Iron
 | ||||||||||||||||||||||||||||||||||||||||||||||
| Iron | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈaɪərn/ | |||||||||||||||||||||||||||||||||||||||||||||
| Allotropes | see Allotropes of iron | |||||||||||||||||||||||||||||||||||||||||||||
| Appearance | lustrous metallic with a grayish tinge | |||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Fe) | ||||||||||||||||||||||||||||||||||||||||||||||
| Iron in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 26 | |||||||||||||||||||||||||||||||||||||||||||||
| Group | group 8 | |||||||||||||||||||||||||||||||||||||||||||||
| Period | period 4 | |||||||||||||||||||||||||||||||||||||||||||||
| Block | d-block | |||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d6 4s2 | |||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 14, 2 | |||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1811 K (1538 °C, 2800 °F) | |||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3134 K (2861 °C, 5182 °F) | |||||||||||||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 7.874 g/cm3 [3] | |||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 6.98 g/cm3 | |||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 13.81 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 340 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.10 J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | common: +2, +3 −4,? −2,[4] −1,[4] 0,? +1,[5] +4,[4] +5,[6] +6,[4] +7[7] | |||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.83 | |||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 126 pm | |||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | Low spin: 132±3 pm High spin: 152±6 pm | |||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 194 [1] pm | |||||||||||||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | α-Fe: body-centered cubic (bcc) (cI2) | |||||||||||||||||||||||||||||||||||||||||||||
| Lattice constant | a = 286.65 pm (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | γ-Fe (912–1394 °C): face-centered cubic (fcc) (cF4) | |||||||||||||||||||||||||||||||||||||||||||||
| Lattice constant | a = 364.68 pm (at 916 °C)[8] | |||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 12.07×10−6/K (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 80.4 W/(m⋅K) | |||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 96.1 nΩ⋅m (at 20 °C) | |||||||||||||||||||||||||||||||||||||||||||||
| Curie point | 1043 K | |||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | ferromagnetic | |||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 211 GPa | |||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 82 GPa | |||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 170 GPa | |||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 5120 m/s (at r.t.) (electrolytic) | |||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.29 | |||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 4 | |||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 608 MPa | |||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 200–1180 MPa | |||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7439-89-6 | |||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | before 5000 BC | |||||||||||||||||||||||||||||||||||||||||||||
| Symbol | "Fe": from Latin ferrum | |||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of iron | ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
Iron (Template:PronEng) is a metallic chemical element with the symbol Fe (Template:Lang-la) and atomic number 26. Iron is a group 8 and period 4 element and is therefore classified as a transition metal. Iron and iron alloys (steels) are by far the most common metals and the most common ferromagnetic materials in everyday use. Fresh iron surfaces are lustrous and silvery-grey in color, but oxidize in air to form a red or brown coating of ferric oxide or rust. Pure single crystals of iron are soft (softer than aluminium), and the addition of minute amounts of impurities, such as carbon, significantly strengthens them. Alloying iron with appropriate small amounts (up to a few per cent) of other metals and carbon produces steel, which can be 1,000 times harder than pure iron.
Iron-56 is the heaviest stable isotope produced by the alpha process in stellar nucleosynthesis; heavier elements than iron and nickel require a supernova for their formation. Iron is the most abundant element in the core of red giants, and is the most abundant metal in iron meteorites and in the dense metal cores of planets such as Earth.
Characteristics
Pure iron is a metal but is rarely found in this form on the surface of the earth because it oxidizes readily in the presence of oxygen and moisture. In order to obtain metallic iron, oxygen must be removed from naturally occurring ores by chemical reduction – mainly of the iron ore hematite (Fe
2O
3) by carbon at high temperature. The properties of iron can be modified by alloying it with various other metals (and some non-metals, notably carbon and silicon) to form steels.
Nuclei of iron atoms have some of the highest binding energies per nucleon, surpassed only by the nickel isotope 62Ni. The universally most abundant of the highly stable nuclides is, however, 56Fe. This is formed by nuclear fusion in stars. Although a further tiny energy gain could be extracted by synthesizing 62Ni, conditions in stars are unsuitable for this process to be favoured. Elemental distribution on Earth greatly favours iron over nickel, and also presumably in supernova element production.[10]
Iron (as Fe2+, ferrous ion) is a necessary trace element used by almost all living organisms. The only exceptions are several organisms that live in iron-poor environments and have evolved to use different elements in their metabolic processes, such as manganese instead of iron for catalysis, or hemocyanin instead of hemoglobin. Iron-containing enzymes, usually containing heme prosthetic groups, participate in catalysis of oxidation reactions in biology, and in transport of a number of soluble gases. See hemoglobin, cytochrome, and catalase.
