ref desk pages. As to your speculative questions, since his cause of death was not publicly stated, how likely is it that such details would be? ←Baseball Bugs What's up, Doc? carrots→ 21:01, 7 July 2020 (UTC)
July 7
Magnetic field intensity: measured in amperes per meter or ampere-turns per meter?
The first reference in the flyback transformer article says the the SI unit for magnetic field intensity is ampere-turns per meter, but the Wikipedia article on magnetic fields says it is amperes per meter. Is the former in error? ZFT (talk) 04:55, 7 July 2020 (UTC)
- Neither is in error, because neither says that the International System (SI) unit of field intensity for magnetic fields is anything other than Tesla (T).
- A search of the first reference in the flyback transformer article did not find the word "meter"
- Our article on Magnetic field says:
- "In the International System of Units, H, magnetic field strength, is measured in the SI base units of ampere per meter (A/m).[1] B, magnetic flux density, is measured in tesla (in SI base units: kilogram per second2 per ampere),[2] which is equivalent to newton per meter per ampere. H and B differ in how they account for magnetization. In a vacuum, B/ and H are the same; but in a magnetized material, B/ and H differ by the magnetization M of the material at that point in the material."
References
- ^ Le Système international d’unités [The International System of Units] (PDF) (in French and English) (9th ed.), International Bureau of Weights and Measures, 2019, ISBN 978-92-822-2272-0, p. 22
- ^ Le Système international d’unités [The International System of Units] (PDF) (in French and English) (9th ed.), International Bureau of Weights and Measures, 2019, ISBN 978-92-822-2272-0, p. 21
- I mean the first article (Magnetics Design Handbook, Section 1) that is in the "References" section. ZFT (talk) 16:32, 7 July 2020 (UTC)
- That page says:
- "Old-time magnetics designers in the U.S. are acclimated to the CGS system, and may prefer magnetics data expressed in Gauss and Oersteds. But newcomers to magnetics design, as well as experienced designers outside the U.S. prefer the internationally accepted SI system - Tesla and Ampere-Turns."
- The page makes no mention of ampere-turns per meter. That being said, Dixon was not being precise in his language. Ampere-turns do not convert to Gauss.
- So:
- Tesla / Gauss measure magnetic flux density,
- Ampere-turn / Gilbert measure magnetomotive force, and
- Ampere per meter / Oersted measure magnetization.
- To confuse things further, there are informal terms. Ampere-turn should actually be Ampere (See Magnetomotive force#Units) but nobody calls it that for obvious reasons. --Guy Macon (talk) 22:37, 7 July 2020 (UTC)
- That page says:
- Turns is dimensionless and not considered to be a unit, and so ampere-turns and amperes are equivalent as far as SI is concerned. catslash (talk) 22:52, 7 July 2020 (UTC). Not quite the same, but Ohms per square is considered to be the same as Ohms. On the other hand radians are SI units despite being dimensionless, whereas apparently whole cycles are not a unit. catslash (talk) 23:42, 7 July 2020 (UTC)
- In Table 1 the SI unit for field intensity is A-T/m. Does the "T" stand for "tesla"? ZFT (talk) 18:34, 8 July 2020 (UTC)
Vent shaft construction method
In this vent shaft build (https://www.youtube.com/watch?v=8xu8d7POPZ4), how is the ground supported as excavation takes place and how is the concrete poured? I don’t see any shuttering. Clover345 (talk) 09:09, 7 July 2020 (UTC)
- Clover345, that link is giving me a "404 not found". {The poster formerly known as 87.81.230.195} 2.122.56.20 (talk) 13:37, 7 July 2020 (UTC)
- Oops, Wikipedia doesn’t let me post you tube links but it should come up if you copy and paste 8xu8d7POPZ4 into the you tube search bar. Clover345 (talk) 16:59, 7 July 2020 (UTC)
- Try Timelapse - Fisher Street shaft completion. It's from the Crossrail project in London. Alansplodge (talk) 18:10, 7 July 2020 (UTC)
- Oops, Wikipedia doesn’t let me post you tube links but it should come up if you copy and paste 8xu8d7POPZ4 into the you tube search bar. Clover345 (talk) 16:59, 7 July 2020 (UTC)
Cluster containment methodology
