From Wikipedia, the free encyclopedia
Flestolol
Names
IUPAC name
3-{[1-(Carbamoylamino)-2-methyl-2-propanyl]amino}-2-hydroxypropyl 2-fluorobenzoate
Identifiers
ChemSpider
UNII
InChI=1S/C15H22FN3O4/c1-15(2,9-18-14(17)22)19-7-10(20)8-23-13(21)11-5-3-4-6-12(11)16/h3-6,10,19-20H,7-9H2,1-2H3,(H3,17,18,22)
Key: ZPLOQFLCMVIWRY-UHFFFAOYSA-N
CC(C)(CNC(=O)N)NCC(COC(=O)C1=CC=CC=C1F)O
Properties
C 15 H 22 F N 3 O 4
Molar mass
−1
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Flestolol is a short-acting beta adrenergic receptor antagonist .[ 1]
Synthesis
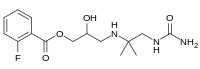
Flestolol synthesis:[ 2] Acylation of glycidol (2 ) with the acid chloride 1 produces the ester 3 . Reaction of that intermediate with amine 4 , obtained by reaction of 1,1-dimethylethylenediamine with urea, gives flestolol (5 ).
References
^ Quon, CY; Stampfli, HF (1993). "Biochemical characterization of flestolol esterase". Research communications in chemical pathology and pharmacology . 81 (3): 309–22. PMID 8235065 . ^ Kam, Sheung Tsam; Matier, William L.; Mai, Khuong X.; Barcelon-Yang, Cynthia; Borgman, Robert J.; O'Donnell, John P.; Stampfli, Herman F.; Sum, Check Y.; Anderson, William G. (1984). "[(Arylcarbonyl)oxy]propanolamines. 1. Novel .beta.-blockers with ultrashort duration of action". Journal of Medicinal Chemistry . 27 (8): 1007. doi :10.1021/jm00374a013 . PMID 6146718 .
α1
Agonists Antagonists
Abanoquil Ajmalicine Alfuzosin Anisodamine Anisodine Atiprosin Atypical antipsychotics (e.g., brexpiprazole , clozapine , olanzapine , quetiapine , risperidone )Benoxathian Beta blockers (e.g., adimolol , amosulalol , arotinolol , carvedilol , eugenodilol , labetalol )Buflomedil Bunazosin Corynanthine Dapiprazole Domesticine Doxazosin Ergolines (e.g., acetergamine , ergotamine , dihydroergotamine , lisuride , nicergoline , terguride )Etoperidone Fenspiride Hydroxyzine Indoramin Ketanserin L-765,314 mCPP Mepiprazole Metazosin Monatepil Moxisylyte Naftopidil Nantenine Neldazosin Niaprazine Niguldipine Pardoprunox Pelanserin Perlapine Phendioxan Phenoxybenzamine Phentolamine Phenylpiperazine antidepressants (e.g., hydroxynefazodone , nefazodone , trazodone , triazoledione )Piperoxan Prazosin Quinazosin Quinidine Silodosin Spegatrine Spiperone Talipexole Tamsulosin Terazosin Tiodazosin Tolazoline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , doxepin , imipramine , trimipramine )Trimazosin Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Urapidil WB-4101 Zolertine
α2
Agonists Antagonists
1-PP Adimolol Amesergide Aptazapine Atipamezole Atypical antipsychotics (e.g., asenapine , brexpiprazole , clozapine , lurasidone , olanzapine , paliperidone , quetiapine , risperidone , zotepine )Azapirones (e.g., buspirone , gepirone , ipsapirone , tandospirone )BRL-44408 Buflomedil Cirazoline Efaroxan Esmirtazapine Fenmetozole Fluparoxan Idazoxan Ketanserin Lisuride mCPP Mianserin Mirtazapine NAN-190 Pardoprunox Phentolamine Phenoxybenzamine Piperoxan Piribedil Rauwolscine Rotigotine Setiptiline Spegatrine Spiroxatrine Sunepitron Terguride Tolazoline Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Yohimbine
β