Baculoviridae
This section may be too technical for most readers to understand. (August 2009) |
| Baculovirus | |
|---|---|
| Virus classification | |
| Group: | Group I (dsDNA)
|
| Family: | Baculoviridae
|
| Genera | |
|
Alphabaculovirus | |
The baculoviruses are a family of large rod-shaped viruses that can be divided to two genera: nucleopolyhedroviruses (NPV) and granuloviruses (GV). While GVs contain only one nucleocapsid per envelope, NPVs contain either single (SNPV) or multiple (MNPV) nucleocapsids per envelope. The enveloped virions are further occluded in granulin matrix in GVs and polyhedrin for NPVs. Moreover, GV have only single virion per granulin occlusion body while polyhedra contains multiple embedded virions. [1]
Baculoviruses have very species-specific tropisms among the invertebrates with over 600 host species having been described. Immature (larval) forms of moth species are the most common hosts, but these viruses have also been found infecting sawflies, mosquitoes, and shrimp. They are not known to replicate in mammalian or other vertebrate animal cells. Baculoviruses contain circular double-stranded genome ranging from 80-180 kbp.
Historical influence
The earliest records of Baculoviruses can be found in the literature from as early as the sixteenth century in reports of “wilting disease” infecting silk-producing larva. Starting in the 1940s they were used and studied widely as biopesticides in crop fields. Since the 1990s they have been utilized for producing complex eukaryotic proteins in insect cell cultures (see Sf21). These recombinant proteins have been used in research and as vaccines in both human and veterinary medical treatments (for example, the most widely used vaccine for prevention of H5N1 avian influenza in chickens was produced in a baculovirus expression vector). More recently it has been found that baculoviruses can transduce mammalian cells with a suitable promoter [2]. These medical and potential medical uses have accelerated the number of publications on baculoviruses since 1995.
Baculovirus life cycle

The baculovirus life cycle involves two distinct forms of virus. Occlusion derived virus (ODV) is present in a protein matrix (polyhedrin or granulin) and is responsible for the primary infection of the host while the budded virus (BV) is released from the infected host cells later during the secondary infection.
Typically, the initial infection occurs when a susceptible host insect feeds on plants that are contaminated with the occluded form of the virus. The protein matrix dissolves in the alkaline environment of the host midgut (stomach), releasing ODV that then fuse to the columnar epithelial cell membrane of the host intestine and are taken into the cell in endosomes. Nucleocapsids escape from the endosomes and are transported to nucleus. This step is possibly mediated by actin filaments. Viral transcription and replication occur in the cell nucleus and new BV particles are budded out from the basolateral side to spread the infection systemically. During budding, BV acquires loosely fitting host cell membrane with expressed and displayed viral glycoproteins.
Baculovirus infection can be divided to three distinct phases, early (0-6 h post-infection), late (6-24 h p.i.) and very late phase (18-24 to 72 h p.i.). While BV is produced in the late phase, the ODV form is produced in the very late phase acquiring the envelope from host cell nucleus and embedded in the matrix of occlusion body protein. These occlusion bodies are released when cells lyse to further spread baculovirus infection to next host. The extensive lysis of cells frequently causes the host insect to literally melt, thus the reason for the historic name "wilting disease." To adapt survival in the wild, ODV-polyhedrin particles are resistant to heat and light inactivation, whereas BV is more sensitive to environment.
Structure of the virion
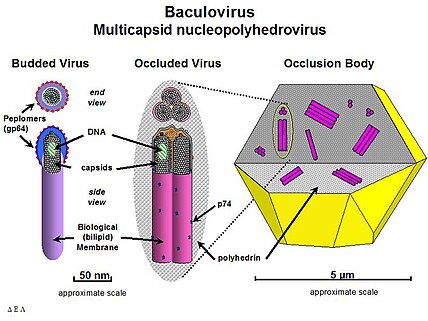
The most studied baculovirus is Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV). The virus was originally isolated from the alfalfa looper (a lepidopteran) and contains a 134-kbp genome with 154 open reading frames (ORF). The major capsid protein VP39 together with some minor proteins forms the nucleocapsid (21 nm x 260 nm) that encloses the DNA with p6.9 protein.