Mechanical properties
| Material | TS (MPa) | BH (Brinell) |
|---|---|---|
| Iron whiskers | 11000 | |
| Ausformed (hardened) steel | 2930 | 850–1200 |
| Martensitic steel | 2070 | 600 |
| Bainitic steel | 1380 | 400 |
| Pearlitic steel | 1200 | 350 |
| Cold-worked iron | 690 | 200 |
| Small-grain iron | 340 | 100 |
| Iron containing dissolved carbon | 140 | 40 |
| Single crystal of pure iron | 10 | 3 |
Mechanical properties of iron and its alloys are evaluated using a variety of tests, such as the Brinell test, Rockwell test, or tensile strength tests, among others; the results are so consistent that tests of iron are often used to relate the results of one test to another.[12][13] Those measurements reveal that mechanical properties of iron crucially depend on purity: Purest research-purpose single crystals of iron are softer than aluminium. Addition of only 10 parts per million of carbon doubles their strength.[11] The hardness increases rapidly with carbon content up to 0.2% and saturates at ~0.6%.[14] The purest industrially produced iron (about 99.99% purity) has a hardness of 20–30 Brinell.[15]
Allotropes
Iron represents perhaps the best-known example of allotropy in a metal. There are three allotropic forms of iron, known as α, γ and δ.
As molten iron cools down it crystallizes at 1538 °C into its δ allotrope, which has a body-centred cubic (bcc) crystal structure. As it cools further its crystal structure changes to face-centred cubic (fcc) at 1394 °C, when it is known as γ-iron, or austenite. At 912 °C the crystal structure again becomes bcc as α-iron, or ferrite, is formed, and at 770 °C (the Curie point, Tc) the iron becomes magnetic. As the iron passes through the Curie temperature there is no change in crystalline structure, but there is a change in "domain structure", where each domain contains iron atoms with a particular electronic spin. In unmagnetized iron, all the electronic spins of the atoms within one domain are in the same direction; however, in neighbouring domains they point in various directions and thus cancel out. In magnetized iron, the electronic spins of all the domains are all aligned, so that the magnetic effects of neighbouring domains reinforce each other. Although each domain contains billions of atoms, they are very small, about 10 microns across.
Iron is of greatest importance when mixed with certain other metals and with carbon to form steels. There are many types of steels, all with different properties; and an understanding of the properties of the allotropes of iron is key to the manufacture of good quality steels.
Alpha iron, also known as ferrite, is the most stable form of iron at normal temperatures. It is a fairly soft metal that can dissolve only a small concentration of carbon (no more than 0.021% by mass at 910 °C).[16]
Above 912 °C and up to 1400 °C α-iron undergoes a phase transition from body-centred cubic to the face-centred cubic configuration of γ-iron, also called austenite. This is similarly soft and metallic but can dissolve considerably more carbon (as much as 2.04% by mass at 1146 °C). This form of iron is used in the type of stainless steel used for making cutlery, and hospital and food-service equipment.
Occurrence

Iron is the sixth most abundant element in the Universe, formed as the final act of nucleosynthesis, by silicon fusing in massive stars. While it makes up about 5% of the Earth's crust, the Earth's core is believed to consist largely of an iron-nickel alloy constituting 35% of the mass of the Earth as a whole. Iron is consequently the most abundant element on Earth, but only the fourth most abundant element in the Earth's crust.[17] Most of the iron in the crust is found combined with oxygen as iron oxide minerals such as hematite and magnetite.
About 1 in 20 meteorites consist of the unique iron-nickel minerals taenite (35–80% iron) and kamacite (90–95% iron). Although rare, iron meteorites are the major form of natural metallic iron on the Earth's surface.
The red color of the surface of Mars is thought to derive from an iron oxide-rich regolith.
Isotopes
Naturally occurring iron consists of four isotopes: 5.845% of radioactive 54Fe (half-life: >3.1×1022 years), 91.754% of stable 56Fe, 2.119% of stable 57Fe and 0.282% of stable 58Fe. 60Fe is an extinct radionuclide of long half-life (2.6 million years).