How does the cluster containment contact tracing which Japan describes here [9] work? What’s different from the prospective contact tracing also described in the presentation? Clover345 (talk) 10:19, 7 July 2020 (UTC)
Guidance on using a headphone port as line out
I'm aware that there can be a difference between the two: line out ports are low power and expect and infinite load. But, is there any practical issue with using a headphone port as line out? In particular, if a headphone port's all that's available, which components, if any, risk damage?--Leon (talk) 19:58, 7 July 2020 (UTC)
- [ https://www.sweetwater.com/insync/headphone-outputs-used-line-outputs-for-line-level-gear/ ] --Guy Macon (talk) 20:56, 7 July 2020 (UTC)
- Okay, thanks!--Leon (talk) 15:29, 8 July 2020 (UTC)
July 8
The colour of the sea
I was told the colour of the sea (blue/green) 'is not really' colourful, and the reason we see it colourful it's because of the light waves. Then my question why these light waves don't work when we take from the same water a glass of water and hold it in our hands? --ThePupil (talk) 00:08, 8 July 2020 (UTC)
- https://www.scientificamerican.com/article/why-does-the-ocean-appear/
- A single glass does not contain enough water to absorb very much light. --Khajidha (talk) 02:14, 8 July 2020 (UTC)
- Quite so. Consider as a parallel, ThePupil, that an empty, clear glass (or plastic) container also does not appear blue, even though the sky (i.e. the atmosphere en masse) does.[ citation not required ] {The poster formerly known as 87.81.230.195} 2.122.56.20 (talk) 12:11, 8 July 2020 (UTC)
- That is a bit misleading though. Water is blue(-ish) because it absorbs non-blue wavelengths more for large enough bodies of water. The sky is blue, not because the atmosphere absorbs blue less than other wavelengths (or at least not in the most part), but because of Rayleigh scattering. See Diffuse sky radiation. TigraanClick here to contact me 15:53, 8 July 2020 (UTC)
Death of conjoined twins: does the second twin always simultaneously die, after the first twin dies?
I was reading about the recent case of conjoined twins, Ronnie and Donnie Galyon. This made me wonder: when you have conjoined twins, if one twin dies, is it also the case that the second twin "must" die, too? (At the same time, I mean.) Is it "theoretically" possible to separate previously "unseparatable" twins, when one dies? Seem like stupid questions, but I have no idea. Also, I guess that I am referring to conjoined twins that actually live for a bit ... not ones who die at birth or shortly thereafter. Thanks. Joseph A. Spadaro (talk) 03:36, 8 July 2020 (UTC)
- Have you read Conjoined twins#Management? {The poster formerly known as 87.81.230.195} 2.122.56.20 (talk) 12:17, 8 July 2020 (UTC)
- Thanks. Yes, I had already read that prior to posting my question. I don't see that it answers my questions. Am I missing something in that link? Thanks. Joseph A. Spadaro (talk) 16:11, 8 July 2020 (UTC)
- Does this article answer your questions?[10] --Guy Macon (talk) 16:21, 8 July 2020 (UTC)
- Ah, extremely interesting! Thanks! I am going to read that now! Thank you. Joseph A. Spadaro (talk) 20:46, 8 July 2020 (UTC)
Can a genuine FFP2 or KN95 respirator have earloops instead of headbands?
Thanks. Apokrif (talk) 11:43, 8 July 2020 (UTC)
- As a person who has worn and been fit-tested for N95 respirators, if the tension in the earloops was strong enough to insure a tight fit, it would be too painful to wear. I don't know if there is a regulation that forbids earloops, but I can't imagine they would work. Jc3s5h (talk) 11:50, 8 July 2020 (UTC)
- The specifications specify things like filter performance, flow rate, and pressure drop. You can design a mask any way you want to as long as it meets the specs.[11]
- This data sheet shows a KN95 with ear loops.[12]
- The reddit thread should be ignored, and instead you should look at the source, which is here:[13] Note that NIOSH-42CFR84 only covers N95 masks. not FFP2 or KN95.
- Any counterfeiter worth his salt will make the counterfeit look like the real thing. That CDC page really tells you how to identify mislabeled masks --which are a much bigger problem -- not counterfeit masks. It takes skill to make a counterfeit. Any idiot can slap a KN95 label on a non-KN95 mask they bought somewhere. --Guy Macon (talk) 13:40, 8 July 2020 (UTC)
covid-19 and personal possessions.....