BV acquires its envelope from the cell membrane and requires a glycoprotein, gp64, to be able to spread systemic infection. This protein forms structures called peplomers on one end of the budded virus particle but is not found on ODV (although several other proteins are only associated with the ODV form). Some differences also exist in the lipid composition of the viral envelope of the two forms. While BV envelope consists of phosphatidylserine, ODV contains phosphatidylcholine and phosphatidylethanolamine.
Major envelope glycoprotein gp64
During the viral evolution, the baculoviral envelope glycoproteins have undergone changes. Ld130, also known as baculovirus F-protein from Lymantria dispar (LdMNPV) is suggested to be an ancestral envelope fusion protein which has been replaced by non-orthologous gene replacement with gp64 in AcMNPV, Bombyx mori (BmNPV) and Orgyia pseudotsugata (OpMNPV) while they still retain the ld130 gene.
Gp64 is a homotrimeric membrane glycoprotein which is polarly present on the rod-shaped virion. It consists of 512 amino acids (aa) with four glycosylation sites at asparagine residues and has N-terminal signal sequence (20 aa), oligomerization and fusion domains and hydrophobic transmembrane domain near the C-terminus (7 aa).
It is produced in both early and late phases of the infection cycle with a maximal rate of synthesis occurring in 24-26 h p.i.. Trimerization with intermolecular cysteine-bonds seems to be a crucial step for protein transport to cell surface, since only 33% of synthesized protein reaches cell surface as monomeric gp64 is degraded within the cells.
Gp64 is essential for efficient budding of the virion and for the cell-to-cell transmission during the infection cycle as well as binding to cell surface i.e. causing viral trophism and endosome mediated uptake to the cell. The major function of the gp64 is causing the pH-mediated envelope fusion to the endosome. Although gp64 has variety of essential functions, it has been reported that gp64-null baculoviruses can be substituted with other viral glycoproteins such as Ld130, G-protein of Vesicular stomatitis virus resulting functional virus.
Applications
Baculovirus expression in insect cells represents a robust method for producing recombinant glycoproteins.[3][4] Baculovirus-produced proteins are currently under study as therapeutic cancer vaccines with several immunologic advantages over proteins derived from mammalian sources.[5]
See also
References
This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. (February 2008) |
Granados, R.R. and Federici, B.A. (eds) (1986). The biology of baculoviruses. CRC Press, Boca Raton, Florida.
Miller, L.K. (ed) (1997). The Baculoviruses. Plenum Press. New York.
- ^ Rohrmann, G.F. (2008).Baculovirus Molecular Biology. Bethesda (MD): National Library of Medicine (US), NCBI; 2008; http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=bacvir
- ^ Lackner A, Genta K et al. A bicistronic baculovirus vector for transient and stable protein expression in mammalian cells. Anal Biochem. 2008 Sep 1;380(1):146-8. Epub 2008 May 22.
- ^ Altmann F, Staudacher E, Wilson IB, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconjugate J 1999;16(February(2)):109–23.
- ^ Kost TA, Condreay JP. Recombinant baculoviruses as expression vectors for insect and mammalian cells. Curr Opin Biotechnol 1999;10(October(5)):428–33.
- ^ Betting DJ, Mu XY, Kafi K, McDonnel D, Rosas F, Gold DP, Timmerman JM. Enhanced immune stimulation by a therapeutic lymphoma tumor antigen vaccine produced in insect cells involves mannose receptor targeting to antigen presenting cells. Vaccine. 2009 Jan 7;27(2):250-9. Epub 2008 Nov 8. PMID: 19000731
External links
Index of Viruses - Baculoviridae (2006). In: ICTVdB - The Universal Virus Database, version 4. Büchen-Osmond, C (Ed), Columbia University, New York, USA. http://www.ncbi.nlm.nih.gov/ICTVdb/Ictv/fs_index.htm
- http://www.blueskybiotech.com/ Blue Sky Biotech, Inc
Baculovirus Molecular Biology