Much of the past work on measuring the isotopic composition of Fe has centred on determining 60Fe variations due to processes accompanying nucleosynthesis (i.e., meteorite studies) and ore formation. In the last decade however, advances in mass spectrometry technology have allowed the detection and quantification of minute, naturally occurring variations in the ratios of the stable isotopes of iron. Much of this work has been driven by the Earth and planetary science communities, although applications to biological and industrial systems are beginning to emerge.[18]
The most abundant iron isotope 56Fe is of particular interest to nuclear scientists. A common misconception is that this isotope represents the most stable nucleus possible, and that it thus would be impossible to perform fission or fusion on 56Fe and still liberate energy. This is not true, as both 62Ni and 58Fe are more stable, being the most stable nuclei. However, since 56Ni is much more easily produced from lighter nuclei in the alpha process in nuclear reactions in supernovae (see silicon burning process), nickel-56 (14 alpha particles) is the endpoint of fusion chains inside extremely massive stars, since addition of another alpha would result in zinc-60, which requires a great deal more energy. This nickel-56, which has a half-life of about 6 days, is therefore made in quantity in these stars, but soon decays by two successive positron emissions within supernova decay products in the supernova remnant gas cloud, to first radioactive cobalt-56, and then stable iron-56. This last nuclide is therefore common in the universe, relative to other stable metals of approximately the same atomic weight.
In phases of the meteorites Semarkona and Chervony Kut a correlation between the concentration of 60Ni, the daughter product of 60Fe, and the abundance of the stable iron isotopes could be found which is evidence for the existence of 60Fe at the time of formation of the solar system. Possibly the energy released by the decay of 60Fe contributed, together with the energy released by decay of the radionuclide 26Al, to the remelting and differentiation of asteroids after their formation 4.6 billion years ago. The abundance of 60Ni present in extraterrestrial material may also provide further insight into the origin of the solar system and its early history. Of the stable isotopes, only 57Fe has a nuclear spin (−1/2).
Chemistry and compounds
Iron forms compounds mainly in the +2 and +3 oxidation states. Traditionally, iron(II) compounds have been called ferrous, and iron(III) compounds ferric. There are many compounds in each of the oxidation states; representative examples would include iron(II) sulfate (FeSO4) and iron(III) chloride (FeCl3). There are also numerous examples of compounds that contain iron atoms in both of these oxidation states, such as magnetite and prussian blue. The ferrate anion [FeO
4]2−
contains an iron(VI) centre, its highest known oxidation state, and is present, for example in potassium ferrate (K2FeO4). There are numerous organometallic compounds (such as iron pentacarbonyl) that contain formally zerovalent (or lower) iron.
History


The first wrought iron used by mankind during prehistory came from meteors. The smelting of iron in bloomeries began in the second millennium BC. Artifacts from smelted iron occur in India from 1800–1200 BC,[19] and in the Levant from about 1500 BC (suggesting smelting in Anatolia or the Caucasus).[20][21]
Cast iron was first produced in China about 550 BC,[22] but not in Europe until the medieval period[citation needed]. During the medieval period, means were found in Europe of producing wrought iron from cast iron (in this context known as pig iron) using finery forges. For all these processes, charcoal was required as fuel.
Steel (with smaller carbon content than pig iron but more than wrought iron) was first produced in antiquity. New methods of producing it by carburizing bars of iron in the cementation process were devised in the 17th century AD. In the Industrial Revolution, new methods of producing bar iron without charcoal were devised and these were later applied to produce steel. In the late 1850s, Henry Bessemer invented a new steelmaking process, involving blowing air through molten pig iron, to produce mild steel. This and other 19th century and later processes have led to wrought iron no longer being produced.
Industrial production

The production of iron or steel is a process unless the desired final product is cast iron. The first stage is to produce pig iron in a blast furnace. The second is to make wrought iron or steel from pig iron by a further process.
Blast furnace
Ninety percent of all mining of metallic ores is for the extraction of iron. Industrially, iron is produced starting from iron ores, principally hematite (nominally Fe2O3) and magnetite (Fe3O4) by a carbothermic reaction (reduction with carbon) in a blast furnace at temperatures of about 2000 °C. In a blast furnace, iron ore, carbon in the form of coke, and a flux such as limestone (which is used to remove impurities in the ore which would otherwise clog the furnace with solid material) are fed into the top of the furnace, while a blast of heated air is forced into the furnace at the bottom.