I remember, back in 2014, hearing that the 2 Nurses in Dallas (Nina Pham and Amber Vinson) who contracted Ebola....had almost all of their possessions destroyed by the CDC for fear they might be contaminated by the Ebola virus. Has anything similar happened to Covid-19 patients? 67.253.78.55 (talk) 20:29, 8 July 2020 (UTC)
- The viability of viral particles on surfaces differs greatly from virus to virus, just as whether a virus is best spread by body fluid contact or respiratory droplets. Comparisons between SARS-CoV-2 mitigation measures and those for Ebola virus or influenza virus may not be apt. --OuroborosCobra (talk) 21:13, 8 July 2020 (UTC)
- May be so, though I recall hearing a few months back that SARS-CoV-2 could persist for up to at least 17 days on some surfaces...My question, however, is have any of the 3 million Americans who have had laboratory been ordered or encouraged to throw away their belongings?67.253.78.55 (talk) 21:49, 8 July 2020 (UTC)
- That's still putting the cart before the horse. Is there any purpose at all in encouraging people to throw away their belongings? There was a lot of misreporting in the media early on about SARS-CoV-2 virus being detectable on surfaces days later, but these tests were not on viability. Detection of virus just means enough intact genetic material in the primer section used for RT-PCR existed for amplification and detection. It doesn't mean that there were intact viral particles viable for infection. There may not have been viral particles at all, just remaining genetic material. --OuroborosCobra (talk) 21:56, 8 July 2020 (UTC)
- Perhaps I have not made my point clear, I am not advocating that SARS-Cov-2 positive patients should be required to discard their possessions for fear of contamination, rather, I am simply asking if this is the policy of public health agencies across the country.In late January when it started to become clear to me that a pandemic was inevitable I moved my many of my possessions (e.g. collections of magazines, photo albums, treasured childhood toys,important documents, books, etc) into plastic tubs and then into the attic lest I contract the virus and be ordered by local public health authorities ( I reside in the USA) to discard all of these items....I am now wondering whether this is a legitimate concern or if I can move these items out of the attic without fear that if I contract the virus I will be compelled to dispose of them. My concern about this was heightened by the fatc that in January it was reported that the Chinese Government was destroying cash in an attempt to curb transmission. 67.253.78.55 (talk) 23:35, 8 July 2020 (UTC)
- ...All of which is stupid government overreaction. If you want to be 100% safe, quarantine the possessions for a week or two. You can speed this up with a UV disinfectant lamp. --Guy Macon (talk) 00:12, 9 July 2020 (UTC)
- No US government agency is advocating destroying property, much less mandating it. If they were stupid enough to try, the courts would shoot them down on constitutional grounds ("The right of the people to be secure in their persons, houses, papers, and effects, against unreasonable searches and seizures, shall not be violated") and lack of scientific evidence (see below). You can take your stuff out of the tubs.
- ...All of which is stupid government overreaction. If you want to be 100% safe, quarantine the possessions for a week or two. You can speed this up with a UV disinfectant lamp. --Guy Macon (talk) 00:12, 9 July 2020 (UTC)
- Perhaps I have not made my point clear, I am not advocating that SARS-Cov-2 positive patients should be required to discard their possessions for fear of contamination, rather, I am simply asking if this is the policy of public health agencies across the country.In late January when it started to become clear to me that a pandemic was inevitable I moved my many of my possessions (e.g. collections of magazines, photo albums, treasured childhood toys,important documents, books, etc) into plastic tubs and then into the attic lest I contract the virus and be ordered by local public health authorities ( I reside in the USA) to discard all of these items....I am now wondering whether this is a legitimate concern or if I can move these items out of the attic without fear that if I contract the virus I will be compelled to dispose of them. My concern about this was heightened by the fatc that in January it was reported that the Chinese Government was destroying cash in an attempt to curb transmission. 67.253.78.55 (talk) 23:35, 8 July 2020 (UTC)
- "The primary and most important mode of transmission for COVID-19 is through close contact from person-to-person. Based on data from lab studies on COVID-19 and what we know about similar respiratory diseases, it may be possible that a person can get COVID-19 by touching a surface or object that has the virus on it and then touching their own mouth, nose, or possibly their eyes, but this isn’t thought to be the main way the virus spreads." --CDC updates COVID-19 transmission webpage to clarify information about types of spread
- "Coronavirus can last for long durations on different metal surfaces, ranging from hours to days.13 , 14 Recent studies show that the coronavirus can last about three days on a plastic surface as well as on stainless steel surface, it can also sustain for a period of whole one day on cardboard, while it can only sustain only for about four hours on a copper surface." --Sustainability of Coronavirus on Different Surfaces
- "The virus that causes coronavirus disease 2019 (COVID-19) is stable for several hours to days in aerosols and on surfaces, according to a new study from NIH, CDC, UCLA and Princeton University scientists published in the New England Journal of Medicine. The scientists found that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was detectable in aerosols for up to 3 hours, up to 4 hours on copper, up to 24 hours on cardboard and up to 2 to 3 days on plastic and stainless steel. The results provide key information about the stability of SARS-CoV-2, which causes COVID-19 disease" --New Coronavirus Stable for Hours on Surfaces
--Guy Macon (talk) 00:08, 9 July 2020 (UTC)
July 9
Scar formation
I am wondering whether exposure of a wound to sunlight increases , decreases or has no effect on the development of a visible, permanent scar? 67.253.78.55 (talk) 00:25, 9 July 2020 (UTC)
- No medical questions here. Abductive (reasoning) 01:59, 9 July 2020 (UTC)


