From Wikipedia, the free encyclopedia
Exaprolol
Names
IUPAC name
1-(2-Cyclohexylphenoxy)-3-(propan-2-ylamino)propan-2-ol
Other names
Esprolol
Identifiers
ChemSpider
KEGG
UNII
InChI=1S/C18H29NO2/c1-14(2)19-12-16(20)13-21-18-11-7-6-10-17(18)15-8-4-3-5-9-15/h6-7,10-11,14-16,19-20H,3-5,8-9,12-13H2,1-2H3
Key: ABXHHEZNIJUQFM-UHFFFAOYSA-N
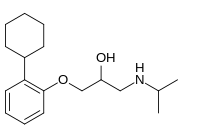
CC(C)NCC(COC1=CC=CC=C1C2CCCCC2)O
Properties
C 18 H 29 N O 2
Molar mass
−1
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Exaprolol is a beta-adrenoceptor antagonist .[ 1]
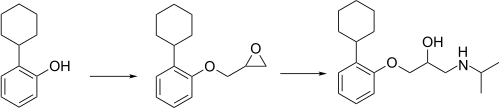
Exaprolol synthesis[ 2]
^ Van Waarde, A; Doorduin, J; De Jong, JR; Dierckx, RA; Elsinga, PH (2008). "Synthesis and preliminary evaluation of (S)-11C-exaprolol, a novel beta-adrenoceptor ligand for PET". Neurochemistry International . 52 (4–5): 729–33. doi :10.1016/j.neuint.2007.09.009 . PMID 17961850 . ^ Carissimi, M; Gentili, P; Grumelli, E; Milla, E; Picciola, G; Ravenna, F (1976). "Basic ethers of cyclohexylphenols with beta-blocking activity: Synthesis and pharmacological study of exaprolol". Arzneimittel-Forschung . 26 (4): 506–16. PMID 8056 .
α1
Agonists Antagonists
Abanoquil Ajmalicine Alfuzosin Anisodamine Anisodine Atiprosin Atypical antipsychotics (e.g., brexpiprazole , clozapine , olanzapine , quetiapine , risperidone )Benoxathian Beta blockers (e.g., adimolol , amosulalol , arotinolol , carvedilol , eugenodilol , labetalol )Buflomedil Bunazosin Corynanthine Dapiprazole Domesticine Doxazosin Ergolines (e.g., acetergamine , ergotamine , dihydroergotamine , lisuride , nicergoline , terguride )Etoperidone Fenspiride Hydroxyzine Indoramin Ketanserin L-765,314 mCPP Mepiprazole Metazosin Monatepil Moxisylyte Naftopidil Nantenine Neldazosin Niaprazine Niguldipine Pardoprunox Pelanserin Perlapine Phendioxan Phenoxybenzamine Phentolamine Phenylpiperazine antidepressants (e.g., hydroxynefazodone , nefazodone , trazodone , triazoledione )Piperoxan Prazosin Quinazosin Quinidine Silodosin Spegatrine Spiperone Talipexole Tamsulosin Terazosin Tiodazosin Tolazoline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , doxepin , imipramine , trimipramine )Trimazosin Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Urapidil WB-4101 Zolertine
α2
Agonists Antagonists
1-PP Adimolol Amesergide Aptazapine Atipamezole Atypical antipsychotics (e.g., asenapine , brexpiprazole , clozapine , lurasidone , olanzapine , paliperidone , quetiapine , risperidone , zotepine )Azapirones (e.g., buspirone , gepirone , ipsapirone , tandospirone )BRL-44408 Buflomedil Cirazoline Efaroxan Esmirtazapine Fenmetozole Fluparoxan Idazoxan Ketanserin Lisuride mCPP Mianserin Mirtazapine NAN-190 Pardoprunox Phentolamine Phenoxybenzamine Piperoxan Piribedil Rauwolscine Rotigotine Setiptiline Spegatrine Spiroxatrine Sunepitron Terguride Tolazoline Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Yohimbine
β