In the furnace, the coke reacts with oxygen in the air blast to produce carbon monoxide:
- 2 C + O2 → 2 CO
The carbon monoxide reduces the iron ore (in the chemical equation below, hematite) to molten iron, becoming carbon dioxide in the process:
- 3 CO + Fe2O3 → 2 Fe + 3 CO2
The flux is present to melt impurities in the ore, principally silicon dioxide sand and other silicates. Common fluxes include limestone (principally calcium carbonate) and dolomite (calcium-magnesium carbonate). Other fluxes may be used depending on the impurities that need to be removed from the ore. In the heat of the furnace the limestone flux decomposes to calcium oxide (quicklime):
- CaCO3 → CaO + CO2
Then calcium oxide combines with silicon dioxide to form a slag.
- CaO + SiO2 → CaSiO3
The slag melts in the heat of the furnace. In the bottom of the furnace, the molten slag floats on top of the denser molten iron, and apertures in the side of the furnace are opened to run off the iron and the slag separately. The iron once cooled, is called pig iron, while the slag can be used as a material in road construction or to improve mineral-poor soils for agriculture.[citation needed]


In 2005, approximately 1,544 million metric tons of iron ore were produced worldwide. China was the top producer of iron ore with at least one-fourth world share followed by Brazil, Australia and India, reports the British Geological Survey.
Further processes
Pig iron is not pure iron, but has 4–5% carbon dissolved in it with small amounts of other impurities like sulfur, magnesium, phosphorus and manganese. As the carbon is the major impurity, the iron (pig iron) becomes brittle and hard. This form of iron is used to cast articles in foundries such as stoves, pipes, radiators, lamp-posts and rails.
Alternatively pig iron may be made into steel (with up to about 2% carbon) or wrought iron (commercially pure iron). Various processes have been used for this, including finery forges, puddling furnaces, Bessemer converters, open hearth furnaces, basic oxygen furnaces, and electric arc furnaces. In all cases, the objective is to oxidize some or all of the carbon, together with other impurities. On the other hand, other metals may be added to make alloy steels.
The hardness of the steel depends upon its carbon content, the higher the proportion of carbon, the greater the hardness and the lesser the ductility. The properties of the steel can also be changed by tempering it. To harden the steel, it is heated to red hot and then cooled by quenching it in the water. It becomes harder and more brittle. This steel is then heated to a required temperature and allowed to cool. The steel thus formed is less brittle.
Applications
Elemental iron
Iron is the most widely used of all the metals, accounting for 95% of worldwide metal production. Its low cost and high strength make it indispensable in engineering applications such as the construction of machinery and machine tools, automobiles, the hulls of large ships, and structural components for buildings. Since pure iron is quite soft, it is most commonly used in the form of steel. Some of the forms in which iron is produced commercially include:
- Pig iron has 3.5–4.5% carbon[23] and contains varying amounts of contaminants such as sulfur, silicon and phosphorus. Its only significance is that of an intermediate step on the way from iron ore to cast iron and steel.
- Cast iron contains 2–4% carbon, 1–6% silicon, and small amounts of manganese. Contaminants present in pig iron that negatively affect material properties, such as sulfur and phosphorus, have been reduced to an acceptable level. It has a melting point in the range of 1420–1470 K, which is lower than either of its two main components, and makes it the first product to be melted when carbon and iron are heated together. Its mechanical properties vary greatly, dependent upon the form carbon takes in the alloy. "White" cast irons contain their carbon in the form of cementite, or iron carbide. This hard, brittle compound dominates the mechanical properties of white cast irons, rendering them hard, but unresistant to shock. The broken surface of a white cast iron is full of fine facets of the broken carbide, a very pale, silvery, shiny material, hence the appellation. In grey iron the carbon exists free as fine flakes of graphite, and also renders the material brittle due to the stress-raising nature of the sharp edged flakes of graphite. A newer variant of grey iron, referred to as ductile iron is specially treated with trace amounts of magnesium to alter the shape of graphite to spheroids, or nodules, vastly increasing the toughness and strength of the material.
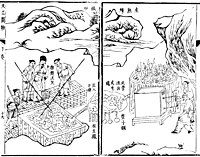
The fining process of smelting iron ore to make wrought iron from pig iron, with the right illustration displaying men working a blast furnace, from the Tiangong Kaiwu encyclopedia, published in 1637 by Song Yingxing.
- Wrought iron contains less than 0.25% carbon.[23] It is a tough, malleable product, but not as fusible as pig iron. If honed to an edge, it loses it quickly. Wrought iron is characterized by the presence of fine fibres of slag entrapped in the metal. Wrought iron is more corrosion resistant than steel. It has been almost completely replaced by mild steel for traditional "wrought iron" products and blacksmithing. Mild steel corrodes more readily that wrought iron, but is cheaper and more widely available.
- Carbon steel contains 2.0% carbon or less,[24] with small amounts of manganese, sulfur, phosphorus, and silicon.
- Alloy steels contain varying amounts of carbon as well as other metals, such as chromium, vanadium, molybdenum, nickel, tungsten, etc. Their alloy content raises their cost, and so they can usually only be justified for specialist uses. Recent developments in ferrous metallurgy have produced a growing range of microalloyed steels, also termed 'HSLA' or high-strength, low alloy steels, containing tiny additions to produce high strengths and often spectacular toughness at minimal cost.
The main disadvantage of iron and steel is that pure iron, and most of its alloys, suffer badly from rust if not protected in some way. Painting, galvanization, passivation, plastic coating and bluing are some techniques used to protect iron from rust by excluding water and oxygen or by sacrificial protection.
Iron compounds

- Iron oxides (FeO, Fe3O4, and Fe2O3) are ores used for iron production (see bloomery and blast furnace). They are also used as a catalyst in the Space Shuttle Solid Rocket Boosters,[25] and in the production of magnetic storage media in computers. They are often mixed with other compounds, and retain their magnetic properties in solution.
- Iron(II) acetate (Fe(CH3CO2)2 (ferrous acetate) is used as a mordant in the dyeing of cloth and leather, and as a wood preservative.
- Iron(III) ammonium citrate (C6H5+4yFexNyO7) is used in blueprints.
- Iron(III) arsenate (FeAsO4) is used in insecticides.
- Iron(III) chloride (FeCl3) is used in water purification and sewage treatment, in the dyeing of cloth, as a colouring agent in paints, as an additive in animal feed, and as an etchant for copper in the manufacture of printed circuit boards.
- Iron(III) chromate (Fe2(CrO4)3) is a yellow pigment for paints and ceramics.
- Iron(III) hydroxide (Fe(OH)3) is used as a brown pigment for rubber and in water purification systems.
- Iron(III) phosphate (FePO4) is used in fertilizers and as an additive in human and animal food.
- Iron(II) gluconate (Fe(C6H11O7)2) is used as a dietary supplement in iron pills.
- Iron(II) oxalate (FeC2O4) is used as yellow pigment for paints, plastics, glass and ceramics, and in photography.
- Iron(II) sulfate (FeSO4) is used in water purification and sewage treatment systems, as a catalyst in the production of ammonia, as an ingredient in fertilizer, herbicide, and moss killer, as an additive in animal feed, in wood preservative, and as an additive to flour to increase nutritional iron levels. Experimental iron fertilization of areas of the ocean using iron(II) sulfate has proven successful in increasing plankton growth.[26][27][28]
The use of iron compounds in organic synthesis is mainly for the reduction of nitro compounds.[29] Additionally, iron has been used for desulfurizations,[30] reduction of aldehydes,[31] and the deoxygenation of amine oxides.[32]
Biological role

Iron is essential to nearly all known organisms. In cells, iron is generally stored in the centre of metalloproteins, because "free" iron (which binds non-specifically to many cellular components) can catalyse production of toxic free radicals. Iron deficiency can lead to iron deficiency anemia.
In animals, plants, and fungi, iron is often the metal ion incorporated into the heme complex. Heme is an essential component of cytochrome proteins, which mediate redox reactions, and of oxygen carrier proteins such as hemoglobin, myoglobin, and leghemoglobin. Inorganic iron also contributes to redox reactions in the iron-sulfur clusters of many enzymes, such as nitrogenase (involved in the synthesis of ammonia from nitrogen and hydrogen) and hydrogenase. Non-heme iron proteins include the enzymes methane monooxygenase (oxidizes methane to methanol), ribonucleotide reductase (reduces ribose to deoxyribose; DNA biosynthesis), hemerythrins (oxygen transport and fixation in marine invertebrates) and purple acid phosphatase (hydrolysis of phosphate esters).
Iron distribution is heavily regulated in mammals, partly because iron has a high potential for biological toxicity[33]. Iron distribution is also regulated because many bacteria require iron, so restricting its availability to bacteria (generally by sequestering it inside cells) can help to prevent or limit infections. This is probably the reason for the relatively low amounts of iron in mammalian milk. A major component of this regulation is the protein transferrin, which binds iron absorbed from the duodenum and carries it in the blood to cells.[34]
Dietary sources
Good sources of dietary iron include red meat, fish, poultry, lentils, beans, leaf vegetables, tofu, chickpeas, black-eyed peas, fortified bread, and fortified breakfast cereals. Iron in low amounts is found in molasses, teff and farina. Iron in meat (haem iron) is more easily absorbed than iron in vegetables,[35] but heme/hemoglobin from red meat has effects which may increase the likelihood of colorectal cancer.[36][37]
Iron provided by dietary supplements is often found as iron (II) fumarate, although iron sulfate is cheaper and is absorbed equally well. Elemental iron, despite being absorbed to a much smaller extent (stomach acid is sufficient to convert some of it to ferrous iron), is often added to foods such as breakfast cereals or "enriched" wheat flour (where it is listed as "reduced iron" in the list of ingredients). Iron is most available to the body when chelated to amino acids - iron in this form is ten to fifteen times more bioavailable[38] than any other, and is also available for use as a common iron supplement. Often the amino acid chosen for this purpose is the cheapest and most common amino acid, glycine, leading to "iron glycinate" supplements.[39] The RDA for iron varies considerably based on age, gender, and source of dietary iron (heme-based iron has higher bioavailability).[40] Infants may require iron supplements if they are bottle-fed cow's milk.[41] Blood donors and pregnant women are at special risk of low iron levels and are often advised to supplement their iron intake.[citation needed]
Regulation of uptake
Iron uptake is tightly regulated by the human body, which has no regulated physiological means of excreting iron. Only small amounts of iron are lost daily due to mucosal and skin epithelial cell sloughing, so control of iron levels is mostly by regulating uptake.[42] Regulation of iron uptake is impaired in some people as a result of a genetic defect that maps to the HLA-H gene region on chromosome 6. In these people, excessive iron intake can result in iron overload disorders, such as hemochromatosis. Many people have a genetic susceptibility to iron overload without realizing it or being aware of a family history of the problem. For this reason, it is advised that people do not take iron supplements unless they suffer from iron deficiency and have consulted a doctor. Hemochromatosis is estimated to cause disease in between 0.3 and 0.8% of Caucasians.[43]
MRI finds that iron accumulates in the hippocampus of the brains of those with Alzheimer's disease and in the substantia nigra of those with Parkinson disease.[44]
Precautions
Large amounts of ingested iron can cause excessive levels of iron in the blood. High blood levels of free ferrous iron react with peroxides to produce free radicals, which are highly reactive and can damage DNA, proteins, lipids, and other cellular components. Thus, iron toxicity occurs when there is free iron in the cell, which generally occurs when iron levels exceed the capacity of transferrin to bind the iron. Damage to the cells of the gastrointestinal tract can also prevent them from regulating iron absorption leading to further increases in blood levels. Iron typically damages cells in the heart, liver and elsewhere, which can cause significant adverse effects, including coma, metabolic acidosis, shock, liver failure, coagulopathy, adult respiratory distress syndrome, long-term organ damage, and even death.[45] Humans experience iron toxicity above 20 milligrams of iron for every kilogram of mass, and 60 milligrams per kilogram is considered a lethal dose.[46] Overconsumption of iron, often the result of children eating large quantities of ferrous sulfate tablets intended for adult consumption, is one of the most common toxicological causes of death in children under six.[46] The Dietary Reference Intake (DRI) lists the Tolerable Upper Intake Level (UL) for adults as 45 mg/day. For children under fourteen years old the UL is 40 mg/day.
The medical management of iron toxicity is complex, and can include use of a specific chelating agent called deferoxamine to bind and expel excess iron from the body.[45][47]
See also
- El Mutún in Bolivia, where 20% of the world's accessible iron and magnesium is located.
- Iron Age
- Iron fertilization - Proposed fertilization of oceans to stimulate phytoplankton growth.
- Iron (metaphor)
- Iron in mythology
- List of countries by iron production
- Pelletising - Process of creation of iron ore pellets.
- Rustproof iron
- Specht Building - A historic landmark in Omaha, Nebraska utilizing an iron facade.
References
- ^ "Standard Atomic Weights: Iron". CIAAW. 1993.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- ^ Ram, R. S.; Bernath, P. F. (2003). "Fourier transform emission spectroscopy of the g4Δ–a4Δ system of FeCl". Journal of Molecular Spectroscopy. 221 (2): 261. Bibcode:2003JMoSp.221..261R. doi:10.1016/S0022-2852(03)00225-X.
- ^ Demazeau, G.; Buffat, B.; Pouchard, M.; Hagenmuller, P. (1982). "Recent developments in the field of high oxidation states of transition elements in oxides stabilization of six-coordinated Iron(V)". Zeitschrift für anorganische und allgemeine Chemie. 491: 60–66. doi:10.1002/zaac.19824910109.
- ^ Lu, J.; Jian, J.; Huang, W.; Lin, H.; Li, J; Zhou, M. (2016). "Experimental and theoretical identification of the Fe(VII) oxidation state in FeO4−". Physical Chemistry Chemical Physics. 18 (45): 31125–31131. Bibcode:2016PCCP...1831125L. doi:10.1039/C6CP06753K. PMID 27812577.
- ^ Cardarelli, François (2008). Materials Handbook: A Concise Desktop Reference. London: Springer. p. 65. ISBN 1-84628-668-9.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ "Iron and Nickel Abundances in H~II Regions and Supernova Remnants". June 14, 1995. Retrieved 2008-05-21..
- ^ a b Walter H. Kohl (1995). Handbook of materials and techniques for vacuum devices. Springer. pp. 164–167. ISBN 1563963876.
- ^ a b prepared under the direction of the ASM International Handbook Committee ; Howard Kuhn, Dana Medlin, volume editors. (2000). ASM Handbook – Mechanical Testing and Evaluation (PDF). Vol. 8. ASM International. p. 275. ISBN 0871703890.
{{cite book}}:|author=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ "Hardness Conversion Chart". Retrieved 2009-07-07.
- ^ V. Raghavan (2004). Materials Science and Engineering. PHI Learning Pvt. Ltd. p. 218. ISBN 8120324552.
- ^ "Properties of Various Pure Irons : Study on pure iron I". Tetsu-to-Hagane. 50 (1): 42–47.
- ^ John Wilson Martin (2007). Concise encyclopedia of the structure of materials. Elsevier. p. 183. ISBN 0080451276.
- ^ "Iron: geological information". Retrieved 2008-05-21..
- ^ Dauphas, N. & Rouxel, O. (2006). "Mass spectrometry and natural variations of iron isotopes" (PDF). Mass Spectrometry Reviews. 25: 515–550. doi:10.1002/mas.20078.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ The origins of Iron Working in India: New evidence from the Central Ganga plain and the Eastern Vindhyas by Rakesh Tewari (Director, U.P. State Archaeological Department)
- ^ E. Photos (1989). "The Question of Meteoritic versus Smelted Nickel-Rich Iron: Archaeological Evidence and Experimental Results". World Archaeology. 20 (3): 403–421.
- ^ Muhly, James D. 'Metalworking/Mining in the Levant' pp. 174-83 in Near Eastern Archaeology ed. S. Richard Winona Lake, IN: Eisenbrauns (2003): 180.
- ^ Donald B. Wagner (2003). "Chinese blast furnaces from the 10th to the 14th century". Historical Metallurgy. 37 (1): 25–37. originally published in Donald B. Wagner (2001). "Chinese blast furnaces from the 10th to the 14th century". West Asian Science, Technology, and Medicine. 18: 41–74.
- ^ a b Camp, James McIntyre (1920). The Making, Shaping and Treating of Steel. Pittsburgh: Carnegie Steel Company. pp. 173–174.
{{cite book}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ "Classification of Carbon and Low-Alloy Steels". Retrieved 2008-01-05.
- ^ Solid rocket boosters
- ^ Vivian Marx (2002). "The Little Plankton That Could…Maybe". Scientific American.
- ^ Melinda Ferguson, David Labiak, Andrew Madden, Joseph Peltier. "The Effect of Iron on Plankton Use of CO2". CEM 181H. Retrieved 2007-05-05.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Dopyera, Caroline (October 1996). "The Iron Hypothesis". EARTH. Retrieved 2007-05-05.
- ^ Fox, B. A.; Threlfall, T. L. (1973). Organic Syntheses. 5: 346
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link).
Fox, B. A.; Threlfall, T. L. (1964). Organic Syntheses. 44: 34{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link). Article - ^ Blomquist, A. T.; Dinguid, L. I. (1947). "Benzothiazoles. II. Nuclear chlorination in the Herz process". J. Org. Chem. 12: 718. doi:10.1021/jo01169a005.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Clarke, H. T.; Dreger, E. E. (1941). Organic Syntheses. 1: 304
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link).
Clarke, H. T.; Dreger, E. E. (1926). Organic Syntheses. 6: 52{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link). Article - ^ den Hertog, J.; Overhoff (1950). Recl. Trav. Chim. 69: 468.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Nanami, M.; et al. (2005). "Tumor necrosis factor-α-induced iron sequestration and oxidative stress in human endothelial cells". Arteriosclerosis, thrombosis, and vascular biology. 25 (12): 2495–2501. doi:10.1161/01.ATV.0000190610.63878.20.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Rouault, Tracey A. (2003). "How Mammals Acquire and Distribute Iron Needed for Oxygen-Based Metabolism". PLoS Biology. 1: e9. doi:10.1371/journal.pbio.0000079.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Food Standards Agency - Eat well, be well - Iron deficiency
- ^ Sesink AL, Termont DS, Kleibeuker JH, Van der Meer R (1999). "Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme". Cancer Research. 59 (22): 5704. PMID 10582688.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Glei M, Klenow S, Sauer J, Wegewitz U, Richter K, Pool-Zobel BL (2006). "Hemoglobin and hemin induce DNA damage in human colon tumor cells HT29 clone 19A and in primary human colonocytes". Mutat. Res. 594 (1–2): 162–71. doi:10.1016/j.mrfmmm.2005.08.006. PMID 16226281.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pineda O, Ashmead HD (2001). "Effectiveness of treatment of iron-deficiency anemia in infants and young children with ferrous bis-glycinate chelate". Nutrition. 17 (5): 381–4. doi:10.1016/S0899-9007(01)00519-6. PMID 11377130.
- ^ Ashmead, H. DeWayne (1989). Conversations on Chelation and Mineral Nutrition. Keats Publishing. ISBN 0-87983-501-X.
- ^ "Dietary Reference Intakes: Elements" (PDF). The National Academies. 2001. Retrieved 2008-05-21.
- ^ "Iron Deficiency Anemia" (web page). MediResource. Retrieved 2008-12-17.
- ^
Kumar, Vinay (2005). "Anemia". Robbins and Cotran: Pathologic Basis of Disease, 7th edition. Elsevier Saunders. Retrieved 2008-03-14.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Durupt S, Durieu I, Nove-Josserand R; et al. (2000). "Hereditary hemochromatosis". Rev Med Interne. 21 (11): 961–71. doi:10.1016/S0248-8663(00)00252-6.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Brar S, Henderson D, Schenck J, Zimmerman EA. (2009). Iron accumulation in the substantia nigra of patients with Alzheimer disease and parkinsonism. Arch Neurol. 66(3):371-4. PMID 19273756
- ^ a b Cheney K, Gumbiner C, Benson B, Tenenbein M (1995). "Survival after a severe iron poisoning treated with intermittent infusions of deferoxamine". J Toxicol Clin Toxicol. 33 (1): 61–6. doi:10.3109/15563659509020217. PMID 7837315.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - ^ a b "Toxicity, Iron". Emedicine. Retrieved 2006-06-19.
- ^ Tenenbein M (1996). "Benefits of parenteral deferoxamine for acute iron poisoning". J Toxicol Clin Toxicol. 34 (5): 485–9. doi:10.3109/15563659609028005. PMID 8800185.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)
Books
- Doulias PT, Christoforidis S, Brunk UT, Galaris D. Endosomal and lysosomal effects of desferrioxamine: protection of HeLa cells from hydrogen peroxide-induced DNA damage and induction of cell-cycle arrest. Free Radic Biol Med. 2003;35:719-28.
- H. R. Schubert, History of the British Iron and Steel Industry ... to 1775 AD (Routledge, London, 1957)
- R. F. Tylecote, History of Metallurgy (Institute of Materials, London 1992).
- R. F. Tylecote, 'Iron in the Industrial Revolution' in J. Day and R. F. Tylecote, The Industrial Revolution in Metals (Institute of Materials 1991), 200-60.




